What is ROCK?
Rho is a molecular switch within a cell and responds to messages from a variety of receptors on the cell surface.1 Rho kinase acts as an effector protein along the Rho pathway and attaches to Rho, forming a complex Rho-associated coiled-coil containing protein kinase, or ROCK. The ROCK modifies Rho through a process of phosphorylation (addition of PO3-), resulting in functional changes that regulates a variety of physiological processes.2 These include:
- Cell contraction
- Cell migration
- Chemotaxis
- Cell mitosis, proliferation and apoptosis
- Angiogenesis
- Neural protection
- Vasodilation
ROCK is expressed in all cell tissues, although the degree of expression varies according to cell tissue.3 Research on Rho kinase has been ongoing for the last 20 years,4,5 with implications in a wide range of systemic diseases including vascular disease, cancer, neuronal degenerative disease,3 asthma and glaucoma. The first clinical treatment (fasudil) was approved in 1995 for systemic use in the prevention of cerebral vasospasm in patients with subarachnoid haemorrhage.6 The up-regulation of the ROCK pathway is associated in the pathogenesis of a number of ocular disorders.7 It was not until 2014 that ROCK inhibitors (such as ripasudil) were approved in Japan for the treatment of primary open angle glaucoma (POAG) and ocular hypertension (OHT).8 The research supporting the involvement of ROCK signalling in glaucoma has now extended to other eye diseases, such as corneal endothelial disease, cataract, age-related macular degeneration, and proliferative vitreous retinopathy.5,8
ROCK molecules and the ROCK pathway have been implicated in a variety of disease processes, including vascular, neurological, renal and neoplastic diseases. Their role in cell proliferation, cell migration and cellular contraction makes them potential therapeutic targets. Over the past two decades, a number of inhibitor molecules have been developed, with different relative potencies and biological functions.3
This article will review the potential clinical benefit for the eye care professional in the inhibition of this pathway in a variety of ocular disorders through the use of ROCK inhibitors.
The Role of ‘ROCK’ In Glaucoma
Aqueous humour (AH) production occurs in the (non-pigmented) ciliary epithelium of the ciliary processes of the ciliary body,8a and is involves three mechanisms of transport of molecules mediated by protein transporters:
- Passive diffusion
- Ultrafiltration
- Active secretion
The active secretion, accounting for 80 to 90% of aqueous production,9 creates an osmotic gradient across the ciliary epithelium, promoting the movement of plasma constituents via diffusion and ultrafiltration.8a Once produced, aqueous travels from the posterior chamber to the anterior chamber via the pupil, and drains via either the ‘conventional’ or the ‘non-conventional’ route.
The majority of AH drainage exits via the conventional (or trabecular) route, so passing through the trabecular meshwork (TM), Schlemm’s canal (SC) and collector channels into the episcleral venous system.10 The non-conventional (or uveoscleral) route accounts for the drainage of the remaining aqueous which exits through spaces in the ciliary muscle and eventually into the suprachoroidal space, leaving the eye through the sclera by means of scleral perforations or the vortex veins.8a The TM accounts for 75% of the aqueous outflow.11
The TM consists of trabeculocytes, extra cellular matrix (ECM) and empty spaces.3 Functionally, these cells shows smooth muscle-like properties (by virtue of actin, myosin and ion channel expression) and provides a contractile ability that is controlled by many factors.3,12 Anatomically (figure 1), the juxtacanalicular tissue (JCT), part of the TM, is adjacent to Schlemm’s canal and offers the greatest resistance to aqueous outflow. The relaxation of the TM results in increased intercellular spaces and greater aqueous outflow.3

Figure 1: The traditional aqueous outflow route10
Glaucoma can be considered to be caused by a dysfunction of the trabecular meshwork outflow pathway causing increased intraocular pressure.13 The majority of anti-glaucoma drugs currently approved do not target the TM.14,15 The exception is pilocarpine which achieves improved TM and SC outflow indirectly through contraction of the ciliary muscle (CM). The use of pilocarpine is, however, associated with significant adverse effects,2 including accommodative spasm (in younger patients), cataract formation and retinal detachment.15
The mechanisms by which most topical therapies achieve IOP reduction is either by increasing the uveoscleral aqueous outflow (as with prostaglandin analogues) or by reducing the production of aqueous humour (as with β-blockers, α-agonists and carbonic anhydrase inhibitors).13,14,15
