This article examines the issues that have led to infection prevention and control rising up the eye health care agenda in recent years, reaching fever pitch in early 2020 as the coronavirus pandemic swept the globe (figure 1). In defining infection and infection control, it is necessary to understand the basics of microbiology, the types of organism that can cause infection, and how infection is transmitted. The various methods of decontamination that practitioners have available to them are discussed, including the most important of them all – hand washing. Look out for a follow-up CET article focusing further on handwashing next week.
Figure 1: The spread of the virus SARS-CoV-2 that causes COVID-19 respiratory illness has been rapid and global
Healthcare-acquired infection has been big news since the Francis Report (2013) into the failings of Mid-Staffordshire NHS Trust between 2005 and 2009. At the time of writing, it is clear that, despite constant news of infection control procedures relating to COVID-19, the full nature of the disease remains poorly understood and measures to prevent its spread are proving very difficult to implement, more so where a practice is unprepared. The priority must be for any eye care practitioner to avoid catching or transmitting any infection that could threaten the sight or life of patients, colleagues or themselves. This is most evident now, but has always been the case.
Context
Until quite recently, infection control was little heard of in community eye care practice. An occasional media-reported scare about, for example, microbial keratitis would make contact lens practitioners in particular sit up and take notice. Yet such infections were often put down to poor patient compliance or simple bad luck, and within a short while it would be back to business as usual.
In the mid-1990s things started to be taken more seriously. UK practitioners saw new professional guidance being issued, each since updated several times. Notable examples include the guidance issued by the College of Optometrists (CoO) and Association of British Dispensing Opticians (ABDO) in response to the epidemic of bovine spongiform encephalopathy (BSE), or ‘mad cow disease,’ and the discovery that the same infective agent caused Creutzfeldt-Jakob Disease (CJD) in humans (human
variant or vCJD).1,2 Tests on donor corneas being used for penetrating keratoplasty (full thickness corneal transplantation) demonstrated that CJD could be spread from the corneas of infected individuals.3
BSE had far-reaching implications that were felt, and continue to be felt, worldwide. The author remembers witnessing the most disturbing infection control procedure while driving from Scotland to North West England at that time. A peculiar meaty smell was in the air as smoke crossed the motorway. Hundreds of infected cattle were being loaded onto funeral pyres in an attempt to halt the progress of the disease. Well over a decade later, the global meat industry has yet to fully recover. On July 17, 2017, the UK edition of China Daily reported on its front page ‘US state sends first batch of steaks to China since ban lifted’ telling of Nebraska’s excitement at restarting the supply of its biggest export to the world’s biggest potential market after a period of 14 years.4
There were also consequences for contact lens practice as clinicians were advised to use disposable trial lenses where possible. This undoubtedly hastened the market penetration of disposable lenses while lessening the use of long-term reusable lenses in all but the most difficult of cases due to the risk of infection and the need for more stringent chemical sterilisation methods. There were also concerns about the re-use of single use diagnostic drops, a tightening of the rules regarding the sterilisation procedures for contact tonometer prisms and other equipment, and the use of disposable versions of any equipment coming into direct contact with the patient became the norm (figure 2).
 Figure 2: Disposable equipment reduces the risk of cross-contamination
Figure 2: Disposable equipment reduces the risk of cross-contamination
In 2007, things moved on in the UK with the publication of Quality in Optometry, a toolkit for clinical governance in optometric practice that enshrined infection prevention and control procedures as a requirement to comply with the General Ophthalmic Services Contract to provide optical services on behalf of the National Health Service. This remains in force today.
Like CJD, many of the infections we seek to prevent in an eye care environment are vanishingly rare. However, it is important to realise that common or garden bugs that are found in, on or around our bodies, homes and gardens, even in tap water, can, under the right circumstances, have consequences that are truly devastating.
At its heart, the scandal that surrounded the Mid-Staffordshire NHS Foundation Trust was a story about infection control, bringing species of bacteria such as Clostridium difficile and methicillin-resistant Staphylococcus aureus (MRSA) into common parlance. When systems break down in a healthcare environment, avoidable infections and increased mortality and morbidity rates are the result. Partly in response to the recommendations of the Francis report and subsequent Berwick and Keogh reports,5 the UK General Optical Council introduced new Standards of Practice for Optometrists and Dispensing Opticians in April 2016. Infection control is specifically mentioned in Standard 12: ‘Ensure a safe environment for your patients’.6
In 2020, infection control became a daily topic of conversation as the global pandemic of coronavirus crossed the planet with devastating consequences, initially on daily life and livelihoods, and then progressively in terms of fatalities as the disease spread around the world aided by international travel, internet fuelled misinformation and poor compliance with recommended infection control procedures.
What is infection?
Infection has been defined by Millodot as ‘an invasion of the body by disease-producing microorganisms.’7 The emphasis is on ‘disease-producing,’ since most microorganisms are completely harmless or even beneficial to humans, which is just as well since we have trillions of them about our bodies at any given time.
Infectious disease still accounts for around a quarter of all human deaths worldwide and is a major cause of morbidity especially in the developing world where it is associated with poverty, overcrowding and a lack of the basic infrastructure that we take for granted in more affluent nations. In recent years, the fall of vaccination rates has also seen the resurgence of conditions that had virtually been eradicated worldwide as the population of many countries, including the UK, have lost the herd immunity conferred by universal vaccination against diseases such as measles, mumps and rubella. The ease with which misinformation is disseminated via modern media is a key factor in this trend.
Often microorganisms will cause infection which our immune system can easily defend against, while other infections need additional intervention with antimicrobial drugs. For a small number of organisms, treatment is only effective if a correct diagnosis is made early, while for others there is no cure and death is often inevitable. Unfortunately, this latter list is growing thanks to the ability of microorganisms to evolve resistance to the most common treatments against them. A further growing list is that of organisms that are new to humans, such as the virus SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) that causes the new human disease known as COVID-19. Such pathogens have evolved or mutated from other similar microorganisms, perhaps in other species, and present a particular problem to healthcare systems. Challenges include insufficient treatment options for a yet-to-be-understood pathogen, inadequate treatment resource capacity, and lack of required reserves of infection control resource, such as suitable personal protective equipment.
Before focusing on contamination and sterilisation, a brief reminder of some key points regarding microbiology and the causes of infection. Microbiology describes the study of organisms that cannot be observed with the naked eye. These organisms are far and away the most abundant on Earth, both in terms of absolute numbers and numbers of species, most of which are yet to be formally identified and classified.
Classification of microorganisms
Living things are classified according to three domains, a biological classification introduced by Carl Woese and colleagues in 1990:
- Bacteria – diverse, widespread single-celled organisms.
- Archaea – once thought to be bacteria, but now known to have distinct molecular characteristics, these single-celled organisms are widespread and found in the most inhospitable environments on the planet.
- Eukarya – all organisms whose cells contain a membrane-bound nucleus.
Both bacteria and archaea are unicellular and prokaryotic, that is they do not have a nuclear membrane. Archaea are thought to be the evolutionary forebears of the eukarya whose cells are eukaryotic, that is they contain a nuclear membrane. Eukaryotes may be multicellular differentiated (animals, plants, complex fungi), multicellular undifferentiated (such as a slime mould), or single celled organisms (such as Acanthamoeba).
The presence of a nuclear membrane is important for the way antibiotics are able to selectively target bacteria without harming the cells of the host. Antibiotics are said to be bactericidal if they kill bacteria, and bacteriostatic if they prevent reproduction while allowing the host’s immune system time to deal with the infection. Antimicrobial chemicals such as benzalkonium chloride, are used in different concentrations as preservatives (which prevent microbes growing and reproducing) and disinfectants (which kill microorganisms).
Infection causing organisms
Infection causing microorganisms include the following:
- Algae – Prototheca wickerhamii has been found to cause ulcerative keratitis.
- Bacteria – such as Pseudomonas aeruginosa and Staphylococcus aureus.
- Fungi – such as Candida albicans and Fusarium species.
- Protozoa – such as Toxoplasma gondii and Acanthamoeba spp.
- Viruses – such as Herpes simplex and Herpes zoster.
- Virus-like proteins (prions) – such as the cause of vCJD.
Algae are photosynthesising eukaryotes, and range from single celled diatoms to seaweeds such as kelp. They cause phytotoxicosis if their toxins are ingested with shellfish or contaminated water.
Bacteria are prokaryotic unicellular organisms that are classified by a number of factors:
- Shape under the microscope – bacilli, cocci and so on.
- Sensitivity to oxygen – aerobic, anaerobic.
- How they take up a variety of stains used in the preparation of microscope slides – most notably the Gram stain. Those taking up the stain are Gram-positive, those not Gram-negative.
