Many optometrists approach heterotropia with a sense of trepidation, especially when it is symptomatic or occurs in children. To some extent this is reasonable as tropias are not a daily presentation and hence we remain relatively inexperienced. Yet they must be managed appropriately, and the optometrist has an obligatory role to play. This article will attempt to clarify this role in non-pathological, comitant tropias (those not changing in size with direction of gaze) in adults and children.
Orthophoria is the ideal situation where the visual axes are perfectly aligned. Heterophoria is a latent deviation of the visual axes and heterotropia is the manifest version. At one end of the spectrum, the patient is asymptomatic because the vergence mechanism is readily capable of maintaining alignment of the visual axes under normal binocular viewing conditions, ie any phoria that is present is fully compensated. If this misalignment is the same size in all directions of gaze, it is described as comitant (or concomitant) and typically should not be interpreted as evidence of an active pathological process. In other words, in compensated heterophoria motor fusion is maintained, therefore sensory fusion is possible and stereopsis should occur. If the phoria is relatively large or the fusional reserves are relatively small, increasing degrees of decompensation of the heterophoria can occur with an increase in symptoms such as asthenopia, headaches and possibly intermittent diplopia (see part 2 in this series, Optician 17.05.19). As decompensation progresses, fusion may break down completely and permanently, resulting in a constant manifest misalignment of the visual axes: we now have a constant heterotropia. When this happens, double vision is an inevitable symptom (see figure 1) and this will need intervention.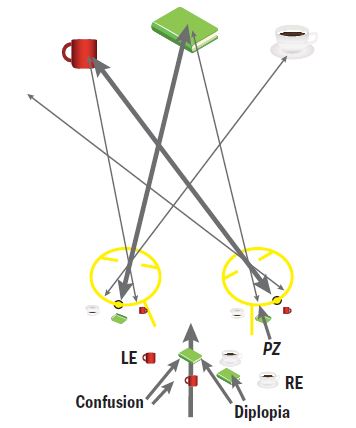
Figure 1: Right esotropia resulting in diplopia. The fixation target (green book) stimulates two different retinal locations (the fovea in the dominant eye, point zero in the tropic eye) and is seen as double. Each fovea receives a different target (green book and mug) so there is confusion. These extend across the whole of the visual field.
There are a number of reasons why decompensation of a comitant phoria may occur, and the age at which decompensation from phoria to tropia occurs can have an impact on the development of the visual/binocular system. These need to be considered and understood in order to arrive at suitable management strategies.
Developmental abnormalities and heterotropia
There are myriad developmental issues that can result in heterotropia. Anomalies in development of the skull or orbital structures, such as for example Apert’s syndrome, can lead to misalignment of the visual axes via orbital misalignment or displacement. The deviations caused can be considerable, increasing the likelihood of breakdown to heterotropia. Also, abnormality of skull development may have a detrimental impact on cerebral development, affecting development of visual and binocular processing. This will further increase the chances of decompensation. Such conditions are typically congenital or infantile in onset and are often associated with varying degrees of physical and/or learning disability and are usually managed within the hospital eye service.
Other non-structural developmental conditions, such as cerebral palsy, may affect the development of vision and/or the control of binocular system, resulting in an increased prevalence of heterotropia with or without learning disabilities. Any impediment to good vision in each eye (eg congenital cataract, corneal scarring, uncorrected anisometropia) is an impediment to sensory fusion, and hence to motor fusion. Another high-risk group is people with Down’s syndrome which is associated with learning impairment and an increased prevalence of both high refractive errors and heterotropia. Such is the potential impact of any systemic developmental condition on visual and binocular development, that all children with any suspected developmental issues should be thoroughly assessed for visual and binocular function.