ROCK inhibitors are unique in increasing the aqueous outflow directly through the trabecular meshwork route outflow.13 ROCK is a kinase that regulates the proteins (actin and myosin) responsible for cellular contraction, as well as producing extracellular proteins. In the TM, the flow of the AH is regulated by the contraction of the TM cells and production of the extracellular matrix proteins.12 The introduction of ROCK inhibition is known to increase the AH outflow by relaxing TM cells by blocking their ability to contract, thereby opening up the empty space and reducing the extracellular matrix products. This results in a reduced resistance to outflow.7
ROCK also offers other beneficial mechanisms in glaucoma management. These include:
- Improved blood flow to the retina and ONH
- Neuroprotective effect16,17,18
- Anti-scarring activity (may have implications following filtration surgery)19,20
There are currently three ROCK inhibitors on the global
market:
- Ripasudil
- Rhopressa
- Rocklatan (formerly known as roclatan)2
These represent the first new class of ocular hypotensive drug since the introduction and FDA approval of prostaglandin analogues back in 1996.15 As of the time of writing, none of the ROCK inhibitors have been authorised for use in the UK and do not currently feature in UK ophthalmology management protocols,21 although this likely to change in the near future.22
Ripasudil
Ripasudil (ripasudil hydrochloride) has been approved for use in Japan since 2014, and is currently used as a second line treatment of glaucoma and OHT. It is available as 0.4% ophthalmic solution, one drop dosed twice daily,23 and is proposed either as a monotherapy24 or, more effectively, in combination.15,24 Ripasudil achieves its IOP-lowering effects through a combination of influencing TM behaviour and disrupting tight junctions in the endothelial cells of Schlemm’s canal.14 Its safety profile is good. The most common adverse reaction is mild conjunctival hyperaemia, a feature common to all ROCK inhibitors as discussed later. The hyperaemia presents within 10 minutes of instillation but resolves within two hours.25 The additional finding of mild to moderate allergic blepharitis has been mentioned,24,26 although the exact mechanism is unknown and not related to type I hypersensitivity.24
Netarsudil
Netarsudil (marketed as Rhopressa) is a 0.02% ophthalmic solution (2.5ml bottle) and, in December 2017, became the first ROCK inhibitor to gain FDA approval for the management of OAG and OHT28 with a qid dosage.29 Netarsudil does not currently have UK marketing authorisation although clinical studies are ongoing21 and NICE guidance is imminent.22
Netarsudil combines action as an inhibitor of the ROCK pathway and a norepinephrine (NET) transporter.30 Norepinephrine (NE) is more widely known in the UK as noradrenaline (NA). The function of the norepinephrine transporter is to inhibit the post-synaptic α-2 signalling. By blocking NET, there is a prolongation of α-2 signalling, (effectively producing an α-2 agonist effect), resulting in reduced aqueous humour production.12 The increased outflow results from a combination of dilation of the episcleral blood vessels and the expansion of the juxtacanalicular connective tissue.31 A number of randomised controlled studies have shown it to be as effective as latanoprost32 and, more recently, the ‘ROCKET’ studies show it be as effective as timolol, with a good safety profile.33,34 The more common adverse effects reported include conjunctival hyperaemia and asymptomatic corneal verticallata (figure 2). As mentioned earlier, this class of drug induces vasodilation, and coupled with the fact the medication is preserved with benzalkonium chloride 0.015%35 it is not surprising that mild conjunctival hyperaemia can occur. Asymptomatic corneal verticallata are a common finding with drugs that are cationic and amphiphilic (including systemic amiodarone and topical gentamacin and tobramycin)36 which promote the formation of the vortex through the process of phosphlipidosis.28

Figure 2: Corneal verticillata may be a common side effect of netarsudil
Rocklatan
Netarsudil has a role as a primary therapy as well as an adjunct to other treatment protocols.13 The most recent formulation is Rocklatan, a combination of netarsudil (0.02%) with latanoprost (0.005%) which gained FDA approval in June 2019.37
Rocklatan appears to be the first anti-glaucoma drop that lowers IOP using all three known mechanisms, thereby:
- Increasing AH outflow (both pathways)12,39
- Reducing AH production
- Reducing episcleral venous pressure2
Clinical trials have shown the combination drug lowers IOP by a further 1 to 3mmHg compared to either netarsudil or latanoprost alone (figure 3).2,37,39

Figure 3: Action of Rocklatan (a combination of netarsudil and latanoprost)
