Statins have been used for controlling blood cholesterol levels and reducing the risk of cardiovascular morbidity and mortality. While their mechanism of action in vascular disease is established, evidence is accumulating regarding alternative immunomodulatory roles that may enhance their cardiovascular effect, as well as play a role in treating inflammatory diseases.1,2
Statins and the Mevalonate Pathway
3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, better known as statins, first became available in 1987 commercially,3 and are a group of medicines that can help lower the level of low-density lipoprotein (LDL) cholesterol (or ‘bad cholesterol’) in the blood. There are currently five types of statins available in the UK,4 which, although varying in variety of ways, all share the same cholesterol lowering effect.5 These are:
- Atorvastatin (Liptor)
- Fluvastatin (Lescol)
- Pravastatin (Lipostat)
- Rosuvastatin (Crestor)
- Simvastatin (Zocor)
It soon became apparent that statins possessed a variety of ‘pleiotropic effects,’ beneficial effects that are independent of the initial one for which they were originally intended. The synthesis of cholesterol occurs via the mevalonate pathway (see figure 1), and it is this pathway, for which statins were originally designed to work, that provides a starting point for understanding some of these pleiotropic effects.6
The enzyme responsible for catalysing the rate-limiting step of the mevalonate pathway is called HMG Co-A reductase. It catalyses the production of mevalonate from HMG-CoA.7 The sole product of the mevalonate pathway is not cholesterol, however, and blocking HMG-CoA reductase has far-reaching consequences that extend beyond decreased cholesterol production.
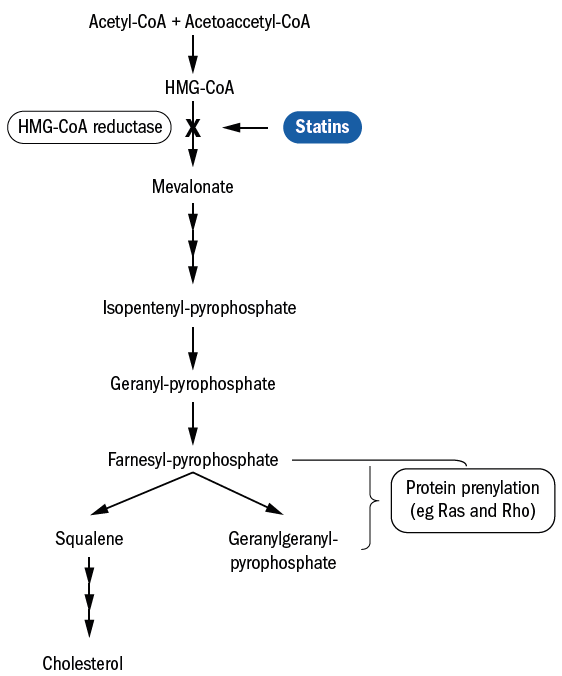
Figure 1: Statins and the cholesterol synthesis pathway – the mevalonate pathway 6
As can be seen from figure 1, there are two ‘intermediates’ of the mevalonate pathway:
- farnesyl pyrophosphate (FPP)
- geranylgeranyl pyrophosphate (GGPP)
By means of a process called isoprenylation, these
intermediates modify certain proteins in ways including:
- Influencing the Rha and Rho families of small G proteins8
- Forming lipophilic attachments for appropriate localisation within cell membranes and appropriate functional biological activity9,10
- Notable effects on inflammatory mechanisms
By blocking the modification of these intermediates, statins are thought to exert anti inflammatory effects.11 By influencing the cytokine balance from pro-inflammatory to anti-inflammatory, statins modulate the immune response and achieve an immunosuppressive effect.1,12 Additionally, statins alter the interaction between the vascular endothelium and lymphocytes by blocking the ICAM1 (intercellular adhesion molecule 1) pathway, which facilities transvascular migration of lymphocytes,13 thereby reducing the number of lymphocytes reaching the sites of inflammation. Figure 2 summarises the immunomodulatory effects of statins.