Further classification is through establishing their ability to break down a range of substances. Most eye infections are bacterial in nature, from acute bacterial conjunctivitis (figure 3) typically caused by Staphylococcus aureus, Streptococcus pneumoniae, and Haemophilus species, to trachoma (figure 4), caused by Chlamydia trachomatis. Trachoma remains the commonest cause of infection-related blindness worldwide. 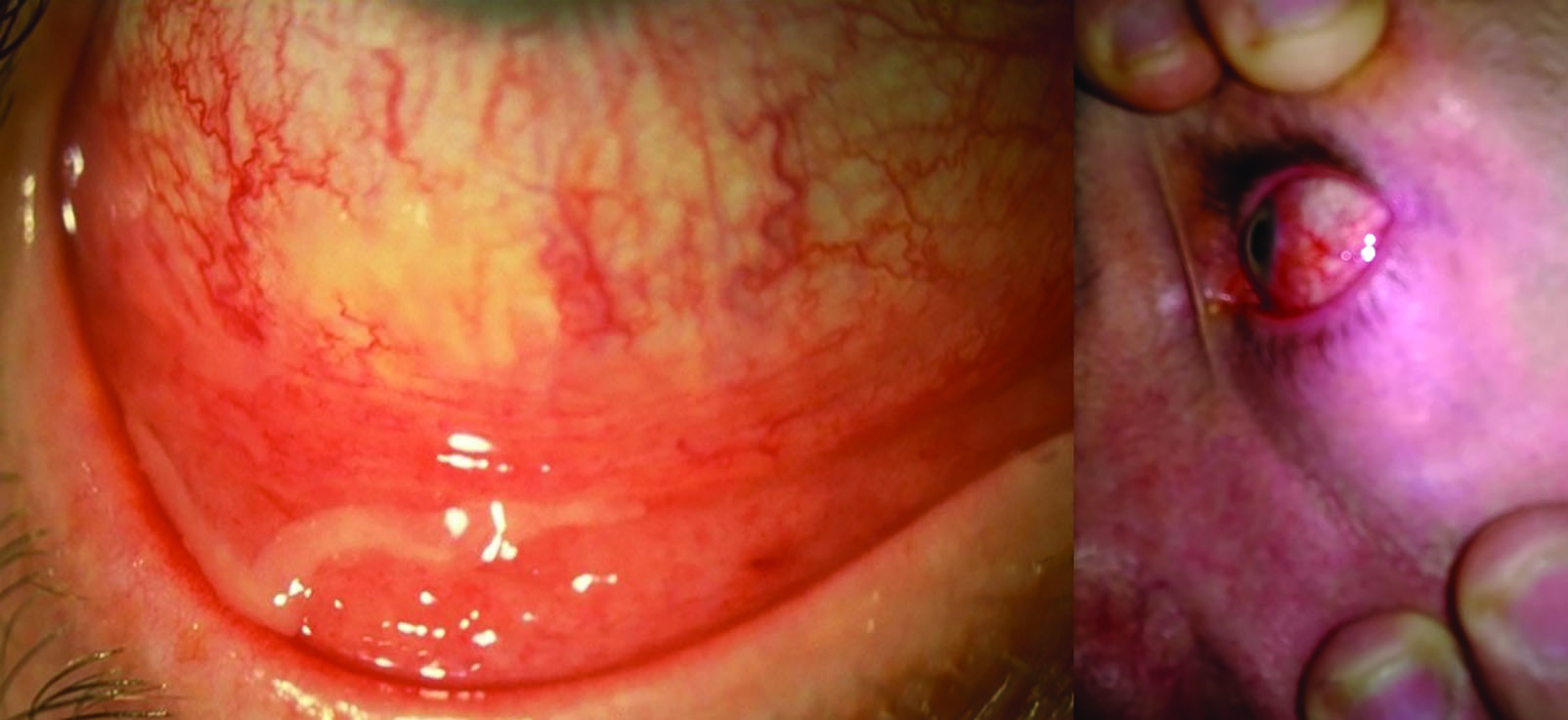 Figure 3: Acute bacterial conjunctivitis
Figure 3: Acute bacterial conjunctivitis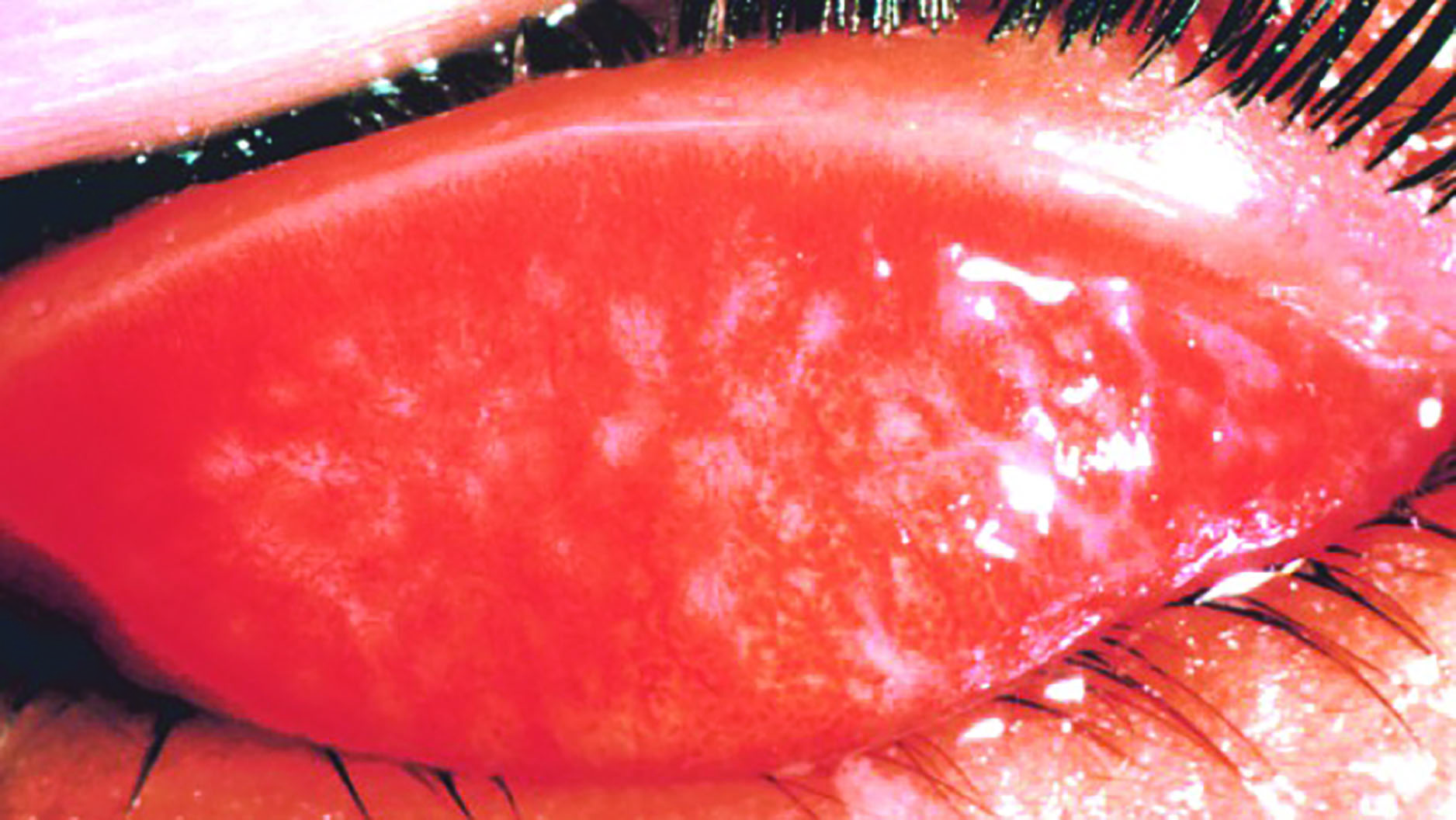 Figure 4: Trachoma
Figure 4: Trachoma
Trachoma is easily prevented by adequate washing facilities and general face hygiene but remains endemic in many developing nations and, notably, in Australia among aboriginal communities where it causes an increase of six times the average population rate of blindness.8 Chlamydia trachomatis infects the inside of the upper lid and, after repeated infection usually over a period of years, the resultant scarring leads to cicatricial entropion and trichiasis. The in-turned lashes abrade the cornea and eventually lead to opacity and blindness. Prior to the lids turning inwards, the infection is easily treated with the antibiotic azithromycin which should be offered to everyone in infected households or communities every six to 12 months.