In summary, ROCK inhibitors are different from other glaucoma medications and represent a new class of glaucoma treatment.40 They are therapeutically effective, albeit with certain side effects by virtue of their mechanism of action, having greater effectiveness as an adjunct or combination drug.15 Further studies are needed to consider reducing the side effects whilst boosting their therapeutic potential40 alongside exploring their neuroprotective potential.15
ROCK and the Corneal Endothelium
The cornea is traditionally described as consisting of five distinct layers:
- Epithelium
- Bowman’s layer
- Stroma
- Descemet’s membrane
- Endothelium
The endothelium is the inner most layer of the cornea and is approximately 4 to 6µm thick with each endothelial cell having a diameter of 20µm.41 The endothelial cells have a high metabolic activity aided by a full complement of organelles. The endothelial cell surface possesses numerous microvilli and lateral inter-digitations with neighbouring cells as well as possessing adhesion, gap and tight junctions. Of particular interest is the fact that actin filaments form a circumferential band which plays a role in maintaining cell shape and mediating cell migration.42
The key function of the endothelium is to maintain corneal transparency. By means of a ‘pump and leak’ mechanism,43 the endothelium keeps the cornea in a relatively dehydrated state. Aqueous humour passes through the incomplete tight junctions of the endothelial cells to supply the stroma with nutrients.44 If this were to continue unchecked, the increase in fluid in the stroma would cause the cornea to swell, so disrupting the precise collagen fibre spacing and organisation within the stroma leading to loss of transparency. To minimise this potential, the endothelial cells activate Na+/K+ pumps, creating local osmotic gradients that draw fluid out of the stroma and back into the anterior chamber, so ensuring equilibrium so that stable corneal transparency is achieved.45
The physiology of the corneal endothelium is unlike that of the epithelium and lacks the ability to regenerate through mitosis.3,46 Cells are stuck in one of the phases (the G1 phase) of the cell cycle (figure 4),47 restricting further mitotic progression resulting in limited regeneration. The endothelial cell count (ECC) normally reduces in a linear fashion (0.6% loss per year) after the third decade of life (figure 5).48
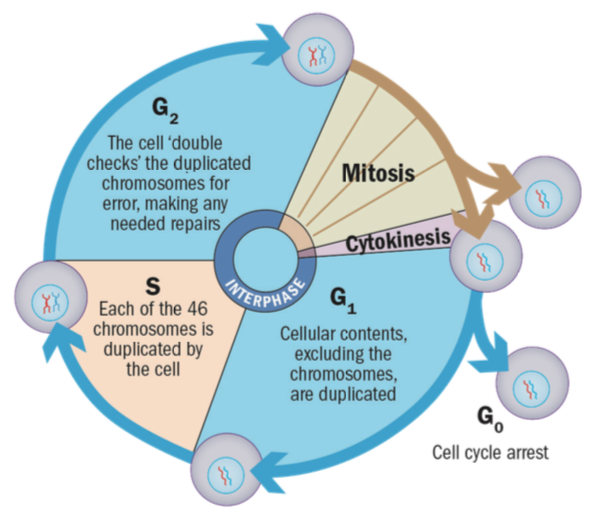
Figure 4: Actively dividing eukaryote cells pass through a series of stages known collectively as the cell cycle: two gap phases (G1 and G2); a synthesis phase (S), in which the genetic material is duplicated; and a mitosis phase (M), in which mitosis partitions the genetic material and the cell divides51
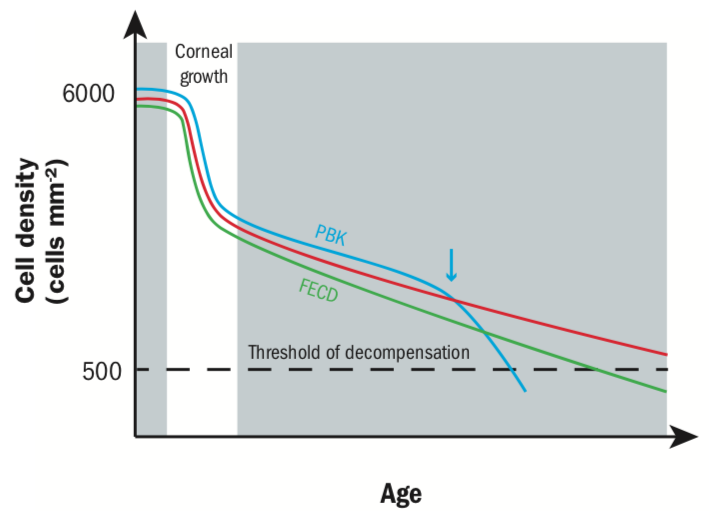
Figure 5: Corneal endothelial cell density with age and disease states. Corneal endothelial cell density is highest in newborns, but dramatically decreases when the corneal surface expands. In a healthy person, cell density drops by 0.6% per year. In cases of trauma, as found in pseudophakic bullous keratopathy (PBK) patients, cell density can suddenly drop (see arrow) under the decompensation threshold of around 500 cells mm-2. In Fuchs endothelial corneal dystrophy (FECD), cell loss will be higher annually in the long run, eventually leading to corneal oedema45
The remaining corneal endothelial cells can ‘recover’ through compensatory mechanisms, whereby the cells migrate and spread to effectively ‘fill in’ any gaps, so ensuring the maintenance of the endothelial pump and retaining corneal transparency.3,47 The typical density in a normal subject being 2,000 to 2,500 cells mm-2,49 whereas the minimum density of endothelial cells (the functional reserve to maintain corneal transparency) is approximately 500 cells mm-2.50
In disease states, such as Fuchs endothelial corneal dystrophy (FECD) or post-operative corneal decompensation after cataract surgery,47 the functional reserve is inadequate and the compensatory mechanism fails, resulting in corneal haze and reduced vision.52
The Rho pathway plays a role in the loss of cell adhesion which promotes cell death through apoptosis.4 By targeting and blocking the Rho pathway, this loss of cell adhesion is counteracted and may play a role in the treatment of diseased corneal endothelial states (figure 6).52

Figure 6: The molecular pathway for a role for ROCK signaling in apoptosis and mechanisms to explain how ROCK inhibitor suppresses apoptosis of the corneal endothelium. Apoptotic stimuli perceived by the corneal endothelium induces the activation of the ROCK pathway stimulating Actomyosin contraction with subsequent loss of cellular adhesion. This is followed by apoptosis. ROCK inhibitor treatment suppresses apoptosis by counteracting ROCK signaling, as well as by activating expression of the focal adhesion complex53