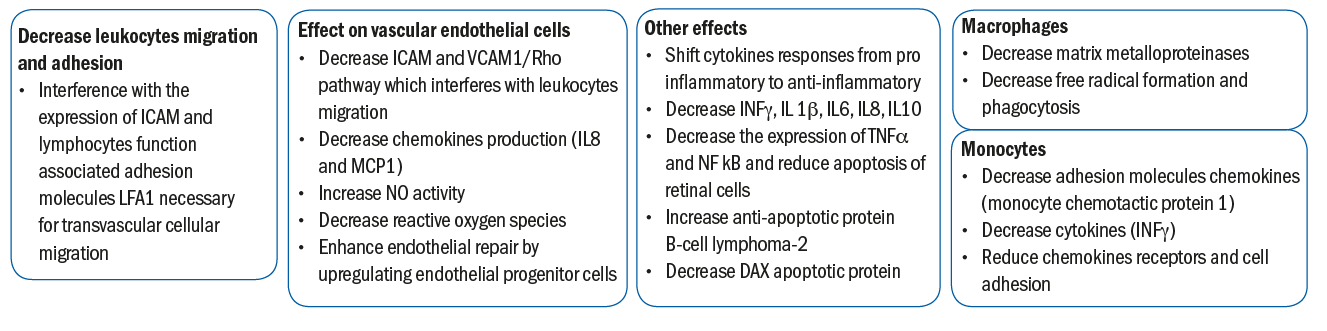
Figure 2: The immunomodulatory effects of statins 1
A full description of the immunological mechanisms is outside the scope of this review. However, special mention should be given to the process whereby white blood cells have to physically move from the circulation, to the target organ to induce an inflammatory response. This is achieved, in part, by adhesion molecules found on endothelial cells binding to adhesion molecules on the leukocyte and slowing the cell down. This interaction is of a relatively low ‘stickiness,’ and is sufficient to slow the leukocyte down into a more rolling movement. There are stronger adhesion molecules (ICAM1) on the endothelial cells lining the venule, which connect with other adhesion molecules (LFA1) on the leukocyte cell membrane allowing the cell to stop, in what is described as a stable arrest (figure 3). The leukocyte, now stopped, flattens out and ‘crawls’ between the gaps of the endothelial cells and into the tissue to exert its pro inflammatory effects. Statins hinder this process by inhibiting the ICAM1 pathway.
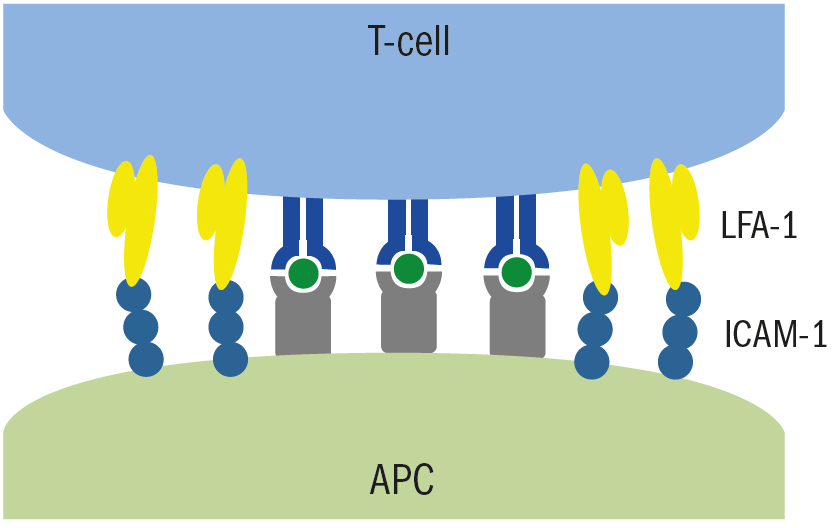
Figure 3: LFA1 on the surface of T-cell white immune cells in the blood connect with ICAM1 molecules on the antigen-activated cell. This adhesions stops the white blood cell from further passage in the blood and remain at the site of the antigen
Statins and Ocular Disease
Dry eye disease
The TFOS DEWS II study14 (Craig 2017) has defined dry eye disease (DED) as follows:
‘Dry eye is a multifactorial disease of the ocular surface characterised by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.’
The aetiology of dry eye disease can be categorised into two (non-mutually exclusive) groups:
- Aqueous deficient (ADDE)
- Evaporative (EDE) – this accounting for most DED
The unifying characteristics of DED is the loss of tear film homeostasis. The core mechanism of DED is an evaporation induced tear film hyperosmolarity, which is the hallmark of DED. This can lead to direct ocular surface damage, as well as the initiation of various inflammatory processes creating a self-perpetuating cycle of DED (figure 4).