Fungi are eukaryotes, and include species ranging from single-celled yeasts, through multicellular filamentous fungi to complex fungi such as our familiar edible mushrooms. Of concern in ophthalmic practice are Fusarium and Aspergillus species and the ubiquitous Candida albicans yeast. Fungal eye infections are opportunistic, usually very rare, but are typically sight-threatening. Wave of sight loss through chorioretinitis due to Candida have been traced to the use of non-sterile needles and substrates among intravenous drug users.
Prions are protein-based infectious agents of animals that do not contain any nucleic acid. Prions cause disease by interacting with the proteins encoded in the host genome, converting a harmless molecule into a damaging protein that causes cell death. Examples of prion illnesses include BSE and vCJD where the prion protein accumulates in the brain, killing neurones and creating sponge-like holes.9 Prion disease is of concern in ophthalmic practice, as it has been shown to be transmitted between patients as a result of ineffective decontamination of contact diagnostic and corneal surgical equipment.
Protozoa are the smallest unicellular animals and much larger than most bacteria. Being eukaryotic, they have a nucleus surrounded by a limiting membrane lying within the cytoplasm which itself is split into ectoplasm (which acquires nutrient sources such as bacteria) and endoplasm (which processes the nutrients). In amoeboid species, the endoplasm flows into finger-like pseudopodia to allow movement and ingestion of external nutrient sources. Other protozoa achieve motility through the action of surface flagella or cilia.10

Plasmodium species cause malaria which infects around 200 million people worldwide each year and is responsible for around half a million deaths.11 In ophthalmic practice, protozoa of concern include Toxoplasma gondii, a common cause of chorioretinitis (figure 5), and Acanthamoeba spp. which causes sight threatening keratitis (figure 6) – see case study. Acanthamoebae have a free living trophozoite form (figure 7) and under adverse conditions form highly resistant cysts (figure 8) which are immune to most disinfection and some sterilisation techniques.