The use of ROCK inhibitors in managing corneal disease is a relatively new area of research and clinical data for their usefulness in FECD treatment remain sparse, so randomised controlled trials are still needed before adoption of this eye drop as a routine therapeutic option.54 Case reports have documented the benefits of topically applied ROCK inhibitor in the early stages of FECD to reduce endothelial apoptosis and increase cell proliferation.49 Interestingly, this technique appears to show promise in later stages of the disease, where a one week course to treatment resulted in significant and sustained vision gain (6/18 to 6/6) over a six-year period.55
FECD is a common reason for corneal transplantation. Surgical management aims to replace the diseased Descemet’s membrane and endothelial cells, so restoring corneal clarity.54 Previously, a full thickness penetrating keratoplasty was undertaken. However, this has been replaced by Descemet stripping endotheial keratoplasty (DSEK), whereby only Descemet’s membrane and the endothelium (with a thin stromal carrier) are removed. This has been further refined to Descemet membrane endothelial keratoplasty (DMEK) which offers a better recovery time and reduces the risk of rejection.44,56 Although these techniques are well established, they involve the use of donor tissue and the prolonged use of steroids which have the associated risk of raised IOP and cataract formation.56
Recently, the technique of Descemet’s stripping only (DSO) has shown promise in some cases of FECD.57 In this procedure, the central endothelium is removed and, provided the peripheral endothelial cell density is >1000mm-2, existing endothelial cells migrate from the periphery and fill in the empty space centrally (with the associated reduction in peripheral endothelial cell density), so restoring corneal clarity and eliminating the need for donor transplantation, graft rejection and prolonged steroid use.58 This DSO technique has been combined with topical use of a ROCK inhibitor (ripasudil) to speed up recovery and increase endothelial cell density than happens with DSO alone.56,57 The ROCK Inhibitors are applied to cultured endothelial cells taken from a donor or patient. The drug aids the proliferation of endothelial cells (in vitro) which are then injected into patient’s anterior chamber (figure 7).59

Figure 7: A simplified view of the hypothesised treatment of corneal endothelial damage using anterior chamber injections and/or topical eye drops of Rho kinase inhibitors5
Corneal decompensation following phacoemulsification is a known adverse finding, although recent advances in surgical technique minimise endothelial cell loss (1.7% to 2.0% at three months) in low risk patients.47 However, endothelial cell loss is more likely in higher risk patients, notably those with shallow anterior chamber depth (12.9%),60 advanced cataract (28.0%),61 small pupil size (16.2%), or intraoperative floppy-iris syndrome risk (12.0%).47
To summarise, recent studies are pointing to the effectiveness of ROCK inhibitors in reducing the rate of apoptosis in corneal endothelial cells. This could offer the possibility of prophylactic treatment by ROCK inhibitors prior to phacoemulsification.47
Diabetic Retinopathy
Diabetic retinopathy (DR), a leading cause of blindness worldwide, is present in over 50% of patients with diabetes mellitus (DM) of more than 20 years duration and is characterised by a variety of vascular changes.62 In particular, microvascular
complications involve hyperpermeability, angiogenesis, micro-thrombosis and inflammation.63 DR also shows similar characteristics of chronic neurodegenerative disease, involving retinal ganglion cells, retinal glial cells, photoreceptors, and the retinal pigment epithelium (RPE).3 Diabetic macular oedema (DMO) is a significant complication in eyes with DR.64
Anti-VEGF agents have become the first-line treatment for DMO,65 although not without some limitations. Treatment often requires ongoing repeated intravitreal injections, with a significant financial and clinical time burden. Adverse events following administration are low, though can be serious with endophthalmitis and cerebrovascular accidents being reported.66 Furthermore, a large percentage of patients still have a poor response to anti-VEGF agents even with frequently repeated injections.7,62,63 An alternative DMO treatment is the use of intravitreal injections of steroids.3,67 The injections are associated with more frequent ocular side effects, such as the development of cataracts and glaucoma, compared to injections of anti-VEGF agents.67 Therefore, interest in other therapeutic modalities with different mechanisms of action that are more cost-effective and safer is growing.3,62,65
The Rho pathway is involved throughout the pathogenesis of diabetic retinopathy,5,68 with particular impact on the retinal microvasculature and VEGF-induced angiogenesis,69 resulting in a direct effect on the retinal endothelial cells and RPE.70 Leukocytes adhere to the retinal endothelium of the blood vessels, via adhesion molecules (ICAM1). This ‘anchoring’ and increase in the number of leukocytes triggers the release of a variety of inflammatory mediators, growth factors and vascular permeability factors that compromise the blood-retinal