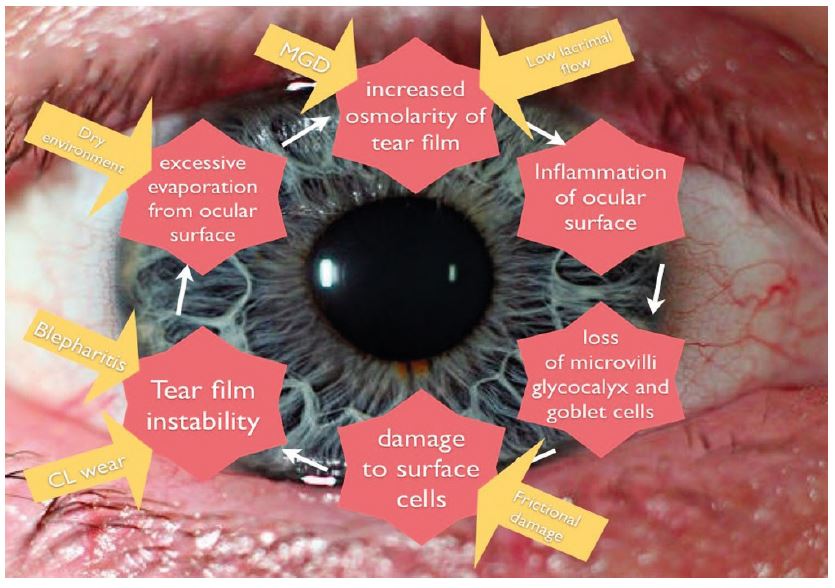
Figure 4 The vicious circle of dry eye (adapted by Farrant 15 after Bron 16)
In EDE related to meibomian gland dysfunction, tear hyperosmolarity occurs due to a tear film lipid layer deficiency which allows loss of the aqueous component.16 Meibomian gland disease results in an excess of free cholesterol and cholesterol esters in tears which disrupt the meibum layer, with resultant tear evaporation, ocular inflammation, and increased tear film osmolarity.17
There is a general up-regulation of the immune system in dry eye and blepharitis.18 In particular, inflammatory cells infiltrate the ocular surface epithelium, along with increases in pro-inflammatory cytokines within the tear film. These include IL-1, IL-6, IL-17, IFN-g, tumor necrosis factor (TNF-α), and proteases such as matrix metalloproteinase (MMP)-9.19 Each of these inflammatory mediators has a specific role in the inflammatory process.
MMP-9 is implicated in corneal epithelial tight junction protein degradation and the pathogenesis of dry eye.19 IL-17 also induces MMPs, and disrupts the corneal barrier during desiccating stress.20
IFN-g can induce conjunctival goblet cell loss and apoptosis. IL-1 and TNF-α are known to amplify the inflammatory response by inducing the expression of intercellular adhesion molecule (ICAM)-1 on epithelial cells in patients with dry eye.21 Given that leukocyte function antigen (LFA)-1 is also up-regulated in the conjunctiva of dry eye patients, the inhibition of the LFA-1 interaction with ICAM-1 has been shown to reduce dry eye. This is potentially through the inhibition of T cell adhesion to endothelial cells and the activation of antigen-presenting cells, thereby reducing the proinflammatory factors.22
Studies have shown HMG-CoA reductase within the meibomian, Zeis, and pilo-sebaceous glands, and also increased cholesterol in meibomian gland dysfunction and chalazion.23 The use of statins, and in particular topical artovostatin known to have good lipophilicity, may inhibit HMG-CoA reductase locally, thereby eliciting a reduction in proinflammatory cytokines and MMP-9. It also results in LFA-1 inhibition and therefore reduces its effects in the sebaceocytes of meibomian, Zeis, and pilo-sebaceous glands. In doing so, statins offer a possible cholesterol-lowering mechanism which may be beneficial in dry eye and meibomian gland dysfunction.18
Uveitis
The process by which immune cells ‘home in’ on target organs, so causing inflammation, is one of the hallmarks of autoimmune disease. One of the mechanisms for this involves leucocyte migration, adhesion and infiltration of tissue is facilitated by LFA1 and ICAM-1 as previously mentioned.25 Reduction in leucocyte infiltration of the eye is a consistent finding in autoimmune uveitis when treated with statins.25
Inflammation is modulated through a process of ‘tolerance’, whereby self-reactive inflammatory cells are ‘tuned down’ to prevent disease. Systemically, this tolerance involves regulatory cells, more specifically, T-Reg lymphocytes,26 which exert a suppressive function on inflammatory cells by the secretion of inhibitory cytokines and cytotoxic factors.27 The level of T-Reg cells in the blood is reduced during active uveitis when compared with healthy controls, with levels being significantly up regulated following treatment.28
Systemic statins appear to have a 48% preventative effect on the development of uveitis.29 Patients with non-infectious uveitis are often required to adhere to steroid therapy for long periods of time, with high initial loading doses of corticosteroids. Studies highlight the use of oral statins, in particular simvastatin,30 as a therapy as it has been shown that those patients on oral statins require fewer steroid drops than the control group who used steroid drops only.31
Cataract
The human crystalline lens has a unique structure, consisting of densely packed fibre cells. Most of these lose their organelles soon after they are formed, and it is thought that this organelle-free zone contributes towards lens transparency.32 The plasma membrane becomes the only membranous structure of the mature fibre cells. The unique biochemical characteristic of this plasma membrane is that it contains the highest cholesterol content of any known membrane in the body.33
The need for a high cholesterol content in the eye lens is not well understood.