 Figure 5: Toxoplasmosis scarring
Figure 5: Toxoplasmosis scarring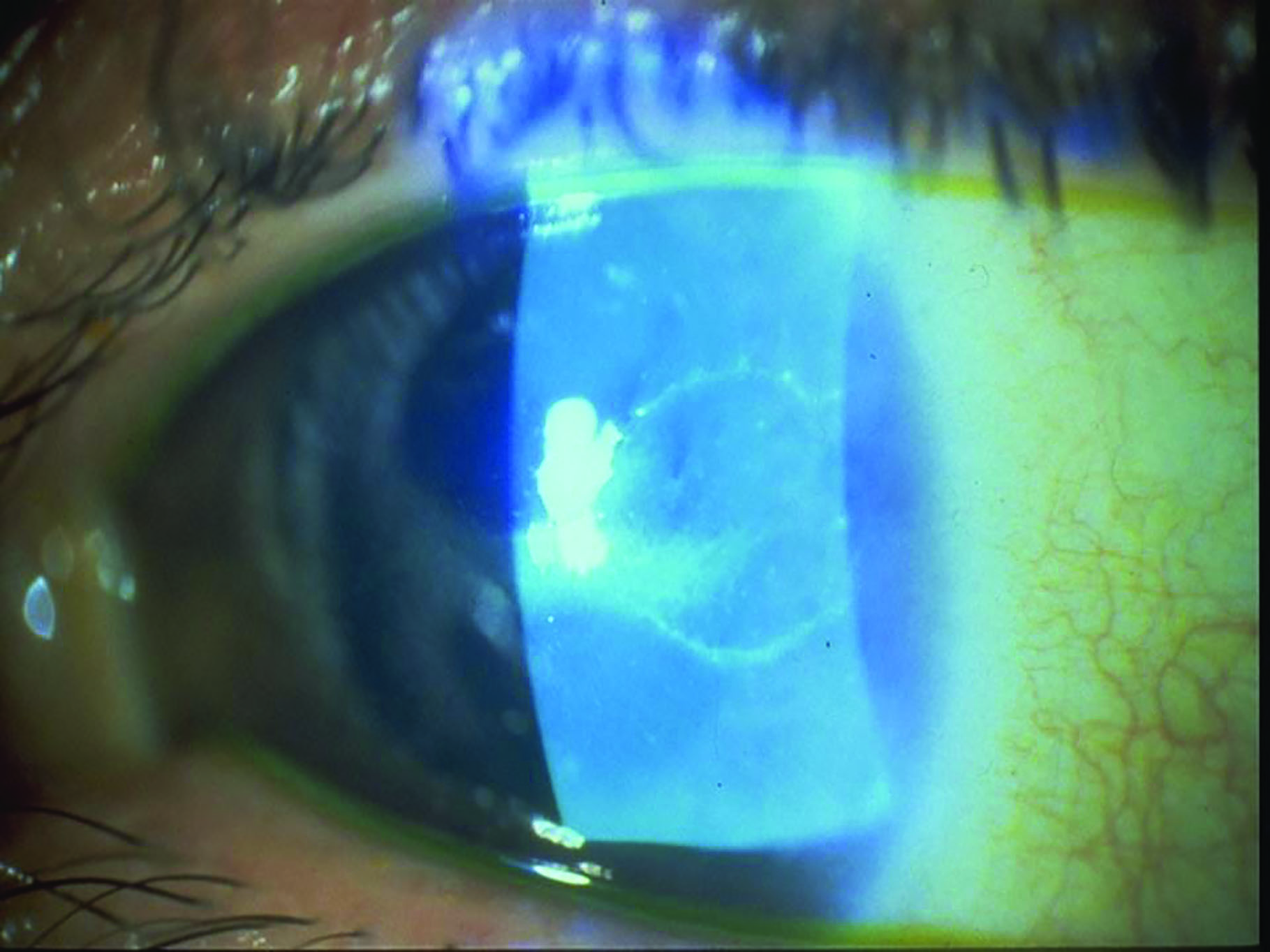 Figure 6: Acanthamoeba keratitis
Figure 6: Acanthamoeba keratitis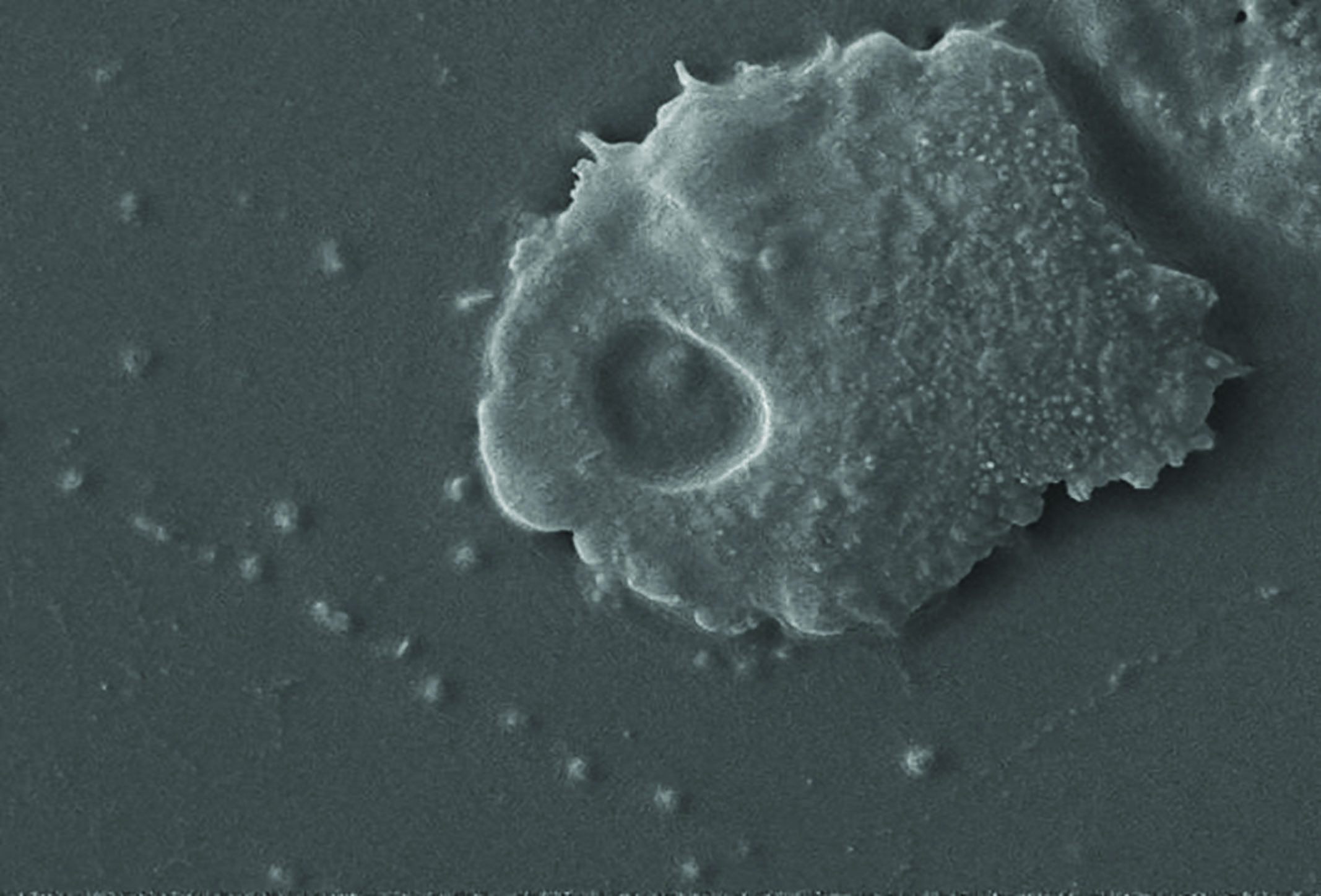 Figure 7: Acanthamoeba in trophozoite form
Figure 7: Acanthamoeba in trophozoite form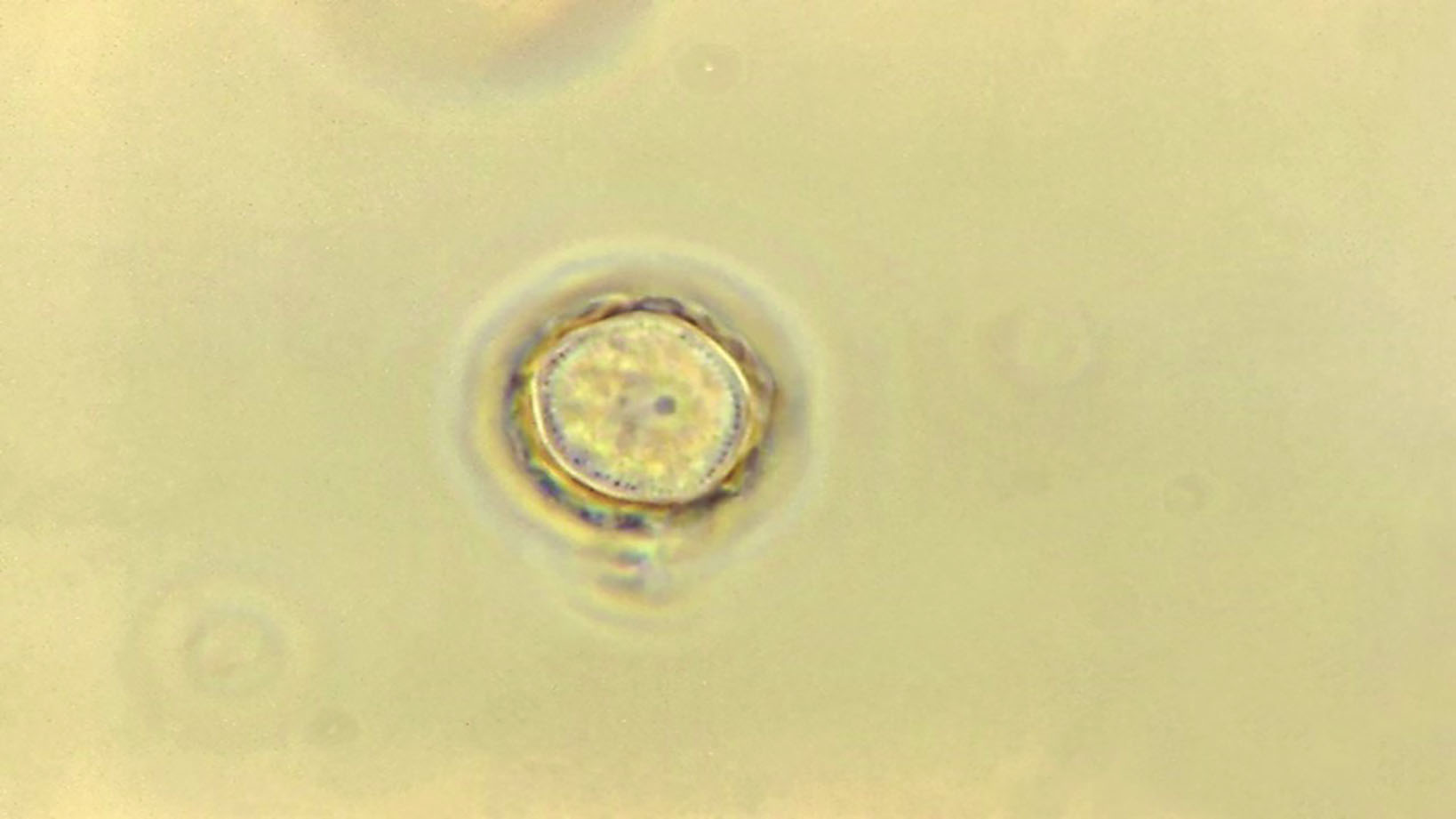 Figure 8: Photomicrograph of an Acanthamoeba protozoan cyst (1125 x magnificiation)
Figure 8: Photomicrograph of an Acanthamoeba protozoan cyst (1125 x magnificiation)
Viruses were most memorably described by Nobel laureate Peter Medawar as ‘a piece of nucleic acid surrounded by bad news’.12 Viruses are so small (typically 20 to 300nm in diameter) that they are beyond the resolution of even the most powerful optical microscope. They consist of a DNA or RNA core surrounded by a protein coat (capsid). Some viruses, such as coronaviruses like SARS-CoV-2, are also enclosed by an envelope of lipid and protein molecules. This is important when considering the role of handwashing.
Viruses cannot be grown in culture (in vitro), only within living cells (in vivo), and remain inert and harmless until introduced into a suitable host cell. Then they take control of the DNA protein synthesis function of the host cell to synthesise new virus particles.
Many viral diseases are highly communicable and have high mortality rates. Smallpox alone killed over 200 million people in the 20th century, despite being eradicated in 1977 following an aggressive campaign by the World Health Organisation. Vaccination means that many viral diseases such as polio, measles, mumps and rubella are highly preventable.
Viruses of ophthalmic note include the following:
- Varicella zoster – the common chicken pox/shingles virus which causes Herpes zoster ophthalmicus and is the largest cause of infection-related blindness in the developed world.
- Herpes simplex – a common cause of viral keratitis (figure 9).
- Adenovirus species – the cause of various forms of conjunctivitis and keratitis, usually concurrent with upper respiratory tract infections (figure 10).