barrier.5,71,72
As previously mentioned, the Rho pathway promotes the ‘contraction’ of actin fibres, causing endothelial cell separation, thereby increasing permeability and impacting the blood retinal barrier (figure 8).72 This localised inflammation and endothelial cell damage induced by leucocytes is not controlled by VEGF inhibition alone and could explain why not all DMO patients are responsive to anti-VEGF therapy.63 Figure 9 summarises the mechanism of ROCK inhibitors in the treatment of DR.

Figure 8: A schematic diagram highlights the cause and effect of specific cytokines in altered vascular permeability in retinal capillaries in diabetes (adapted from 71). The leukocytes release cytokines which bind to receptors on the endothelial cell surface, activating the Rho/ROCK pathway, which results in a significant increase in actin stress fibre density and thickness. This leads to the endothelial separation with subsequent alteration in tight junctions72 and increased endothelial permeability with subsequent alteration of the blood retinal barrier (BRB)
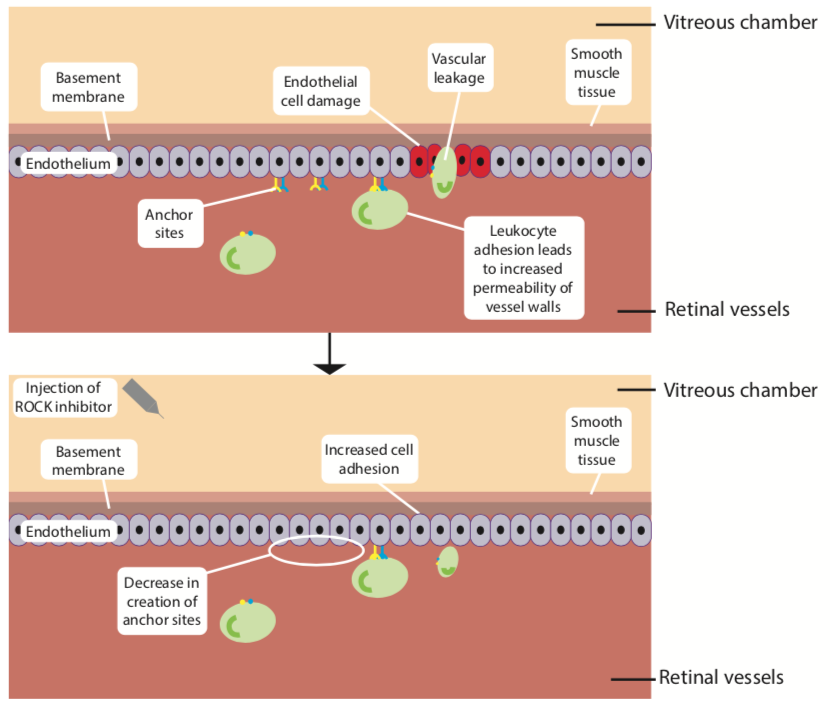
Figure 9: The mechanism of ROCK inhibitors in the treatment of diabetic retinopathy5
The Rho pathway is also linked with the down-regulation of nitric oxide levels (via the nitric oxide synthase pathway), hindering vasodilation and promoting apoptosis, further increasing leukocyte-induced damage.3,69 The introduction of ROCK
inhibitors intravitreally reduces the anchor sites for leukocytes and limits further damage to the endothelium7,73,74 and improves the functioning of tight junctions.75
Promoting nitric oxide and inducing vasodilation restores the blood flow to the cells in the retina, and particularly the macula, without the risk of increased vessel permeability, reducing the risk of apoptosis.73
Case reports63,65,74 have demonstrated the effect of dual use of the ROCK inhibitors fasudil74 and ripasudil65 with anti-VEGF as being more effective in treating DMO that anti-VEGF alone (figure 10), indicating a different mechanism of action,74 although larger clinical trials are required.65
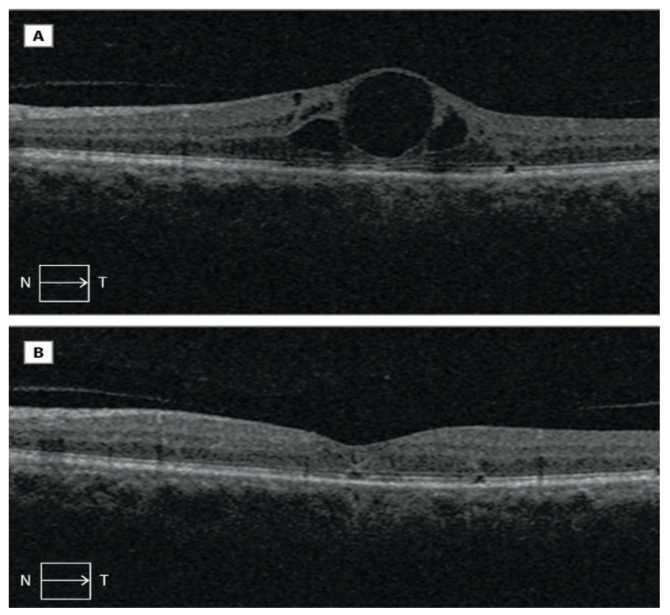
Figure 10: Appearance of the retina on OCT before and after treatment of DMO with combined therapy of intravitreal fasudil hydrochloride and bevacizumab
Conclusion
Table 1 summarises some key points about ROCK inhibitors. Although more clinical trials are needed,7 there is growing evidence that supports the usefulness of ROCK inhibitors as effective therapeutic modalities for the treatment of glaucoma, corneal disease and several further ophthalmological
disorders.2,8

Table 1: Key points about ROCK inhibitors
Dr Rohit Narayan is a therapeutic optometrist and Clinical Scientist at Aston University
References
- Bar-Sagi, D. and Hall, A., 2000. Ras and Rho GTPases: a family reunion. Cell, 103(2), pp.227-238.
- Berrino, E. and Supuran, C.T., 2019. Rho-kinase inhibitors in the management of glaucoma. Expert Opinion on Therapeutic Patents, 29(10), pp.817-827.
- Moura-Coelho, N., Ferreira, J.T., Bruxelas, C.P., Dutra-Medeiros, M., Cunha, J.P. and Proença, R.P., 2019. Rho kinase inhibitors—a review on the physiology and clinical use in Ophthalmology. Graefe’s Archive for Clinical and Experimental Ophthalmology, 257(6), pp.1101-1117.
- Okumura, Naoki, et al. “Activation of the Rho/Rho kinase signaling pathway is involved in cell death of corneal endothelium.” Investigative ophthalmology & visual science 57.15 (2016): 6843-6851.
- Moshirfar, M., Parker, L., Birdsong, O.C., Ronquillo, Y.C., Hofstedt, D., Shah, T.J., Gomez, A.T. and C Sr, P., 2018. Use of Rho kinase Inhibitors in Ophthalmology: A Review of the Literature. Medical Hypothesis, Discovery and Innovation in Ophthalmology, 7(3), p.101.
- Cohen, P., 2002. Protein kinases—the major drug targets of the twenty-first century?. Nature reviews Drug discovery, 1(4), p.309.
- Nourinia, R., Nakao, S., Zandi, S., Safi, S., Hafezi-Moghadam, A. and Ahmadieh, H., 2018. ROCK inhibitors for the treatment of ocular diseases. British Journal of Ophthalmology, 102(1), pp.1-5.
- Okumura, N., Nakao, S., Inoue, T. and Pattabiraman, P., 2017. Rho Kinase in Eye Disease. Journal of ophthalmology, 2017.
8a. Goel, M., Picciani, R.G., Lee, R.K. and Bhattacharya, S.K., Aqueous humor dynamics: a review. Open Ophthalmol J. 2010; 4: 52–9. - Mark, H.H., 2010. Aqueous humor dynamics in historical perspective. Survey of ophthalmology, 55(1), pp.89-100.
- Skalicky S.E. (2016) The Ciliary Body and Aqueous Fluid Formation and Drainage. In: Ocular and Visual Physiology. Springer, Singapore
- Grant, W.M., 1958. Further studies on facility of flow through the trabecular meshwork. AMA archives of ophthalmology, 60(4), pp.523-533.