Despite this uncertainty, it is thought that cholesterol depletion has a negative effect upon the lens microarchitecture and increases the risk of cataract.34 It would seem reasonable to conclude that statins would therefore promote cataract change. However, the literature provides conflicting findings.35 Some studies have found an increased risk of cataracts in statin users,36,37 while others show beneficial effects of statins that decrease the risk of cataracts or no effects at all.38,39 All these studies indicate, however, that cholesterol plays an important physiological role in the eye lens.
Glaucoma
The greater the level of total serum cholesterol, the greater risk of developing primary open angle glaucoma (POAG), with the risk of POAG increasing by 7% for every 20mg/dL increase in total serum cholesterol.40,41
Statins appear to have a role in reducing both glaucoma incidence and its progression.18 The suggested mechanisms appear to be based around reducing the resistance to aqueous outflow through the trabecular meshwork (TM) and Schlemm’s canal (SC).42 The glycoprotein known as SPARC (secreted protein acidic and rich in cysteine) is found in this region and impacts upon aqueous flow. Statins appear to down-regulate SPARC and facilitate aqueous outflow.42
The trabecular meshwork possess smooth muscle-like properties which are impacted by the intermediary, rho-kinase, which is actively involved in the regulation of aqueous humour outflow and intraocular pressure.44 Statins are known to inhibit rho-kinase, resulting in a ‘relaxation’ of the cells lining SC and the TM 45 resulting in increased aqueous outflow.45-47 This inhibition of rho-kinase (often reduced to the acronym ROCK) has recently led to the introduction of two topical ROCK inhibitors;
- Netarsudil; available in the US since 2017
- Ripasudil; available in Japan since 2018
Both of these agents have been approved for the management of POAG and ocular hypertension.
A further mechanism through which statins may have a beneficial effect upon POAG is the upregulation of nitric oxide on vascular endothelial cells.42 Nitric oxide is a potent vasodilator, and leads to increased retinal and choroidal blood flow which may benefit the optic nerve and retinal nerve fibre layer.18
Statin use in patients who have been taking them orally for two or more years has been associated with more than a 20% reduced risk of POAG. This effect was achieved with low dose (<40mg) statins, and no additional benefit at higher doses was found.48
There appears to be a protective effect against visual field progression associated with a history of statin use. This has been found in both normal tension glaucoma 49 and POAG glaucoma, with patients having reduced visual field progression rates compared with those not taking statins. 50
Macular degeneration
Although the exact mechanism of age-related macular degeneration (AMD) remains unknown, inflammation and oxidative damage have been implicated in playing a role in this disease.51 AMD and cardiovascular disease share a number of risk factors, such as smoking, elevated serum cholesterol, atherosclerosis and hypertension.52,53
It has been suggested that drugs lowering the risk of cardiovascular disease might also confer a protective effect for AMD. Statins reduce low-density lipoprotein (LDL) levels and exert anti-inflammatory effects, in addition to modifying dyslipidaemia (abnormal amounts of lipids). These effects are relevant in the development of AMD, indicating that statin use might play a potential role in reducing the risk of disease (figure 5).54,55
Figure 5: There may be several mechanisms whereby statins may reduce the risk and progression of AMD
Thus, attention to the possibility that cholesterol-lowering medications, such as statins already known to be effective in reducing cardiovascular disease, might also be beneficial in delaying the onset and progression of AMD.