Fully and partially accommodative heterotropia
Convergence and accommodation are inextricably linked, such that an increase in one will automatically result in an increase in the other with a typical ratio of three to five prism dioptres of convergence per dioptre of accommodation. This is logical as both need to increase by fixed amounts for increasingly close working distances. However, this often causes problems in hypermetropia. A child with good accommodation will accommodate over the hypermetropia to maintain clear vision. The accompanying convergence is likely to leave the child esophoric (assuming they started approximately orthophoric) and because negative fusional reserves are relatively small this induced phoria can easily break down to a tropia. The greater the hypermetropia, the greater the convergence and hence the higher the risk of heterotropia. If correction of the hypermetropia results in the restoration of orthophoria, the eso-deviation is described as fully accommodative and if binocularity is successfully restored, no further intervention may be necessary beyond refractive error correction. If the correction only partially corrects the eso-deviation, the deviation is described as partially accommodative. The eso component remaining after refractive error correction must be investigated with regards to the degree of compensation if it is a phoria. If it is a tropia, further investigation will be needed to establish if binocularity can be restored.
General health conditions
Some childhood infections, such as measles, are implicated in a direct breakdown of binocularity which may be permanent. Any systemic conditions that result in an overall decrease in wellbeing and energy levels can also precipitate a decompensation of an underlying phoria in children or adults and should be explored. This may include such conditions as stress or fatigue. Resolution or remediation of any such conditions may be enough to restore normal binocular function without further optometric intervention, although short term relief of symptoms may be appropriate. If no adequate predisposition presents itself for remedy, or the tropia persists beyond remediation, direct intervention will be required.
Idiopathic and sensory deficit heterotropia
Uncorrected refractive error or underlying ocular/systemic abnormality are not pre-requisites for either the presence of heterophoria or its breakdown to heterotropia, it can occur in children spontaneously. This is typically due to a fundamental developmental abnormality of the sensory component of binocularity, meaning sensory fusion is not robust, which in turn means motor fusion cannot be maintained. A predisposing heterophoria may be suspected, but a lack of optometric history may mean this cannot be confirmed. As this is considered to be a developmental abnormality, such a spontaneous breakdown is much less likely in adults.
The exact cause of the tropia may not always be easy to ascertain, but whatever the cause, there are some basic concepts in investigation and management.
Basic investigation and management of recent onset heterotropia in adults
Let us start with a simple example, where a patient presents with constant diplopia as the primary symptom and that any underlying causes for decompensation have been addressed, eg general health is good and an appropriate, up-to-date refractive correction is worn. That the diplopia is due to heterotropia rather than one of the monocular causes (eg some cataracts, corneal distortion) can be confirmed by demonstrating that occlusion of each eye in turn eradicates the diplopia. The direction and degree of heterotropia may be readily evaluated by simple observation (aided by observation of the corneal reflexes, ie the Hirschberg test) when it is large enough. This should be refined by the cover test, where the deviation can be more accurately measured by prism neutralisation (ie the prism cover test) although deviations smaller than 2 to 4 Δ can be difficult to spot. These smaller deviations are most readily detected and measured by the Maddox rod and Maddox wing for distance and near deviations respectively.
Once the motor status has been classified and measured, consideration needs to be given to the sensory status. If the patient is reporting diplopia, they have simultaneous perception but no flat fusion. The next step would be to establish if this can be improved upon and so to establish the patient’s binocular potential. Correcting the tropia by incorporating the neutralising prism identified on the prism cover test or the Maddox rod / wing will realign the foveae and hence allow for the assessment of any improvement in the binocular status. This is done by assessing the patient’s sensory status while aligned ie do they now have single vision (as would be hoped for) and if so, do they have stereopsis? This can be assessed by any appropriate stereo test. If there is no stereopsis, do they at least have flat fusion? This is perhaps best assessed using the Mallett unit (see figure 2) with the appropriate filters in place.