- Bucolo, C., Platania, C., Drago, F., Bonfiglio, V., Reibaldi, M., Avitabile, T. and Uva, M., 2018. Novel therapeutics in glaucoma management. Current neuropharmacology, 16(7), pp.978-992.
- Kahook, M.Y., Serle, J.B., Mah, F.S., Kim, T., Raizman, M.B., Heah, T., Ramirez-Davis, N., Kopczynski, C.C., Usner, D.W., Novack, G.D. and ROCKET-2 Study Group, 2019. Long-term Safety and Ocular Hypotensive Efficacy Evaluation of Netarsudil Ophthalmic Solution: Rho Kinase Elevated IOP Treatment Trial (ROCKET-2). American journal of ophthalmology, 200, pp.130-137.
- Garnock-Jones, K.P., 2014. Ripasudil: first global approval. Drugs, 74(18), pp.2211-2215.
- Tanna, A.P. and Johnson, M., 2018. Rho kinase inhibitors as a novel treatment for glaucoma and ocular hypertension. Ophthalmology, 125(11), pp.1741-1756.
- Van de Velde, S.; De Groef, L.; Stalmans, I.; Moons, L.; Van Hove, I. Towards axonal regeneration and neuroprotection in glaucoma: Rho kinase inhibitors as promising therapeutics. Prog. Neurobiol., 2015, 131, 105-119.
- Goldhagen, B., Proia, A.D., Epstein, D.L. and Rao, P.V., 2012. Elevated levels of RhoA in the optic nerve head of human eyes with glaucoma. Journal of glaucoma, 21(8), pp.530-538.
- Yamamoto, K., Maruyama, K., Himori, N., Omodaka, K., Yokoyama, Y., Shiga, Y., Morin, R. and Nakazawa, T., 2014. The novel Rho kinase (ROCK) inhibitor K-115: a new candidate drug for neuroprotective treatment in glaucoma. Invest ophthalmology & visual science, 55(11), pp.7126-7136.
- Van de Velde, S; Van Bergen, T.; Vandewalle, E.; Kindt, N.; Castermans, K.; Moons, L.; Stalmans, I. Rho kinase inhibitor AMA0526 improves surgical outcome in a rabbit model of glaucoma filtration surgery. Prog. Brain Res., 2015, 220, 283-297.
- Rao, P.V., Pattabiraman, P.P. and Kopczynski, C., 2017. Role of the Rho GTPase/Rho kinase signaling pathway in pathogenesis and treatment of glaucoma: Bench to bedside research. Experimental eye research, 158, pp.23-32.
- NICE: Proposed Health Technology Appraisal Netarsudil for treating open angle glaucoma or ocular hypertension (Sept 2019) https://www.nice.org.uk/guidance/gid-ta10431/documents/draft-scope-pre-referral
- NICE Netarsudil for treating open angle glaucoma or ocular hypertension [ID1078]Proposed [GID-TA10431] https://www.nice.org.uk/guidance/proposed/gid-ta10431/documents
- KOWA Ripasudil drug information sheet: https://medical.kowa.co.jp/asset/item/67/94/7-pse_168.pdf
- Tanihara, H., Inoue, T., Yamamoto, T., Kuwayama, Y., Abe, H., Fukushima, A., Suganami, H., Araie, M., K-115 Clinical Study Group, Uchino, M. and Iwasaki, M., 2016. One-year clinical evaluation of 0.4% ripasudil (K-115) in patients with open-angle glaucoma and ocular hypertension. Acta ophthalmologica, 94(1), pp.e26-e34.
- Sakamoto, E., Ishida, W., Sumi, T., Kishimoto, T., Tada, K., Fukuda, K., Yoneda, T., Kuroiwa, H., Terao, E., Fujisawa, Y. and Nakakura, S., 2019. Evaluation of offset of conjunctival hyperemia induced by a Rho-kinase inhibitor; 0.4% Ripasudil ophthalmic solution clinical trial. Scientific reports, 9(1), p.3755.
- Maruyama, Y., Ikeda, Y., Mori, K., Yoshii, K., Ueno, M., Yoshikawa, H., Sotozono, C. and Kinoshita, S., 2019. Safety and efficacy of long-term ripasudil 0.4% instillation for the reduction of intraocular pressure in Japanese open-angle glaucoma patients. Investigative Ophthalmology & Visual Science, 60(9), pp.2394-2394
- Okeke “New Topical Medications for Glaucoma Treatment” https://collaborativeeye.com/articles/apr-19/new-topical-medications-for-glaucoma-treatment/
- FDA Advisory briefing document (2017): Rhopressa https://www.fda.gov/media/108389/download
- Rhopressa drug insert:https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208254lbl.pdf
- Wang, R.F.; Williamson, J.E.; Kopczynski, C.; Serle, J.B. Effect of 0.04% AR-13324, a ROCK, and norepinephrine transporter inhibi- tor, on aqueous humor dynamics in normotensive monkey eyes. J. Glaucoma, 2015, 24(1), 51-54.
- Ren R, Li G, Le TD, et al. Netarsudil increases outflow facility in human eyes through multiple mechanisms. Invest Ophthalmol Vis Sci 2016;57:6197–209.
- Bacharach, J., Dubiner, H.B., Levy, B., Kopczynski, C.C., Novack, G.D. and AR-13324-CS202 Study Group, 2015. Double-masked, randomized, dose–response study of AR-13324 versus latanoprost in patients with elevated intraocular pressure. Ophthalmology, 122(2), pp.302-307.