There are a number of mechanisms by which statins may exert protective effects in AMD.1 These include:
- Statins lower serum lipid that may alter basement membrane lipid deposition56
- Statins preserve the vascular supply to the outer retina, thus maintaining the clearance of lipoproteins produced by the retinal pigment epithelium (RPE) and delaying the development of drusen57
- Statins have anti-inflammatory properties that may further affect the inflammatory processes underlying the development and progression of AMD
- The effect of statins upon the induction of heme oxygenase 1 and down-regulation of LDL and peroxidised lipids58 may protect the outer retina, choroid, and RPE from oxidative damage59
- Inhibition of the activation of macrophages by statins and the subsequent release of pro-inflammatory cytokines and matrix metalloproteinases (MMP 2), found in a choroidal neovascular complex (CNV) in wet AMD, delays CNV development1,59
The statin hypothesis has been evaluated in several clinical trials to assess risk reduction of disease onset and progression.1,55 Some studies have shown that the role of statins does appear to have a protective effect in both early disease as well as reducing the risk of CNV development, while having no effect on the development of geographic atrophy.60 However, the results remain mixed. A recent Cochrane review suggested insufficient evidence to support the hypothesis that statins prevent or delay AMD progression.61 However, there is also insufficient evidence to put the question to rest.55 Given the large potential benefit to patient quality of life and cost of care that even a modest protective effect would provide, further study is reasonable and warranted.55
Diabetic retinopathy
Diabetic retinopathy (DR) is a frequent complication of diabetes and increases in prevalence with disease duration. The most common causes of vision loss in diabetes are macular oedema and complications related to proliferative diabetic retinopathy.
There is mounting evidence regarding the role of endothelial dysfunction in the inflammatory processes leading to the development of DR.62 High glucose levels lead to an overproduction of free radicals, endothelial cell injury and a significant up regulation of proinflammatory cytokines (such as TNF-α, IL-1, ICAM1 and IL-6).63,64
This can lead to a breakdown of the blood-retina barrier (BRB), resulting in a loss of vascular integrity and increased permeability, leading to macular oedema and compromised blood supply. The latter is due, in part, to reduced levels of endothelium-dependent nitrous oxide and retinal ischaemia.65 Statins have a role in maintaining the stability of the BRB by reducing reactive oxygen species, increasing nitrous oxide levels and helping to regenerate the endothelial lining of blood vessels. This results in an improvement in vascular resistance, blood flow velocity and retinal perfusion of subjects with diabetic retinopathy.66 Moreover, statins (specifically atorvastatin) as discussed earlier, have pleiotropic functions on RPE cells, including anti-proliferation, anti-contraction, and anti-adhesion.67
Statins have demonstrated some effect in reducing the incidence of DR,68 as well as reducing the severity of background retinopathy,69 delaying the progression of DR70 and reducing the severity of diabetic macular oedema71 when used as an adjunct therapy.
Side Effects of Statins
Statins are generally well-tolerated and safe, and considered among the safest drugs used in medical practice.72 However, adverse effects are known and the most common is drug-induced muscle damage (myopathy), with patients presenting with symptoms of muscle soreness. However, in many cases, this resolves spontaneously. Routine blood tests have been advocated to monitor renal function of statin users, as there is a rare potential for the myopathy to lead to renal failure.73
Patients on more intensive statin therapy are at a greater risk of developing type 2 diabetes,74 although the benefits of reduced stroke and myocardial infarction outweigh the risks of developing DM.72
As well as systemic side effects, there is also the potential of ocular effects which account for 1.8% of statin reported adverse reactions.75 The most common ocular adverse events are blurred vision (48.8% of the effects reported) and visual impairment (25.7%). More recently, a possible relationship between statin therapy and myositis of the extraocular muscles and/or the levator palpebrae superioris muscles have been noted.75
Conclusion
The anti-inflammatory and immunomodulatory effects of statins are now widely accepted. The impact of such effects is supported by randomised controlled studies which have demonstrated that, further to their lipid-lowering ability, statins also exert anti-inflammatory effects in a wide variety of disease states. Statins are well tolerated and have a favourable side effect profile compared to other medications.
Dr Rohit Narayan is a therapeutic optometrist and Clinical Scientist at Aston University.