Figure 2: The Mallett Unit illustrating bifoveal vision, or ARC if a tropia is present and the target is large enough to exceed the suppression scotoma. Maintenance of this appearance when prism is introduced demonstrates motor and hence sensory fusion. 2a and b) illustrate suppression (either local or global) of one or the other eye. Alternating suppression would alternate between 2a and 2b, diplopia would see both simultaneously.
Both sets of dichoptic bars being visible establishes simultaneous perception, and if this is maintained along with single vision while small amounts of (base out) prism are added then motor fusion is occurring, therefore flat fusion is occurring. Immediate breakdown to diplopia would imply simultaneous perception only, ie no improvement in binocularity. Failure to demonstrate any improvement in the binocular status suggests that correction of the tropia is not justified unless on purely cosmetic terms. If, however, full binocularity is restored then some form of correction is required. As the tropia is almost certainly the result of decompensation of a pre-existing phoria, the prescribing approach is similar to that in decompensating heterophoria in that the minimum amount of prism that maintains stable binocularity should be prescribed, ie partial correction of the deviation. However, there seems to be less obvious guidance as to how this minimum amount should be arrived at.
One approach is to prescribe the full correcting prism, reducing the deviation to zero, to guarantee the re-establishment of binocularity, then seek to reduce the prism over time. This approach seeks to get around the fact that the fusional reserves are likely to be low (and the longer the tropia has been present the lower these reserves will be due to lack of exercise of the unused vergence mechanism) but will improve with time once fusion has been re-established. An option to try to reduce the magnitude of the first-prescribed prism is to prescribe the minimum amount that eliminates fixation disparity on the Mallett unit and/or the minimum amount that gives maximal stereo performance. Again, this can be reduced over time. As with heterophoria, spherical manipulation should be considered as a treatment option. Also, once fusion has been firmly re-established, vergence exercises can be considered to improve the fusional reserves.
Basic investigation and management of recent onset heterotropia in young children
For the purposes of binocular development, a young child is considered to be no more than approximately nine years old. After that they are treated as an adult. The basic principles with children are similar to those with adults, but there are some slight alterations needed for a less cooperative child. For all, a thorough refraction must be carried out, and if an eso deviation is not fully resolved (ie appears to be only partially accommodative) then a cyclo refraction is required. (Note that a full binocular assessment with the new cannot take place until the cyclo has worn off, hence a post-cyclo appointment will be necessary to assess the binocular potential.) Investigations may have to lean more on objective techniques. For example, classification/measurement will rely on Hirschberg and cover test rather than Maddox rod or wing. When assessing binocular potential with the deviation corrected (either by refractive error correction, prism or a combination of both) stereopsis may be difficult to demonstrate, but sensory fusion can be readily demonstrated with minimal cooperation at near, using the 10 (or 20) base out Δ test. While fixating a near target, the 10/20 base out prism is inserted and a convergence response is looked for, as is the complementary divergence on removal of the prism. A robust binocular system should quickly and automatically overcome such a prismatic challenge. A positive result confirms motor fusion and therefore sensory fusion; a negative result suggests its absence.
For young children still within the plastic or sensitive period of development, immediate correction with restoration of binocularity is essential in order to allow the restoration of development of a robust binocular system with stereopsis. Therefore, less emphasis is placed on ascertaining the ‘minimum’ prism required, and full prism correction is usually given. If binocularity has been restored via refractive correction alone then technically no further intervention (other than monitoring) may be needed, although the presence of amblyopia may need to be addressed and referral for all first-time presentations is recommended by some ophthalmologists to exclude any underlying pathology (check local protocols for referral and prescribing guidance). If restoration of binocularity can only be achieved by the addition of prism, referral for consideration of surgical correction is indicated. This restoration may not occur as there is likely to be an underlying sensory deficit. If no binocularity can be demonstrated under any circumstances, referral further investigation is required, but a simple failure of sensory development is the likely diagnosis. Surgery may now be considered for cosmetic purposes, but this may be delayed until the child is a little older. For all cases of referral, it is recommended that any significant refractive error be prescribed prior to the hospital appointment, as the impact of this refractive error on the binocular status needs to be known.