- Serle, J.B., Katz, L.J., McLaurin, E., Heah, T., Ramirez-Davis, N., Usner, D.W., Novack, G.D. and Kopczynski, C.C., 2018. Two phase 3 clinical trials comparing the safety and efficacy of netarsudil to timolol in patients with elevated intraocular pressure: Rho kinase elevated IOP treatment trial 1 and 2 (ROCKET-1 and ROCKET-2). American journal of ophthalmology, 186, pp.116-127.
- Khouri, A.S., Serle, J.B., Bacharach, J., Usner, D.W., Lewis, R.A., Braswell, P., Kopczynski, C.C., Heah, T. and Rocket-4 Study Group, 2019. Once-Daily Netarsudil vs Twice-Daily Timolol in Patients with Elevated Intraocular Pressure, the Randomized Phase 3 ROCKET-4 Study. American journal of ophthalmology
- Chaglassian E.L. (2018) ROCK and whorl. Review of Contact Lenses https://www.reviewofcontactlenses.com/article/rock-and-whorl
- Raizman, M.B., Hamrah, P., Holland, E.J., Kim, T., Mah, F.S., Rapuano, C.J. and Ulrich, R.G., 2017. Drug-induced corneal epithelial changes. Survey of ophthalmology, 62(3), pp.286-301.
- FDA Prescribing label ROCKLATAN (2019) https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/ 208259s000lbl.pdf
- Bergmann Koury, C. Rx Perspective: A new option for optimal IOP lowering. https://www.ophthalmologymanagement.com/issues/2019/august-2019/rx-perspective
- Abdelmseih M. “Roclatan: The next revolution in the medical management of Glaucoma”. Ophthalmology Research and Reports (2019) https://www.gavinpublishers.com/articles/editorial/Ophthalmology-Research-and-Reports/roclatan-the-next-revolution-in-the-medical-management-of-glaucoma
- Honjo, M. and Tanihara, H., 2018. Impact of the clinical use of ROCK inhibitor on the pathogenesis and treatment of glaucoma. Japanese journal of ophthalmology, 62(2), pp.109-126.
- Waring III, G.O., Bourne, W.M., Edelhauser, H.F. and Kenyon, K.R., 1982. The corneal endothelium: normal and pathologic structure and function. Ophthalmology, 89(6), pp.531-590.
- Dudley, C.E., Morell, A.J., Duffey, M.E. and Patel, S.P., 2019. Effects of amantadine on corneal endothelium. Experimental eye research, 181, pp.208-212.
- Barry, P.A., Petroll, W.M., Andrews, P.M., Cavanagh, H.D. and Jester, J.V., 1995. The spatial organization of corneal endothelial cytoskeletal proteins and their relationship to the apical junctional complex. Investigative ophthalmology & visual science, 36(6), pp.1115-1124.
- Feizi, S., 2018. Corneal endothelial cell dysfunction: etiologies and management. Therapeutic advances in ophthalmology, 10, p.2515841418815802.
- Van den Bogerd, B., Dhubhghaill, S.N., Koppen, C., Tassignon, M.J. and Zakaria, N., 2018. A review of the evidence for in vivo corneal endothelial regeneration. Survey of ophthalmology, 63(2), pp.149-165.
- Joyce NC. Proliferative capacity of corneal endothelial cells. Exp Eye Res 2012; 95: 16–23.
- Achiron, A., Feldman, A., Karmona, L., Avizemer, H., Barequet, I.S., Rosner, M., Knyazer, B., Bartov, E., Burgansky, Z. and Vishnevskia-Dai, V., 2018. Prophylactic exposure of human corneal endothelial cells to Rho-associated kinase inhibitor reduced apoptosis rate after phacoemulsification: Ex vivo study. Journal of Cataract & Refractive Surgery, 44(10), pp.1261-1266.
- Wilson, R.S. and Roper-Hall, M.J., 1982. Effect of age on the endothelial cell count in the normal eye. The British journal of ophthalmology, 66(8), p.513.
- Okumura, N., Kinoshita, S. and Koizumi, N., 2017. Application of Rho kinase inhibitors for the treatment of corneal endothelial diseases. Journal of ophthalmology, 2017.
- Agarwal, A., Jacob, S., Agarwal, A., Agarwal, S. and Kumar, A., 2006. Iatrogenic descemetorhexis as a complication of phacoemulsification. Journal of Cataract & Refractive Surgery, 32(5).
- University of Leicester Virtual Genetics Education Centre: “The Cell cycle, Mitosis and Meiosis” https://www2.le.ac.uk/projects/vgec/highereducation/topics/cellcycle-mitosis-meiosis. Accessed Dec 10th, 2019
- Joyce, N.C., 2003. Proliferative capacity of the corneal endothelium. Progress in retinal and eye research, 22(3), pp.359-389.
Okumura N, Koizumi N, Kay EP, et al. The ROCK inhibitor eye drop accelerates corneal endothelium wound healing. Invest Ophthalmol Vis Sci. 2013;54:2439–2502. - Okumura, N., Fujii, K., Kagami, T., Makiko, N., Kitahara, M., Kinoshita, S. and Koizumi, N., 2016. Activation of the Rho/Rho kinase signaling pathway is involved in cell death of corneal endothelium. Investigative ophthalmology & visual science, 57(15), pp.6843-6851.
- Okumura, Naoki et al. “Perspective of Future Potent Therapies for Fuchs Endothelial Corneal Dystrophy.” The open ophthalmology journal vol. 12 154-163. 23 Jul. 2018, doi:10.2174/1874364101812010154
- Koizumi, N., Okumura, N., Ueno, M., Nakagawa, H., Hamuro, J. and Kinoshita, S., 2013. Rho-associated kinase inhibitor eye drop treatment as a possible medical treatment for Fuchs corneal dystrophy. Cornea, 32(8), pp.1167-1170.
