One of the things that was well established before anyone had even heard of the Covid-19 disease and coronavirus infection was the importance of maintaining the highest standard of cleanliness in practice. And, if asked to name any example of an ocular condition that is highly contagious and justifies such high standards in eye care practice, I am confident that almost everyone would have cited viral conjunctivitis in response. It may have been the discovery of prion material in tears in the early 1990s that drove a move towards new guidance concerning cross-contamination with contact assessment techniques, but viral conjunctivitis has long been known to be both common and highly contagious. This is well known by anyone who has received a ticking off for referring the condition to secondary care and risked its further spread.
From the beginning of 2020, reports have appeared describing the various signs and symptoms of a disease caused by a novel coronavirus. As implied by its name, the virus SARS-CoV-2, newly introduced to humans in 2019, is a coronavirus (CoV) that causes severe acute respiratory syndrome identified as Covid-19.
Of particular interest to our profession has been the apparent susceptibility of eye care practitioners to the disease. Indeed, it was an ophthalmologist who first broke cover from the wall of silence surrounding the first cases of Covid-19 to appear in the Chinese city of Wuhan in late 2019, an act which rightly secures Li Wenliang’s place in history for his bravery. The proximity of eye care professionals (ECPs) to patients, the highly contagious nature of SARS-CoV-2 via aerosol droplets, and the human body’s access point for infection offered by the tears and their drainage all provide a good explanation for the high prevalence of the disease in this group of health workers.
Initial studies understandably focused on the severity of the impact of the new disease upon the upper respiratory tract, and any ocular manifestations were rarely heard of. As time goes by, evidence is growing to support the view that the tears act as a major route for infection, that some patients with Covid-19 can develop viral conjunctivitis (either in later stages of the disease or at first presentation), and that this may be caused by the novel virus itself rather than the usual suspects acting opportunistically upon an immune-compromised person.
Bearing this in mind, as ECPs move back into primary care practice, the ability to diagnose and manage viral conjunctivitis, to recognise the symptoms of possible Covid-19 and to triage accordingly must be considered important. It is with this in mind that a review of the diagnosis and management of viral conjunctivitis and of what we currently know regarding links with Covid-19 would seem timely.
Definition of Viral Conjunctivitis
Conjunctivitis is a non-specific term describing an inflammation of the conjunctiva. This is the thin, transparent membrane covering the ocular surface between the lid margin and the limbus, and within which there is a dilation of the blood vessels leading to a hyperaemic (red) eye (figure 1). 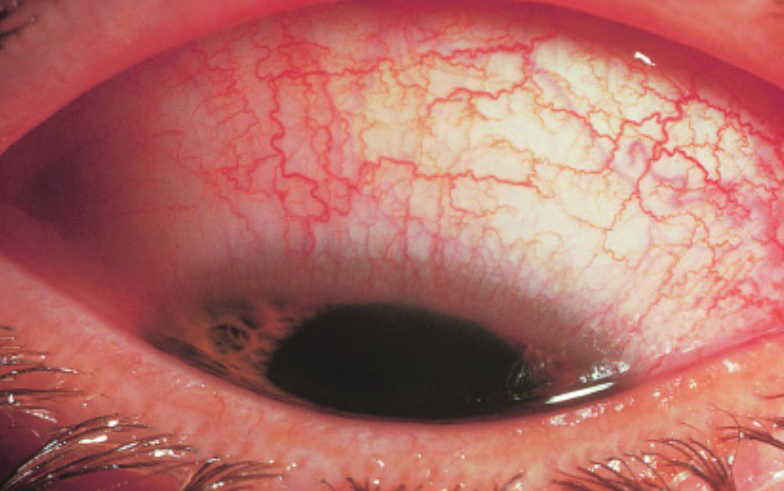 Figure 1: Hyperaemia is characteristic of conjunctivitis
Figure 1: Hyperaemia is characteristic of conjunctivitis
Many of the causes of conjunctivitis are due to an external infective agent or pathogen, including some viruses. Though some causes of conjunctivitis cause a disease of more than two weeks, resulting in what is described as chronic conjunctivitis, the most common infective forms of conjunctivitis, due to bacteria or viruses, cause an acute form of the disease which is short-lived.