- DNA pox viruses – cause molluscum contagiosum and associated conjunctivitis
 Figure 9: Herpes simplex keratitis
Figure 9: Herpes simplex keratitis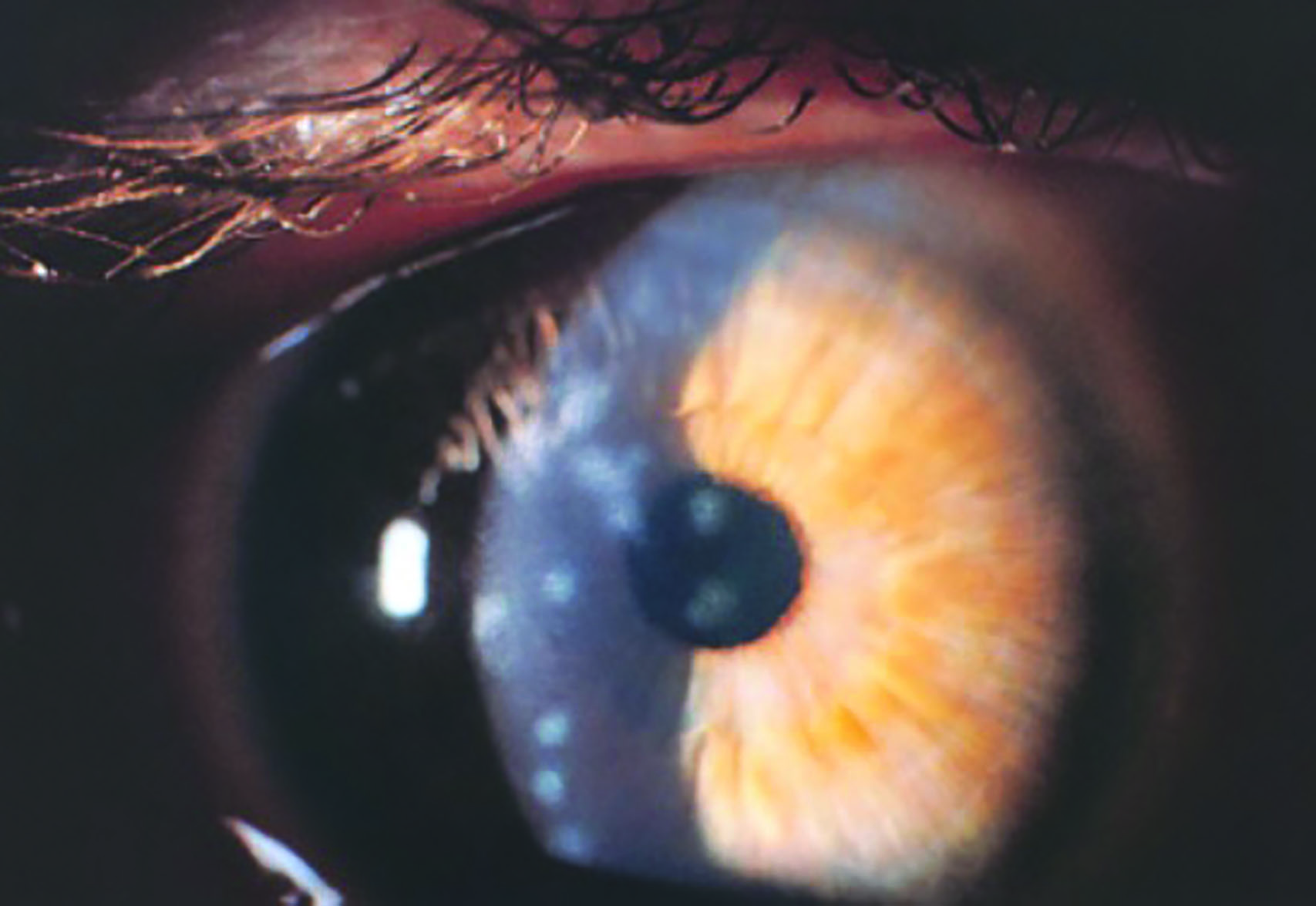 Figure 10: Stromal scarring subsequent to adenovirus keratoconjunctivitis
Figure 10: Stromal scarring subsequent to adenovirus keratoconjunctivitis
Helminths are worms, the largest organisms to infect the human body and so, strictly speaking, not usually classed as micro-organisms. Onchocerca volvulus causes onchocerciasis, or river blindness, the second largest infective cause of blindness worldwide.13 The nematode worm is endemic to parts of Africa, Central and South America, it is transmitted by day-biting flies of Simulium species. The 0.5mm diameter adult worms can live for up to 15 years and reach lengths of 50cm. The disease is occasionally seen in developed nations in refugee communities, overseas workers and practitioners returning from charity missions to provide healthcare abroad. In the UK, people, usually youngsters, who accidentally ingest the Toxocara species nematodes found in the faeces of cats and dogs may lose sight through a severe (often macula-centred) chorioretinitis triggered by the worm (figure 11).
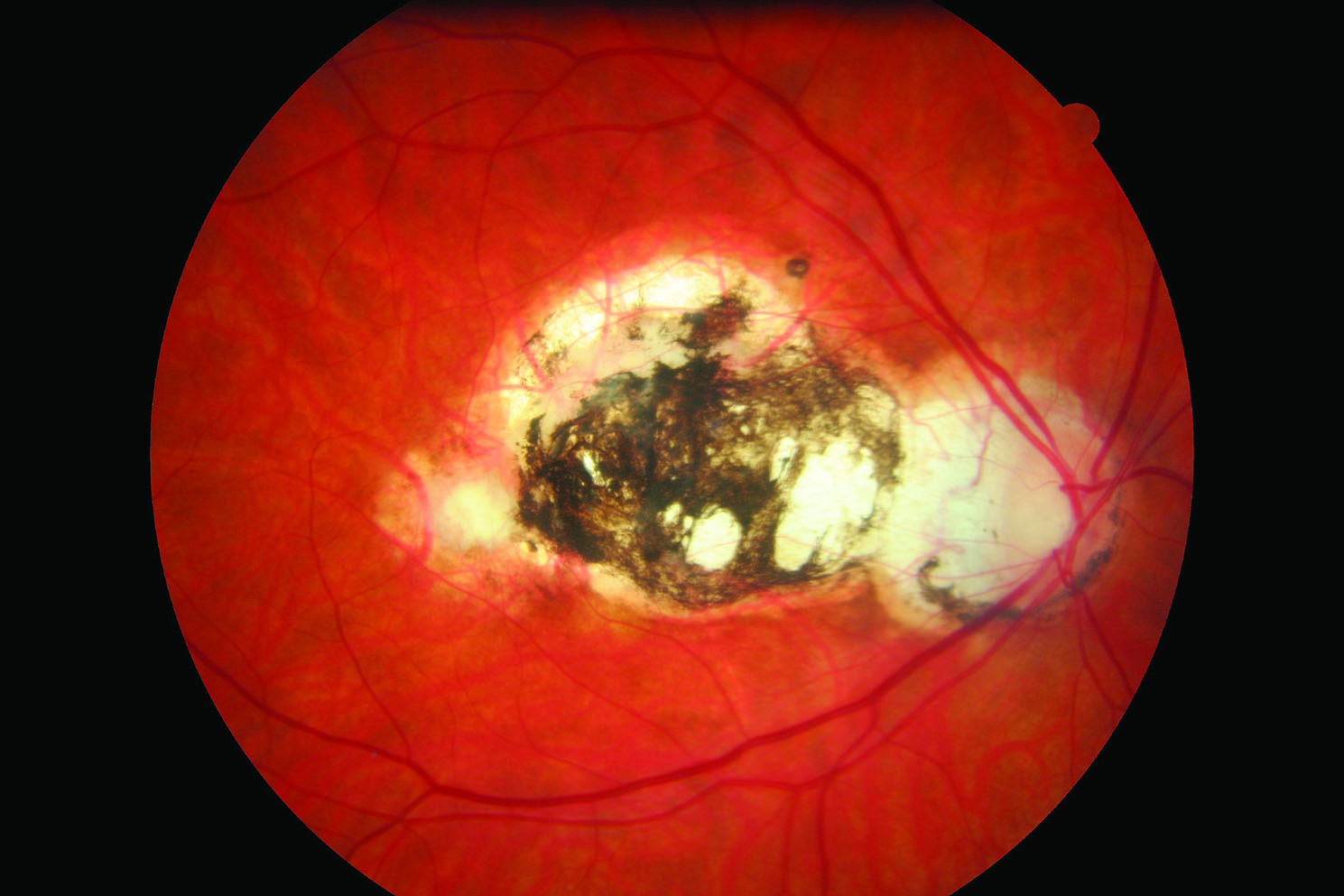 Figure 11: Toxocara scarring
Figure 11: Toxocara scarring
Infection control in optometric practice
Clinicians must strive to prevent infections being transmitted between patients, and from themselves to their patients and vice versa. They should be on a heightened state of alert when dealing with immune compromised patients such as those living with cancer, HIV/AIDS or advanced diabetes.
Practical decontamination
In order to understand how infection is controlled, it is necessary to understand how it is transmitted and how microbiological contamination can be ameliorated. After hand washing and hygiene (which will be covered in more detail next week), regular decontamination of equipment and the practice environment is the next most important step in infection prevention and control.
Decontamination terminology
- Clean – physically remove dirt. If appropriate, drying is an important part of cleaning. Cleaning dramatically reduces the number of microorganisms present and effectively prevents their multiplication by removing their food source.
- Disinfect – kill majority of microorganisms but not spores.
- Sanitise – clean and disinfect.
- Pasteurise – disinfect, typically foodstuffs or utensils, by heating to 65°C to 80°C.
- Sterilise – kill or remove all microorganisms and their spores by filtration, heating (such as in an autoclave), irradiation (such as with ultraviolet exposure) or chemical means.
Infection transmission methods
There are several means of transmission of infective agents.
Direct transmission
This involves direct contact with a person, or something they have touched, including faeces. The prime method of reducing transmission of disease by direct contact is to prevent direct contact through isolation.
In a clinical, interactive environment, the commonest method of avoidance is through regular cleaning of soiled areas and, most importantly, hand washing (see Box on this page). Equipment such as keyboards and telephones should be regularly sanitised, and ophthalmic equipment that comes into contact with the patients, such as chin rests, should be disinfected before every patient. Reusable items, such as tonometer prisms, should be sterilised between patients using 2% sodium hypochlorite, and wherever possible replaced by disposable equivalents. Single use products such as eye drops should not be used more than once.
Airborne transmission
This is the spread of infective agents through air-borne droplets from coughs and sneezes, dust or liquid vapour spray. Practitioners with a cold or cough should use disposable tissues, wash hands after every episode and consider wearing a mask. Recently, Perspex breath screens have been highlighted as important to minimise airborne transmission during slit-lamp examination (see next week for more details). Practices should also be well ventilated and air conditioning serviced regularly. Dust should not be allowed to accumulate.