- Macsai, M.S. and Shiloach, M., 2019. Use of Topical Rho Kinase Inhibitors in the Treatment of Fuchs Dystrophy After Descemet Stripping Only. Cornea, 38(5), pp.529-534.
- Garcerant, D., Hirnschall, N., Toalster, N., Zhu, M., Wen, L. and Moloney, G., 2019. Descemet’s stripping without endothelial keratoplasty. Current opinion in ophthalmology, 30(4), pp.275-285.
- Borkar DS, Veldman P, Colby KA. Treatment of Fuchs endothelial dystrophy by Descemet stripping without endothelial keratoplasty. Cornea. 2016;35:1267–1273.
- Kinoshita, S., Koizumi, N., Ueno, M., Okumura, N., Imai, K., Tanaka, H., Yamamoto, Y., Nakamura, T., Inatomi, T., Bush, J. and Toda, M., 2018. Injection of cultured cells with a ROCK inhibitor for bullous keratopathy. New England Journal of Medicine, 378(11), pp.995-1003.
- Cho, Y.K., Chang, H.S. and Kim, M.S., 2010. Risk factors for endothelial cell loss after phacoemulsification: comparison in different anterior chamber depth groups. Korean Journal of Ophthalmology, 24(1), pp.10-15.
- Bourne, R.R., Minassian, D.C., Dart, J.K., Rosen, P., Kaushal, S. and Wingate, N., 2004. Effect of cataract surgery on the corneal endothelium: modern phacoemulsification compared with extracapsular cataract surgery. Ophthalmology, 111(4), pp.679-685.
- Sugimoto, M., Ichio, A., Mochida, D., Tenma, Y., Miyata, R., Matsubara, H. and Kondo, M., 2019. Multiple effects of intravitreal aflibercept on microvascular regression in eyes with diabetic macular edema. Ophthalmology Retina.
- Ahmadieh, H., Nourinia, R., Hafezi-Moghadam, A., Sabbaghi, H., Nakao, S., Zandi, S., Yaseri, M., Tofighi, Z. and Akbarian, S., 2019. Intravitreal injection of a Rho-kinase inhibitor (fasudil) combined with bevacizumab versus bevacizumab monotherapy for diabetic macular oedema: a pilot randomised clinical trial. British Journal of Ophthalmology, 103(7), pp.922-927.
- Moss, S.E., Klein, R. and Klein, B.E., 1988. The incidence of vision loss in a diabetic population. Ophthalmology, 95(10), pp.1340-1348.
- Minami, Y., Song, Y.S., Ishibazawa, A., Omae, T., Ro-mase, T. and Yoshida, A., 2019. Effect of ripasudil on diabetic macular edema. Scientific reports, 9(1), p.3703.
- Avery, R.L. and Gordon, G.M., 2016. Systemic safety of prolonged monthly anti–vascular endothelial growth factor therapy for diabetic macular edema: a systematic review and meta-analysis. JAMA ophthalmology, 134(1), pp.21-29.
- Lattanzio, R., Cicinelli, M.V. and Bandello, F., 2017. Intravitreal steroids in diabetic macular edema. In Management of Diabetic Retinopathy (Vol. 60, pp. 78-90). Karger Publishers.
- Yamaguchi, M., Nakao, S., Arima, M., Wada, I., Kaizu, Y., Hao, F., Yoshida, S. and Sonoda, K.H., 2017. Rho-kinase/ROCK as a potential drug target for vitreoretinal diseases. Journal of ophthalmology, 2017.
- Arita, R, Hata, Y and Ishibashi, T, 2010. ROCK as a therapeutic target of diabetic retinopathy. Journal of ophthalmology, 2010.
- Rothschild, P.R., Salah, S., Berdugo, M., Gélizé, E., Delaunay, K., Naud, M.C., Klein, C., Moulin, A., Savoldelli, M., Bergin, C. and Jeanny, J.C., 2017. ROCK-1 mediates diabetes-induced retinal pigment epithelial and endothelial cell blebbing: Contribution to diabetic retinopathy. Scientific reports, 7(1), p.8834.
- Monickaraj, F., McGuire, P.G., Nitta, C.F., Ghosh, K. and Das, A., 2015. Cathepsin D: an Mϕ-derived factor mediating increased endothelial cell permeability with implications for alteration of the blood-retinal barrier in diabetic retinopathy. The FASEB Journal, 30(4), pp.1670-1682.
- Cong, X. and Kong, W., 2019. Endothelial tight junctions and their regulatory signaling pathways in vascular homeostasis and disease. Cellular signalling, p.109485.
- Arita, R., 2011. Mechanism of diabetes-induced microvascular damage and therapeutic potential of ROCK inhibition. Nippon Ganka Gakkai Zasshi, 115(11), pp.985-997.
- Ahmadieh H, Nourinia R, Hafezi-Moghadam A (2013) Intravitreal fasudil combined with bevacizumab for persistent di- abetic macular edema: a novel treatment. JAMA Ophthalmol 131(7):923–924. https://doi.org/10.1001/jamaophthalmol.2013. 143
- Hida, Y., Nakamura, S., Nishinaka, A., Inoue, Y., Shimazawa, M. and Hara, H., 2018. Effects of ripasudil, a ROCK inhibitor, on retinal edema and nonperfusion area in a retinal vein occlusion murine model. Journal of pharmacological sciences, 137(2), pp.129-136.