Anatomy of the conjunctiva
Of similar etymology to words such as ‘conjoined’ or ‘conjunction,’ the conjunctiva describes the thin, translucent, mucous membrane that joins the eyeball to the eyelid. It comprises a superficial epithelium overlying a loose connective tissue stroma. The conjunctiva is continuous with the corneal epithelium which it meets at the limbus and, at its other extremity, with the skin at the mucocutaneous junction along the lid margin (figure 2).
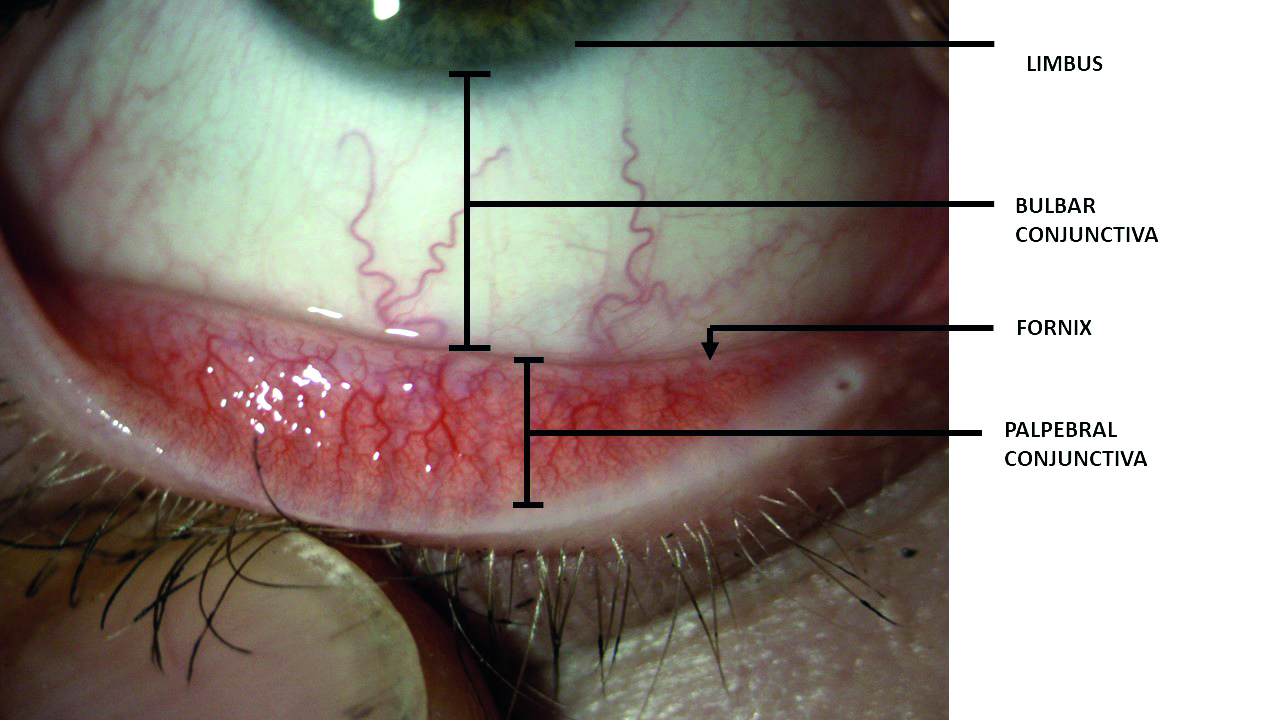 Figure 2: The three main regions of conjunctiva. Note, the fornix represents the outer limiting point at which the conjunctiva leaves the lid to move onto the bulbar surface
Figure 2: The three main regions of conjunctiva. Note, the fornix represents the outer limiting point at which the conjunctiva leaves the lid to move onto the bulbar surface
The point at which the conjunctiva leaves the eyeball to pass onto the inner lid surface is called the fornix and, at this point, the forniceal conjunctiva has some loose connection with the underlying fascial sheaths of the levator palpebrae superioris (upper lid) and the extraocular rectus muscles. It is also the region into which the lacrimal glands empties all and the accessory glands most of their secretion. The term fornix has a similar derivation to ‘fornication,’ both stemming from the Latin for archway or vaulted roof, structures commonly associated with places where prostitution took place.
The palpebral conjunctiva is tightly bound to the lid and the lacrimal puncta (superior and inferior) open onto it (figure 3). This is important to remember, as the palpebral conjunctiva is, therefore, continuous with the lining of the inferior meatus of the nasal cavity and, as such, offers a direct route for infective materials to pass from the ocular surface into the respiratory tract. It is also why some eye drops leave a bad taste.