The Royal College of Ophthalmologists (RCOphth) updated its guidance to ophthalmologists on 30 March 2020. It is now recommending that clinicians should wear standard surgical masks when examining or treating patients at the slit lamp. The College of Optometrists subsequently recommended that optometrists who are seeing patients follow the same advice. Fluid-resistant face masks are worn to protect the wearer from the transmission of a virus by respiratory droplets, which may be released from the mouth during coughing, sneezing or speaking. They may also reduce the risk of contamination of oral and nasal mucosa by accidental touching of your mouth or nose with a contaminated hand. They can be worn for sessional or single-use and must be fitted properly around the nose, but do not require a ‘fit test’. Gowns and gloves are not recommended unless aerosol-generating procedures are being conducted (which optometrists will not be doing). The RCOphth also recommends that plastic breath shields (figure 13) are attached to slit lamps and that these are disinfected between patients. This is because studies show that the COVID-19 virus is viable for up to 72 hours on plastic surfaces. They also recommend that ophthalmologists should avoid speaking at the slit lamp.
 Figure 13: Plastic breath shield for mounting on a slit lamp (Photo: BIB)
Figure 13: Plastic breath shield for mounting on a slit lamp (Photo: BIB)
The same surgical mask may be worn for examining multiple patients, but you must be fastidious to avoid transmitting the virus on the front of the mask via your hands or your clothes. Do not take the mask on and off between patients and do not allow it to dangle on your chest.
Fomite transmission
A fomite describes any inanimate object that, when contaminated with or exposed to infectious agents can transfer disease to a new host. Skin cells, hair, clothing, and bedding are common fomites in a clinical setting. Fomite transmission is, therefore, transmission via direct contact with contaminated objects.17 If someone with a respiratory tract infection sneezes without covering their face, droplets containing infective material may land on surfaces and be picked up by someone touching the surface. If they then touch their eyes, nose or mouth they are at risk of catching the infection. Similarly, if someone coughs into their hand and fails to wash their hands, they too may spread the disease on everything they subsequently touch.
Sanitisation is essential to combat diseases such as COVID-19, as the SARS-CoV-2 virus can remain viable for up to three days on hard surfaces.
Although handwashing and facemasks are key weapons, sanitisation of any item touched by multiple people is also vital. Likely surfaces for infection risk in practice include:
- Door handles
- Locks
- Keys
- Alarm panels
- Banister rails
- Credit card machines
- Phones
- Keyboards
- Computer mice
- Touchscreens
- Optometric and dispensing equipment
- Stock and display frames
Beyond the practice, sanitisation should include:
- Shared vehicles – steering wheels, gear sticks, grab handles
- Household controls – switches, controls, taps, toilet flushers
Using one’s elbow to cover one’s mouth during sneeze and cough episodes, and using one’s sleeve to handle grab rails, door handles and so on can help reduce infection where tissues and handwashing facilities are unavailable. Fastidious hygiene during the current COVID-19 pandemic is also intended to reduce the incidence of other infectious diseases and thereby reduce the overall burden on the health system. Freeing up resources remains a priority for us all, whether or not a household has contracted the coronavirus.
Water-born infection
This may be from contaminated drinking or bathing water, or from areas left permanently damp or wet. Water coolers should be serviced and sanitised regularly. Particular attention should be paid to providing the correct advice to contact lens wearers and ensuring the practice upholds the highest standards of cleanliness in hand washing areas so that water does not stand and harbour infection.
Food contamination
This is typically due to poor storage or direct contamination with faecal bacteria if hand hygiene rules are not obeyed. If coffee or food is ever provided to patients, then the appropriate food hygiene standards must be observed. If staff have use of a refrigerator, then it should be at the correct temperature and they should be trained to ensure they do not contaminate cooked food with, for example, liquids or meat juices. This is relevant if they shop at lunchtime to buy food to take home.
Inoculation
Inoculation directly into the blood stream via a wound, injection or bite is a greater risk in community practice than might at first be supposed. Screwdriver injuries are a common occurrence – see case 2. First aid kits, with sticking plasters and a means of cleaning wounds, should always be available and easily accessible. In a hospital environment, care should be taken that sharps are disposed of appropriately.

Waste disposal
As well as sharps, there should be appropriate measures for the disposal of clinical waste including diagnostic drugs, face masks and gloves. Poor systems with regard to the disposal of general and food waste can encourage vermin such as rodents and cockroaches which dramatically increases the risk of human infection.
How hygienic is your practice?
On a practice visit to assess a trainee dispensing optician, the author witnessed a handover from the optometrist for a patient who needed new spectacles because the nose pad had been lost from her previous ones. The patient had an open sore, clearly inflamed, where the pad arm had been digging into the side of her nose. After dispensing, the author intervened to replace the nose pad with a poorly matching one and advised the patient to clean the area with an antiseptic wipe and apply a sticking plaster to aid healing.
In discussion with the trainee and the optometrist supervisor later, it was apparent that both the trial frame and pupilometer were heavily contaminated with make-up and had not been subject to regular infection control procedures. Neither practitioner had recognised the risk of causing the patient with the open wound a serious infection, or indeed of passing an infection from the patient to another, perhaps immune-compromised, patient. While most practices wipe down instrument chin rests, there is often little regard to trial frames, pupilometers or indeed stock frames that have been tried on by many patients. This will undoubtedly change as a result of recent events.
Infection in contact lens practice
Contact lens opticians and optometrists have a duty of care to their patients to ensure they do not unwittingly cause their patients to contract an eye infection. Handwashing, discarding contact lens solutions at the appropriate time, using disposable diagnostic lenses where possible, and ensuring sterilisation of reusable trial lenses are all important measures.
Perhaps more importantly contact lens practitioners must also ensure their patients are aware of the risks of eye infection posed by contact lens wear and have sufficient information to make an informed choice with regard to the lenses they wear, how they wear them, and the lens care regime they follow. A presentation from Moorfields Eye Hospital that the author attended in February 2015 stated that 9% of all ophthalmic accident and emergency cases were contact lens-related keratitis.
The US Centre for Disease Control (USCDC) and the UK Loveyourlenses.com offer contact lens wearers very similar advice.21,22 However, it is interesting to compare the presentation. Should infection control be separate from other wear and care advice to lend it more importance? According to the USCDC practitioners have a duty to ensure they tell their contact lens patients the following:
- Wash your hands with soap and water. Dry them well with a clean cloth before touching your contact lenses every time.
- Do not sleep in your contact lenses unless prescribed by your contact lens practitioner.
- Keep water away from your contact lenses. Avoid showering in contact lenses, and remove them before using a hot tub or swimming.
- Rub and rinse your contact lenses with contact lens disinfecting solution – never water or saliva – to clean them each time you remove them. Daily disposables should be disposed of once worn.
- Never store your contact lenses in water.
- Replace your contact lenses as often as recommended by your contact lens practitioner.
- Rub and rinse your contact lens case with contact lens solution – never water – and then empty and dry with a clean tissue. Store upside down with the caps off after each use.
- Replace your contact lens case at least once every three months.
- Do not ‘top off’ solution. Use only fresh contact lens
disinfecting solution in your case – never mix fresh solution with old or used solution. - Dispose of solution at the directed time from opening and by the use by date.
- Use only the contact lens solution recommended by your contact lens practitioner.
- Visit your contact lens practitioner yearly or as often as he or she recommends.
The above list shows conflicting information. For example, if a patient uses extended wear lenses, is it realistic to expect them to remove their lenses for showering or swimming? Loveyourlenses.com tries to address this by urging patients to keep their eyes firmly shut in the shower and to wear watertight goggles when swimming. Patients need to be given the facts, relative risks and the danger signs to look out for so they do not put themselves at risk, and should an adverse incident occur, they act appropriately.
With new lens wearers, or experienced patients who are new to your practice, it is wise to assess their understanding of infection control and their compliance with instructions. Asking a patient to remove their own lenses and observing their inclination to wash and dry their hands is a good start. How practitioners communicate and ask questions is also important. For example, ‘do you ever sleep in your lenses?’ is likely to elicit a negative response, whereas ‘when did you last sleep in your lenses?’ is likely to obtain a more truthful answer. Giving written information as well as verbal instruction is vital.
The advice to contact lens wearers during the COVID-19 pandemic has been little different to that in normal times. However, in the UK the GOC has relaxed rules with regard to supplying lenses to minimise patient practitioner contact. This balances the higher risk of COVID-19 against the lower risk of adverse issues relating to contact lens wear. Nevertheless, practitioners should establish that patients are not having any obvious problems by telephone before agreeing to continue supply beyond the usual date.