Sensory adaptations to early onset heterotropia
The visual system at birth is poorly developed with minimal visual acuity and virtually no recognisable binocular function. Rapid development occurs over what is known as the ‘sensitive’ or ‘plastic’ period. This rapid rate of development slows until, at around nine years of age, no significant further development can occur and the system can be considered to be ‘hard wired’. Any breakdown of binocularity after this age will result in the situation similar to that in an adult. If heterotropia develops during the sensitive period, then the course of development of both vision and binocularity may be altered. These alterations in development act to remove the troublesome symptom of diplopia and are collectively known as ‘sensory adaptations’ to heterotropia. Some, such as suppression and anomalous retinal correspondence (ARC), can be described as binocular adaptations (see figure 3) because they only occur (and hence can only be assessed) under binocular viewing conditions to eliminate the diplopia. A patient with these binocular adaptations will therefore be largely asymptomatic, but at the cost of reduced or absent stereopsis. If the dominant eye is occluded, these binocular adaptations should switch off to allow for foveal fixation with the non-dominant eye under these monocular conditions.
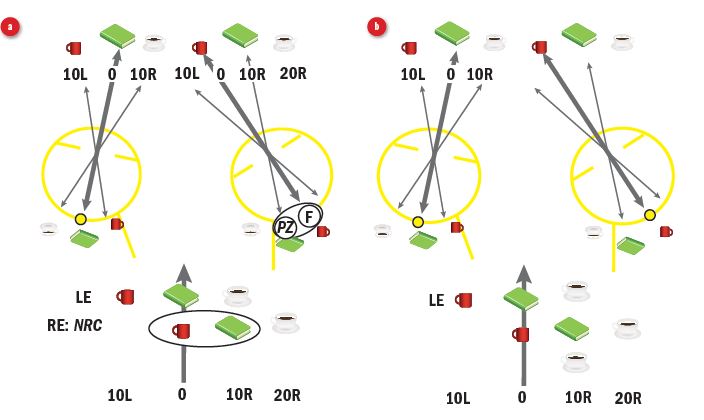
Figure 3: (a) Suppression of point zero and the fovea of the tropic eye eliminates the most troublesome central diplopia and confusion, but normal retinal correspondence (NRC) leaves diplopia in the periphery (eg two teacups). At this point in time, retinal correspondence has not adapted. (b) Changing the directional sense of the tropic eye to match that of the dominant eye shifts the perception to allow for restoration of single vision. Correspondence has adapted to ARC. Note maintenance of central suppression scotoma.
The other adaptations, namely amblyopia and eccentric fixation (EF), are described as ‘monocular adaptations.’ They are present under binocular conditions but they also persist under monocular viewing conditions, such as when the dominant eye is occluded, despite the occlusion automatically eliminating the troublesome diplopia.
Suppression and ARC
Perhaps the easiest adaptation to understand is global suppression. This involves the brain ‘ignoring’ the whole of the retinal image from the tropic eye, therefore abolishing simultaneous perception and therefore diplopia (in central suppression, only the central portion of vision is suppressed, with the peripheral retinal image still being available). But global suppression sacrifices all binocular function, as there can be no flat fusion and therefore no stereopsis. If the tropia alternates, so does the suppression. If the tropic eye is occluded, diplopia is eliminated and the temporarily redundant suppression will be switched off in order to allow the unoccluded eye to take up foveal fixation, otherwise there would be no available retinal image (and no vision). Global suppression is typically found in early onset exotropias and vertical tropias, and also in intermittent, unstable or large esotropias.