References
- Al-Janabi, Ahmed, Sue Lightman, and Oren Tomkins-Netzer. Statins in retinal disease. Eye 32.5 (2018): 981-991
- Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discovery, 2005;4:977–87
- Endo A. A historical perspective on the discovery of statins. Proceedings of the Japanese Academy Ser B Phys Biol Sci. 2010;86(5):484-93
- NICE Clinical Guideline 181, available at; www.nice.org.uk/guidance/cg181
- Chong PH, Seeger JD, Franklin C. Clinically relevant differences between the statins: implications for therapeutic selection. Am J Med. 2001 Oct 1;111(5):390-400.
- Gilbert, Rose, et al. ‘Statins as anti-inflammatory agents: A potential therapeutic role in sight-threatening non-infectious uveitis.’ Porto Biomedical Journal 2.2 (2017): 33-39
- Liao JK, Laufs U. Pleiotropic effects of statins. Annual Review of Pharmacological Toxicology. 2005;45:89-118
- Bustelo XR, Sauzeau V, Berenjeno IM. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. Bioessays. 2007 Apr; 29(4): 356–370
- Casey PJ (1995) Protein lipidation in cell signalling. Science 268:221–225
- Argmann CA et al. Regulation of macrophage cholesterol efflux through hydroxymethylglutaryl-CoA reductase inhibition: a role for RhoA in ABCA1-mediated cholesterol efflux. J Biol Chem. 2005 Jun 10;280(23):22212-21
- Jameel, Ashmal, et al. Statin modulation of human T-cell proliferation, IL-1 and IL-17 production, and IFN-T cell expression: synergy with conventional immunosuppressive agents. International Journal of Inflammation 2013 (2013)
- Kwak B, Mulhaupt F, Myit S, Mach F. Statins as a newly recognized type of immunomodulator. Nature Med. 2000;6:1399-402
- Yoshida M, Sawada T, Ishii H, Gerszten RE, Rosenzweig A,Gimbrone MA, et al. HMG-CoA reductase inhibitor modulates monocyte–endothelial cell interaction under physiological flow conditions in vitro. Arterioscler, Thromb, Vasc Biol. 2001;21:1165–71
- Craig J, et al. TFOS DEWS2. Available at; www.tearfilm.org/public/TFOSDEWSII-Executive.pdf
- Farrant, S. TFOS DEWS2 – part one. Optician, 08.09.2017
- Bron, Anthony J., et al. TFOS DEWS 2 pathophysiology report. The ocular surface 15.3 (2017): 438-510
- Arciniega, Juan C., Eduardo Uchiyama, and Igor A. Butovich. ‘Disruption and Destabilization of Meibomian Lipid Films Caused by Increasing Amounts of Ceramides and Cholesterol.’ Investigative Ophthalmology & Visual Science 54.2 (2013): 1352
- Ooi, Kenneth GJ, et al. Statins in ophthalmology. Survey of Ophthalmology 64 (2019).401-432
- Acera, Arantxa, et al. Inflammatory markers in the tears of patients with ocular surface disease. Ophthalmic Research 40.6 (2008): 315-321
- De Paiva CS, Chotikavanich S, Pangelinan SB, et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009;2(3):243e53
- Gao J, Morgan G, Tieu D, et al. ICAM-1 expression predisposes ocular tissues to immune-based inflammation in dry eye patients and Sjogrens syndrome-like MRL/lpr mice. Exp Eye Res. 2004;78(4):823e35
- Perez VL, Pflugfelder SC, Zhang S, et al. Lifitegrast, a Novel Integrin Antagonist for Treatment of Dry Eye Disease. Ocular Surface. 2016;14(2):207e15
- Knop E, Knop N, Millar T, et al. The International Workshop on Meibomian Gland Dysfunction: Report of the Subcommittee on Anatomy, Physiology, and Pathophysiology of the Meibomian Gland. Investigative Ophthalmology & Visual Science. 2011;52(4):1938e78
- Weitz-Schmidt, Gabriele, et al. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nature Medicine 7.6 (2001): 687
- Greenwood, John, Lawrence Steinman, and Scott S. Zamvil. Statin therapy and autoimmune disease: from protein prenylation to immunomodulation. Nature Reviews Immunology 6.5 (2006): 358
- Singh, Nevil J., and Ronald H. Schwartz. Primer: mechanisms of immunologic tolerance. Nature Reviews Rheumatology 2.1 (2006): 44
- Chavele, Konstantia-Maria, and Michael R. Ehrenstein. Regulatory T-cells in systemic lupus erythematosus and rheumatoid arthritis. FEBS letters 585.23 (2011): 3603-3610