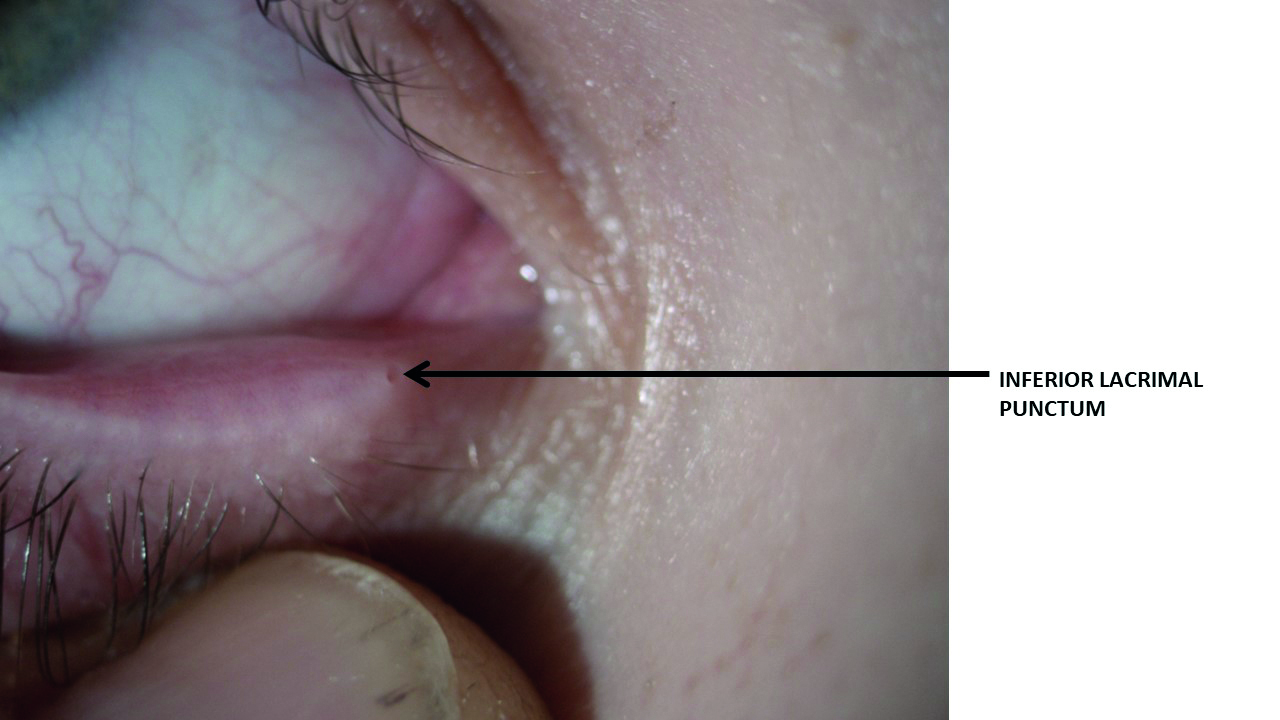 Figure 3: Tears drain through the lacrimal puncta towards the outer edge of the palpebral conjunctiva, both superior and (as shown here) inferior
Figure 3: Tears drain through the lacrimal puncta towards the outer edge of the palpebral conjunctiva, both superior and (as shown here) inferior
The healthy bulbar conjunctiva reveals the white sclera beneath and covers the anterior part of the eyeball from limbus (where it is tightly bound to the globe) to the fornix, passing over the insertions of the extraocular muscles and Tenon’s capsule. It loses its attachment to the globe further from the limbus where there is also to be found a loose episcleral tissue layer. This incorporates the peri-corneal vascular plexus and it is these vessels that become dilated and contribute towards the red eye appearance we associate with inflammation or trauma.
The epithelium, as with all epithelia, is a layered structure and varies significantly from region to region, from between two to seven cell layers, but always with a basal, an intermediate and a superficial zone. Of the many specialised cells to be found in the epithelial, the following are worth remembering:
- Goblet cells; mucus-secreting cells, particularly concentrated around the fornices and the plica semilunaris (the floppy layer seen at the inner canthal region).
- Melanocytes; their concentration, and therefore degree of melanin production, is greatly influenced by ethnicity.