ARC involves the brain realigning the sense of direction of the tropic eye in order to make it match the sense of direction of the dominant eye, hence restoring a semblance of ‘normal’ binocularity. Figure 3 illustrates the basic concept, where the peripheral retinal location of ‘point zero’ in the tropic eye must be given a direction of straight ahead to match the fovea of the dominant eye, therefore eliminating the peripherally perceived second image and restoring binocular single vision. This realignment needs to happen across the whole of the visual field to eliminate peripheral as well as central diplopia. In reality this only occurs for the peripheral retina of the tropic eye, and is accompanied by a central suppression scotoma that covers point zero, the fovea and all points in between. This peripheral-only binocularity allows for coarse stereopsis, but this seems to be a better alternative than the total absence of binocularity in diplopia or global suppression. It typically occurs in small, stable, early onset esotropias.
ARC is best evidenced by use of the Bagolini lenses, a set of plano lenses with orthogonal fine striations (figure 4). When viewing a spotlight target bifoveally, each striated lens generates a feint line which combine to generate a feint cross superimposed upon the normal undistorted visual background (typically the test chart), centred upon the spotlight. In the presence of heterotropia and diplopia, there will be two spotlights and the cross will pass above or below these lights. With ARC, singularity has been restored and hence a single spot with a cross passing through it will be seen: virtually identical to the normal bifoveal percept (the line from the tropic eye may appear feinter and observant subjects may report a gap in this line either side of the spotlight that corresponds to the suppression scotoma that always accompanies ARC). If Bagolini lenses are not available, then the Mallett unit or stereopsis can be considered. If, with a tropic patient, both sets of bars are seen and are aligned on the Mallett unit, ARC is implied (but usually the bar for the tropic eye will fall within the central suppression scotoma and not be seen, figure 2). Coarse stereo may be evidenced by the Titmus fly or by the TNO screening plates. Such stereo in a tropic patient can be logically explained by ARC.
Subjectively, suppression is suggested by the absence of diplopia in the presence of a tropia, but this could be global suppression, or central suppression with ARC. Suppression can be assessed by any dichoptic test, where the targets that are available only to the tropic eye are not seen (the Worth Four Dot test is the textbook standard, but the more ubiquitous Mallett unit can perform the same function). As these targets are relatively small, they will fall within a typical suppression scotoma and hence will not be able to distinguish between a central scotoma and global suppression. To ascertain if the periphery is also being suppressed (and therefore if the suppression is global) the Bagolini lenses can be used, where global suppression will allow only one line to be seen. Alternatively, a 10/20 prism can be used. If this prism induces diplopia, then the periphery is in use and the suppression is central. If no diplopia is induced, it can be assumed that global suppression is present.
Amblyopia and eccentric fixation: the monocular adaptations
In the context of early onset heterotropia, amblyopia can be considered as a ‘hangover’ from suppression (note that there are numerous non-strabismic causes for amblyopia). If one eye is constantly suppressed from an early age, the neural connections within the visual cortex that serve that eye will atrophy. This will lead to poor visual acuity, even after refractive error correction and elimination of any pathology. There may also be an exaggerated crowding affect, where single letter acuity (uncrowded) is noticeably better than chart (crowded) acuity. Similarly, eccentric fixation can be considered a hangover of ARC. In this case, the fovea has lost its sense of ‘straight-ahead’ and when the dominant eye is covered, fixation with the tropic eye is not taken up by the fovea but by an eccentric retinal location, typically somewhere in between the fovea and point zero. This can be assessed by asking the patient to look into an ophthalmoscope graticule (if one is available) or into the centre of the macular stop. The centrality and stability can be compared between the dominant and the tropic eye. Eccentric fixation necessarily implies amblyopia and poor vision.
Management decisions when adaptations are present
The binocular adaptations form in order to eliminate diplopia, therefore some caution needs to be exercised in managing these patients because disruption of their adaptations could lead to the return of diplopia, which may be intractable. This situation should be avoided at all costs. The aims in children and adults can be very different. In young children, restoration of bifoveality, with an accompanying improvement in binocular status is a clear indication that the tropia should be corrected on a permanent basis, either by prism or surgery. Treatment of any accompanying amblyopia (ie patching) may be necessary. If caught very young and early, restoration of normal development patterns may result in further improvements in binocularity over time, although expectations should be kept modest and high-level stereopsis is unlikely.