- Ruggieri, Simona, et al. T-reg lymphocytes in autoimmune uveitis. Ocular Immunology and Inflammation 20.4 (2012): 255-261
- Borkar, Durga S., et al. Association between statin use and uveitis: results from the Pacific Ocular Inflammation study. American Journal of Ophthalmology 159.4 (2015): 707-713
- Jameel A et al. Statin Modulation of Human T-Cell Proliferation, IL-1β and IL-17 Production, and IFN-γ T Cell Expression: Synergy with Conventional Immunosuppressive Agents. Int J Inflam. 2013; 2013: 434-586
- Shirinsky, Ivan V., Anastasia A. Biryukova, and Valery S. Shirinsky. Simvastatin as an adjunct to conventional therapy of non-infectious uveitis: a randomized, open-label pilot study. Current Eye Research 42.12 (2017): 1713-1718
- Wride, M A. Lens fibre cell differentiation and organelle loss: many paths lead to clarity. Philosophical Transactions of the Royal Society B: Biological Sciences 366.1568 (2011): 1219-1233
- Cenedella, Richard J. ‘Cholesterol and cataracts.’ Survey of Ophthalmology 40.4 (1996): 320-337
- Herman, Gail E. Disorders of cholesterol biosynthesis: prototypic metabolic malformation syndromes. Human Molecular Genetics, 12.suppl_1 (2003): R75-R88
- Alves, Carlos, Diogo Mendes, and Francisco Batel Marques. Statins and risk of cataracts: A systematic review and meta‐analysis of observational studies. Cardiovascular Therapeutics, 36.6 (2018): e12480
- Lai, Chao-Lun, et al. ‘Statin use and cataract surgery: a nationwide retrospective cohort study in elderly ethnic Chinese patients.’ Drug Safety 36.10 (2013): 1017-1024.
- Leuschen, Jessica, et al. ‘Association of statin use with cataracts: a propensity score–matched analysis.’ JAMA Ophthalmology 131.11 (2013): 1427-1434
- Chodick, Gabriel, et al. ‘Persistence with statins and incident cataract: a population-based historical cohort study.’ Annals of Epidemiology 20.2 (2010): 136-142
- Tan, Jennifer SL, et al. ‘Statin use and the long-term risk of incident cataract: the Blue Mountains Eye Study.’ American Journal Of Ophthalmology 143.4 (2007): 687-689
- Widomska J, Subczynski WK, Mainali L, Raguz M. Cholesterol Bilayer Domains in the Eye Lens Health: A Review. Cell Biochem Biophys. 2017;75(3-4):387–398. doi:10.1007/s12013-017-0812-7
- Kang, Jae H., et al. ‘Association of statin use and high serum cholesterol levels with risk of primary open-angle glaucoma.’ JAMA ophthalmology (2019)
- Stein JD, Newman-Casey PA, Talwar N, et al. The relationship between statin use and open-angle glaucoma. Ophthalmology. 2012;119(10):2074e81
- Villarreal, Guadalupe, et al. Pharmacological regulation of SPARC by lovastatin in human trabecular meshwork cells. Investigative ophthalmology & Visual Science 55.3 (2014): 1657-1665
- Wiederholt, Michael, Hagen Thieme, and Friederike Stumpff. The regulation of trabecular meshwork and ciliary muscle contractility. Progress in retinal and Eye Research 19.3 (2000): 271-295
- Rao, P. Vasantha, et al. Modulation of aqueous humor outflow facility by the Rho kinase–specific inhibitor Y-27632. Investigative Ophthalmology & Visual Science 42.5 (2001): 1029-1037
- McGwin, Gerald, et al. Statins and Other Cholesterol-Lowering Medications and the Presenceof Glaucoma. Archives of Ophthalmology 122.6 (2004): 822-826
- Song, Julia, et al. Effects of cholesterol-lowering statins on the aqueous humor outflow pathway. Investigative Ophthalmology & Visual Science 46.7 (2005): 2424-2432
- Talwar, Nidhi, David C. Musch, and Joshua D. Stein. ‘Association of daily dosage and type of statin agent with risk of open-angle glaucoma.’ JAMA ophthalmology 135.3 (2017): 263-267
- Leung, Dexter YL, et al. Simvastatin and disease stabilization in normal tension glaucoma: a cohort study. Ophthalmology 117.3 (2010): 471-476
- Whigham B et al. The influence of oral statin medications on progression of glaucomatous visual field loss: A propensity score analysis. Ophthalmic Epidemiol. 2018 Jun;25(3):207-214