Contact lens disinfecting solutions
Contact lens solutions are tested against a number of microorganisms that are used to test disinfection products generally or are known to cause eye infections. They usually include:
- Gram-negative bacteria: Pseudomonas aeruginosa, Serratia marcescens, Escherichia coli
- Gram-positive bacteria: Staphylococcus aureus, Bacillus subtilis
- Fungi: Candida albicans, Fusarium solani
- Protozoa: Acanthamoeba castellani
Nonetheless, contact lens solutions have been implicated in epidemics of serious sight threatening eye disease, notably of Fusarium keratitis, on several occasions since the turn of the 21st century. While this has partly been due to contamination of manufacturing facilities, it is important to note that several manufacturers for a time did not advocate a rub and rinse step for their multipurpose solutions. This has since been shown to reduce disinfection efficacy. Furthermore, solutions are only tested against free living organisms in solution and not against resistant biofilms such as are likely found on contact lenses and storage cases. Figure 14 shows either side of a Petri dish inoculated with the right and left contact lenses (visible on the culture medium) of a keratitis-patient, and had subsequently grown a filamentous colony of Fusarium oxysporum fungal organisms.
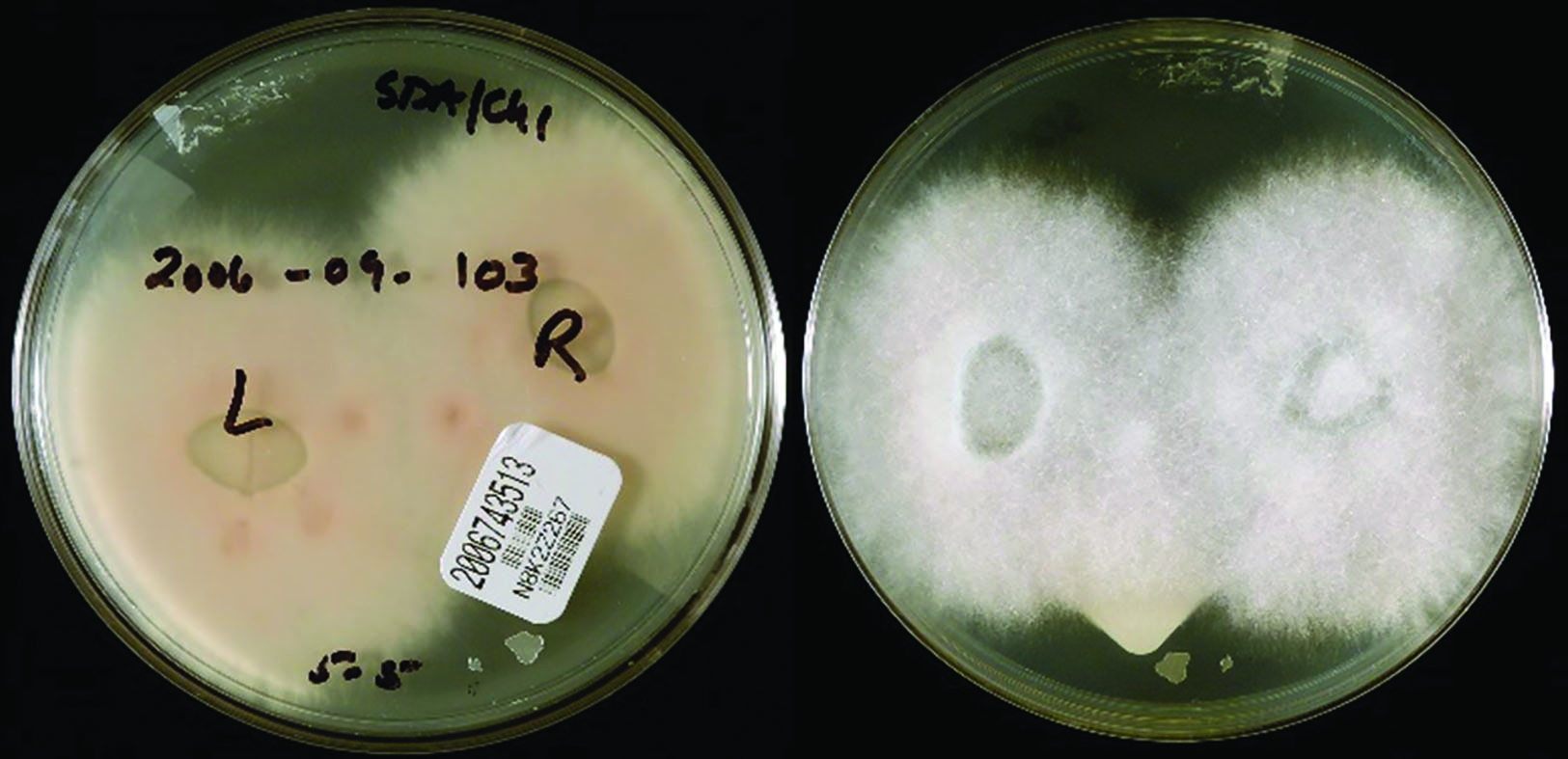 Figure 14: Fusarium cultured from a keratitis patient’s contact lenses (Public Health Image Library, Center for Disease Control)
Figure 14: Fusarium cultured from a keratitis patient’s contact lenses (Public Health Image Library, Center for Disease Control)
Anti-microbial medicines for ophthalmic use
It is beyond the scope of this article to go into detail with regard to the treatment of infectious eye disease. However, it is incumbent upon all practitioners to understand their own scope of practice, the likely course of treatment and possible side effects and complications associated with eye infections and in particular the incorrect use of antimicrobials.
Chloramphenicol is an antibiotic produced by bacteria of the genus Streptomyces to help them compete against Gram-negative bacteria, such as Pseudomonas aeruginosa.23 UK registered opticians and optometrists can issue chloramphenicol for acute bacterial conjunctivitis only. The reason they should not issue chloramphenicol for keratitis is that they have no means of being certain that the infection has been caused by a Gram-negative bacterium. If it has not, then the elimination of Gram-negative bacteria will make culturing and identification impossible or may serve to promote a different causative organism and make the infection worse.
A note on preservatives
Preservatives, such as benzalkonium chloride, get a bad press in eye care as potential causes of allergy and toxic reactions when used long term. It is a good thing that they have been removed from many dry eye and glaucoma preparations, but only where alternative protection from microbiological contamination is in place. This might be through unit dose vials (figure 15) or novel multi-dose bottles, such as the Abak system (figure 16), that maintain the sterility of the contents without need of chemical preservative. That said, preservatives keep us safe from infection and are another weapon in our armoury that need to be balanced against risks of infection, patient non-compliance, allergy and cost of treatment.