The presence of monocular adaptations is a poor prognostic sign. Amblyopia may make the restoration of bifoveality difficult due to uneven VAs, and it may be almost impossible with eccentric fixation, without significant, intensive and potentially controversial treatment. Intervention that fails to restore binocularity runs the risk of intractable diplopia and a subsequent consecutive exotropia, which is cosmetically worse that an esotropia. Although optometrists are technically qualified to manage such cases, these interventions and treatments are risky and should not be carried out by those with limited experience of paediatric eye care. A more appropriate role for the optometrist is to correct any significant refractive error, and if this is not enough to restore normality, to refer for further investigation.
In older children and adults with adaptations, restoration of bifoveality is unlikely, and hence correction of the tropia is not likely to be done on the grounds of visual performance. If the adaptations include global suppression rather than ARC, then surgical correction for cosmetic reasons can be considered as the risk of intractable diplopia is low. If, on the other hand, ARC is present then extreme caution is advised. Any alteration in the angle of deviation could potentially result in intractable diplopia: the motor fusion is weak and cannot cope with the change, and the mature visual system does not have the flexibility to re-adapt the ARC to a new angle. An alteration could occur due to a change in hypermetropic correction resulting in a change in the vergence position, or due to changing the optical centration of the lenses in the false belief that the amblyopic/tropic eye can be ignored: it cannot if there is ARC and the peripheral retinal image is available. Changing the power of a ‘balance’ lens could also cause problems due to induced aniseikonia. The presence of monocular adaptations in such cases suggests that even if the alteration is carried out in an attempt to correct the tropia (either by prism or surgery) and results in visual axes alignment, sensory fusion will not occur with the possibility of diplopia and, over time, a consecutive exotropia developing. In all cases of adult tropia without diplopia, the potential impact of any changes should be carefully assessed before management decisions are made because once lost, restoration of single vision may be extremely problematic. Such a breakdown can also occur spontaneously. If single vision cannot be restored, occlusion of the tropic eye may be necessary via an occlusive contact lens, a frosted spectacle lens, or perhaps extreme fogging.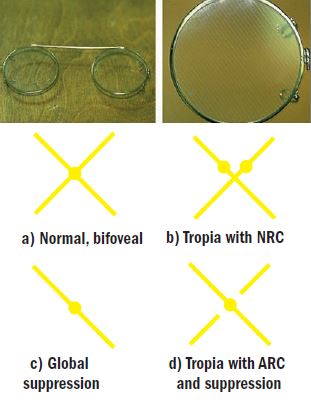
Figure 4: Bagolini lenses and their interpretation. The orthogonally-striated lenses each generate a line which combine to form a cross in normal, bifoveal, binocularity. The four typical views reported by patients are illustrated in a-d.
Conclusion
Heterotropias are not the most common presenting scenarios in optometric practice, but when they do present they can be problematic and need careful management. Recent onset diplopia should trigger an attempt to restore binocularity as quickly and fully as possible and can be approached as an extreme form of decompensating phoria. Tropias in children that are not readily resolved by refractive correction should be referred for further investigation, but expectations must be modest. Any significant refractive error should be prescribed at the outset so that its impact can be fully explored. Identification of adaptations suggests that management should be handed over to experienced paediatric practitioners who may or may not instigate treatment in an attempt to restore binocularity. For adults with an asymptomatic tropia, the emphasis is to avoid any potentially detrimental change. These patients may enquire about surgery for cosmetic reasons. The impact of such a change in angle must be carefully considered in order to avoid the inadvertent onset of intractable diplopia.
Dr Fergal Ennis is a Senior Lecturer (Teaching and Scholarship) at Cardiff University.