- van Lookeren C et al. Mechanisms of age‐related macular degeneration and therapeutic opportunities. The Journal of Pathology. 232.2 (2014): 151-164
- Erke, MG et al. Cardiovascular risk factors associated with age‐related macular degeneration: the Tromsø Study. Acta Ophthalmologica 92.7 (2014): 662-669
- Wu J et al. Age-related macular degeneration and the incidence of cardiovascular disease: a systematic review and meta-analysis. PLoS One 9.3 (2014): e89600
- Fong DS, Contreras R. Recent statin use and 1-year incidence of exudative age-related macular degeneration. Am J Ophthalmology, 149, 955–958 (2010)
- Roizenblatt M, Naranjit N, Maia M, Gehlbach PL. The Question of a Role for Statins in Age-Related Macular Degeneration. Int J Mol Sci. 2018;19(11):3688
- Rader, Daniel J., and Cyrille Maugeais. Genes influencing HDL metabolism: new perspectives and implications for atherosclerosis prevention. Molecular Medicine Today 6.4 (2000): 170-175
- Guymer RH, Chiu AW, Lim L, Baird PN. HMG CoA reductase inhibitors (statins): do they have a role in age-related macular degeneration? Surveys in Ophthalmology. 2005;50:194–206
- Curcio, Christine A., et al. Subretinal drusenoid deposits in non-neovascular age-related macular degeneration: morphology, prevalence, topography, and biogenesis model. Retina (Philadelphia, Pa.) 33.2 (2013)
- Guymer RH et al. Proof of concept, randomized, placebo-controlled study of the effect of simvastatin on the course of age-related macular degeneration. PloS one 8.12 (2013): e83759
- Ma L et al. The association between statin use and risk of age-related macular degeneration. Scientific reports 5 (2015): 18280
- Gehlbach P, Li T, Hatef E. Statins for age-related macular degeneration. Cochrane Database Syst Rev 2015;2:CD006927
- Landmesser U, Hornig B, Drexler H: Endothelial dysfunction in hypercholesterolemia: mechanisms, pathophysiological importance, and therapeutic interventions. Semin Thromb Hemost 26 :529 –537,2000
- Li, J et al. Systemic administration of HMG-CoA reductase inhibitor protects the blood–retinal barrier and ameliorates retinal inflammation in type 2 diabetes. Experimental eye research 89.1 (2009): 71-78
- Tang J, Kern TS. Inflammation in diabetic retinopathy. Progress Retin Eye Res. 2011;30:343–58
- Joussen AM et al. Non-steroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-alpha suppression. FASEB J. 2002;16:438–40
- Ozkiris A, Erkilic K, Koc A, Mistik S. Effect of atorvastatin on ocular blood flow velocities in patients with diabetic retinopathy. Br J Ophthalmol. 2007;91:69-73
- Wu WC et al. Pleiotropic role of atorvastatin in regulation of human retinal pigment epithelial cell behaviors in vitro. Exp Eye Res. 2011;93:842–51
- Nielsen, Sune F., and Børge G. Nordestgaard. Statin use before diabetes diagnosis and risk of microvascular disease: a nationwide nested matched study. The Lancet Diabetes & Endocrinology 2.11 (2014): 894-900
- Gordon B et al. The effects of lipid lowering on diabetic retinopathy. Am J Ophthalmol. 1991;112:385–91
- Sen K, Misra A, Kumar A, Pandey RM. Simvastatin retards progression of retinopathy in diabetic patients with hypercholesterolemia. Diabetes Res Clin Pract. 2002;56:1–11
- Gupta S. Does aggressive statin therapy offer improved cholesterol-independent benefits compared to conventional statin treatment? Int J Cardiol. 2004 Aug;96(2):131-9
- Guyton JR, Bays HE, Grundy SM, Jacobson TA, The National Lipid Association Statin Intolerance Panel. An assessment by the Statin Intolerance Panel: 2014 update. J Clin Lipidol. 2014;8 Suppl.:S72–81
- Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97:52c–60c
- Thakker D, Nair S, Pagada A, Jamdade V, Malik A. Statin use and the risk of developing diabetes: a network meta-analysis. Pharmacoepidemiol Drug Saf. 2016
- Mizranita V, Pratisto EH. Statin-associated ocular disorders: the FDA and ADRAC data. Int J Clin Pharm. 2015;37:844–50