 Figure 15: Unit dose application
Figure 15: Unit dose application
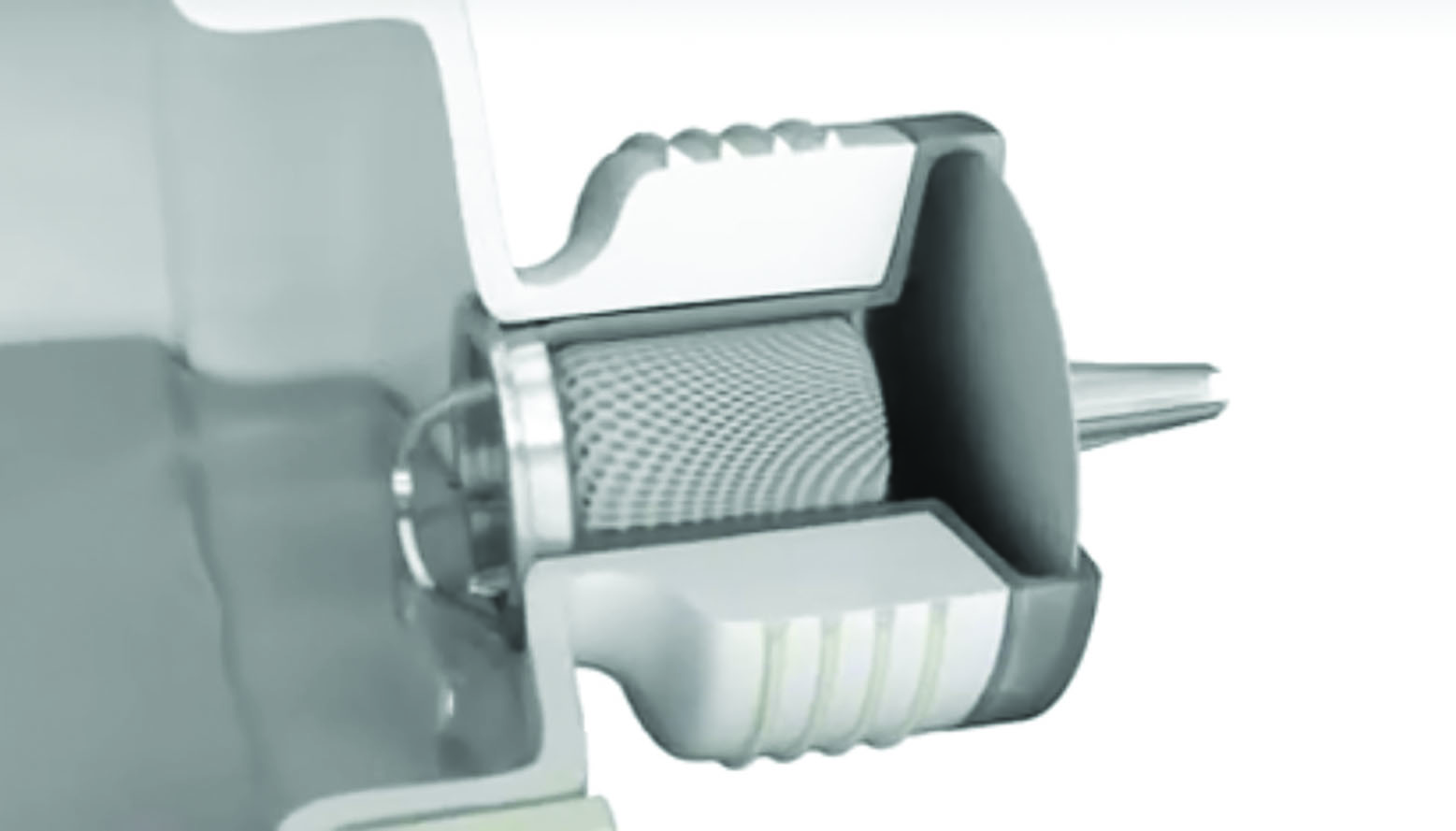 Figure 16: The Abak system of preservative-free repeat drop use (courtesy of Thea)
Figure 16: The Abak system of preservative-free repeat drop use (courtesy of Thea)
Conclusions
If there is one take home message from this article, it is that hand hygiene is the most important aspect of infection control (see next week for more on this subject). However, there is increasing evidence that in cost conscious times it is unfair to blame poor hand hygiene alone for the rise of healthcare acquired infection.24 It is important to ensure that all items that come into direct contact with patients and practitioners (from door handles to chin rests) are regularly sanitised.
Eye care practitioners must take extra care when dealing with patients who are likely to be immune compromised, especially when conducting invasive tests such as contact tonometry, as they are at a greatly increased risk of contracting ocular, respiratory, skin or gastrointestinal infection from normally harmless organisms. Contact lens practitioners should recognise that their patients are at the greatest risk of serious sight threatening eye infection. They should be appraised of these risks and given information in writing so that they can take mitigating action such as wearing their spectacles while they sip prosecco in the hot-tub. Finally, practitioners are strongly advised to familiarise themselves with the detailed advice and guidance issued by the NHS and their professional bodies, and especially during global pandemics revisit this regularly to avoid putting themselves, their colleagues or their patients at risk of sight or life-threatening infections.
Peter Black MBA FBDO FEAOO is senior lecturer in ophthalmic dispensing at the University of Central Lancashire, Preston, and is a practical examiner, practice assessor, exam script marker, and past president of the Association of British Dispensing Opticians.
Tina Arbon Black BSc (Hons) FBDO CL is director of accredited CET provider Orbita Black Limited, an ABDO practical examiner, practice assessor and exam script marker, and a distance learning tutor for ABDO College.
• The information presented here is accurate at the time of publication. Readers are advised to monitor daily for any changes to guidance regarding cross-contamination.
References
- Association of British Dispensing Opticians. ABDO Advice and Guidelines – Infection control. [Online] [Cited: 28 March 2020.] https://www.abdo.org.uk/regulation-and-policy/advice-and-guidelines/regulatory/infection-control/.
- College of Optometrists. Guidance for Professional Practice. [Online] [Cited: 28 March 2020.] https://guidance.college-optometrists.org/guidance-contents/safety-and-quality-domain/infection-control/.
- Kumar P, Clark M. Clinical Medicine. 5th. Edinburgh : WB Saunders, 2002. ISBN: 0-7020-2579-8.
- He A, Zhou M. Nebraska has stake in beef exports. China Daily. 217, 2017, Vol. 1, ISSN: 2398-8975.
- Responding to Francis, Keogh and Berwick reviews. NHS Leadership Academy. [Online] [Cited: 28 March 2020.] https://www.leadershipacademy.nhs.uk/about/responding-francis-keogh-berwick-reviews/.
- General Optical Council. Standards for optometrists and dispensing opticians. www.optical.org. [Online] April 2016. [Cited: 28 March 2020.] https://standards.optical.org/standards/ensure-a-safe-environment-for-your-patients/.
- Millodot, M. Dictionary of Optometry and Visual Science. 7th Edition. Edinburgh : Butterworth Heinemann Elsevier, 2009. ISBN: 978-0-7020-2958-5.
- Taylor H, Stanford E, Lange F. Why is trachoma blinding aboriginal children when mainstream Australia eliminated it 100 years ago? http://theconversation.com/. [Online] 13 September 2016. [Cited: 28 March 2020.] http://theconversation.com/why-is-trachoma-blinding-aboriginal-children-
when-mainstream-australia-eliminated-it-100-years-ago-63526. - Money, NP. Microbiology a very short introduction. s.l.:Oxford, 2014. ISBN 978-0-19-968168-6.
- McCulloch, J. Infection control. Science, management and practice. London:Whurr, 2000. ISBN 1-86156-053-2.
- World Health Organisation. World Malaria Report 2016. s.l.WHO, 2016.
- Bryson, B. A short history of nearly everything. s.l.:Transworld Publishers, 2003. Chapter 20 – Small World. ISBN: 0385 408188.
- Kanski, JJ and Bowling, B. Synopsis of clinical ophthalmology. 3rd Edition. s.l.:Elsevier Saunders, 2013. 978-0-7020-5036-7.
- Siddiqui R, Aqeel Y, Khan NA. The Development of Drugs against AcanthamoebaInfections. National Center for Biotechnology Information. [Online] [Cited: 28 March 2020.] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5075048/.
- Acanthamoeba keratitis cluster: an increase in Acanthamoeba keratitis in Australia. Ku JY, Chan FM, Beckinsale P. Brisbane:Clin Exp Ophthalmol, 2009 Mar:37(2):181-90. doj:10.1111/j.1442-9071.2008.01910.x.Epub 2008 Dec 29, 2009.
- RNIB. Campaigning to stop blindness from contact lenses. www.rnib.org.uk. [Online] 12 April 2018. [Cited: 28 March 2020.] https://www.rnib.org.uk/rnibconnect/campaigning-stop-blindness-contact-lenses.
- Gordon, L. Methylisothiazolinone allergy. https://www.dermnetnz.org. [Online] 31 January 2016. [Cited: 28 March 2020.] https://www.dermnetnz.org/topics/methylisothiazolinone-allergy/.
- Centers for Disease Control and Prevention. Healthy contact lens wear and care. [Online] 14 September 2016. [Cited: 28 March 2020.] https://www.cdc.gov/contactlenses/show-me-the-science.html#habits.
- General Optical Council. How to love your lenses. Love your lenses. [Online] [Cited: 28 March 2020.] http://www.loveyourlenses.com/how-to-love-your-lenses/.
- Amyes, S. Bacteria a very short introduction. s.l.:Oxford, 2013. ISBN: 978 0 19 957876 4.
- Gawande, A. Complications. A surgeon’s notes on an imperfect science. s.l.:Profile Books, 2003. ISBN: 978-1846681325.
- Wilson, J. Infection control in clinical practice. s.l.:Bailliere Tindall Elsevier, 2007. ISBN 13: 978 0 7020 2761 1.
- McCartney, M. The patient paradox. Why sexed up medicine is bad for your health. s.l.:Pinter & Martin , 2016. ISBN: 978-1-78066-000-4.
Further Reading
A number of books have been read during the compilation of this article, in addition to those quoted and cited above these have included:
- Gawande, A. The checklist manifesto. How to get things right. Profile Books, 2011. ISBN: 978-184668-3145
- Gawande, A. Better. A surgeon’s notes on performance. Profile Books, 2008. ISBN: 978-1861976574
- Gawande, A. Being mortal. Illness, medicine and what matters in the end. Profile Books, 2015. ISBN: 978-1846685828
- Dictionary of science and technology. Chambers, 2007. ISBN-13: 978-0550-100719
- Stedman’s concise medical and allied health dictionary. Williams & Wilkins, 1997. ISBN: 0-683-23125-1