- Intraepithelial MHC class II-positive dendritic cells; sometime called Langerhans cells, these trap and ingest antigens and pathogens, transport them to concentrations of immune cells (such as the preauricular lymph nodes, conjunctival-associated lymph tissue, or follicles) and trigger an immune response from T-cells, maturation of antigen-presenting B-cells, and production of immunoglobulins.
- Intraepithelial lymphocytes; these immune cells increase in number during inflammation or infection.
The loose subepithelial tissue contains many immune-active cells, including mast cells and more lymphocytes, and the aggregations of these form as a response to inflammation or infection, when they are then described as follicles. The stroma also contains many blood vessels, being highly vascularised in the same way as the eyelids and also receiving supply from the anterior ciliary arteries. Again, inflammation and infection cause vasodilation, contributing to the reddening of conjunctivitis.
So, it can be seen that the conjunctiva, a tissue very much exposed to external environmental infectious threat, offers a second layer of defence against pathogens, after they have first completed successful passage through what the tear film has to offer.
Types of conjunctivitis
As mentioned previously, chronic conjunctivitis is far less likely to be caused by an infection. Bacterial and simple viral infections usually cause acute conjunctivitis. Infective causes of conjunctivitis are as follows:
- Bacteria; there are many, but the commonest are Staphylococcal species (S. aureus, S. epidermis and S. pneumoniae), Haemophilus influenzae and Moraxella lacunata.
- Chlamydia; these inclusion bodies can cause a severe conjunctival infection in new-borns, after transmission of the STD-causing pathogen during the birth process.
- Phthiraptera (lice); pubic lice can cause a rare inflammatory response of the conjunctiva known as phthiriasis palpebrarum.
- Viral conjunctivitis; there are many viruses with the potential to cause conjunctivitis, as will be discussed later.
Of the many non-infective causes of conjunctivitis, these are the ones to know and so to rule out during diagnosis:
- Allergy; further classified as seasonal, vernal, giant papillary or atopic, itching is the classic characteristic symptom. The condition may be chronic and occasionally requires far greater medical intervention than mere removal of any allergen. A characteristic sign of allergic disease is the appearance of papillae, closely packed, flat-topped projections, with numerous eosinophils, lymphocytes, plasma cells, and mast cells in the stroma which, unlike follicles already discussed, surround a central vascular channel. Papillae are to be found on the palpebral conjunctiva after lid eversion.
- Dry eye disease; blepharitis, evaporative or aqueous deficient dry eye disease, from environment or endogenous disease such as Sjögren’s syndrome, commonly causes conjunctival inflammation.
- Immunological disease; examples include ocular cicatricial pemphigoid or Stevens-Johnson syndrome, diseases which can cause a fusing of the palpebral and bulbar conjunctivae, something known as symblepharon.
- Toxins; conjunctival inflammation may result from external chemical insult, sensitivity to external pharmaceuticals, or non-chemical injury such as in a photokeratopathic response to UV light.
- Mechanical insult; eye rubbing or contact lens friction are both worryingly common causes of conjunctival
inflammation. - Nasolacrimal duct obstruction; chronic dacryocystitis is an infection of the tear drainage apparatus and can have associated conjunctivitis response.
Most cases of conjunctivitis may be categorised as either papillary or follicular, according to the macroscopic and microscopic appearance of the conjunctiva. Neither type is pathognomonic for a particular disease entity. That said, just as the nature of any discharge helps to diagnose the underlying cause of infection of the conjunctiva, papillary conjunctivitis typifies allergic disease, and may also result from bacterial infection as well as allergy. However, follicular response is instead far more likely to indicate a viral or chlamydial infection (table 1).
 Table 1: Some key distinguishing clinical features of the main types of infective conjunctivitis
Table 1: Some key distinguishing clinical features of the main types of infective conjunctivitis
Follicular conjunctivitis, very much associated with viral infection, is also seen in a variety of other conditions including:
- Atypical bacterial infection
- Exposure to toxins
- Adverse response to topical medications, such as the anti-glaucoma drug brimonidine or some over-the-counter decongestants.
In contrast to papillae, follicles are small, dome-shaped nodules without a prominent central vessel. Because of this, a papilla appears to be more red on its surface and more pale at its base (figure 4), whereas a follicle appears more pale on its surface and more red at its base (figure 5). The follicles in follicular conjunctivitis are typically most prominent in the inferior palpebral and forniceal conjunctiva. Blood vessels may overly the follicles, but are not a prominent component within them.
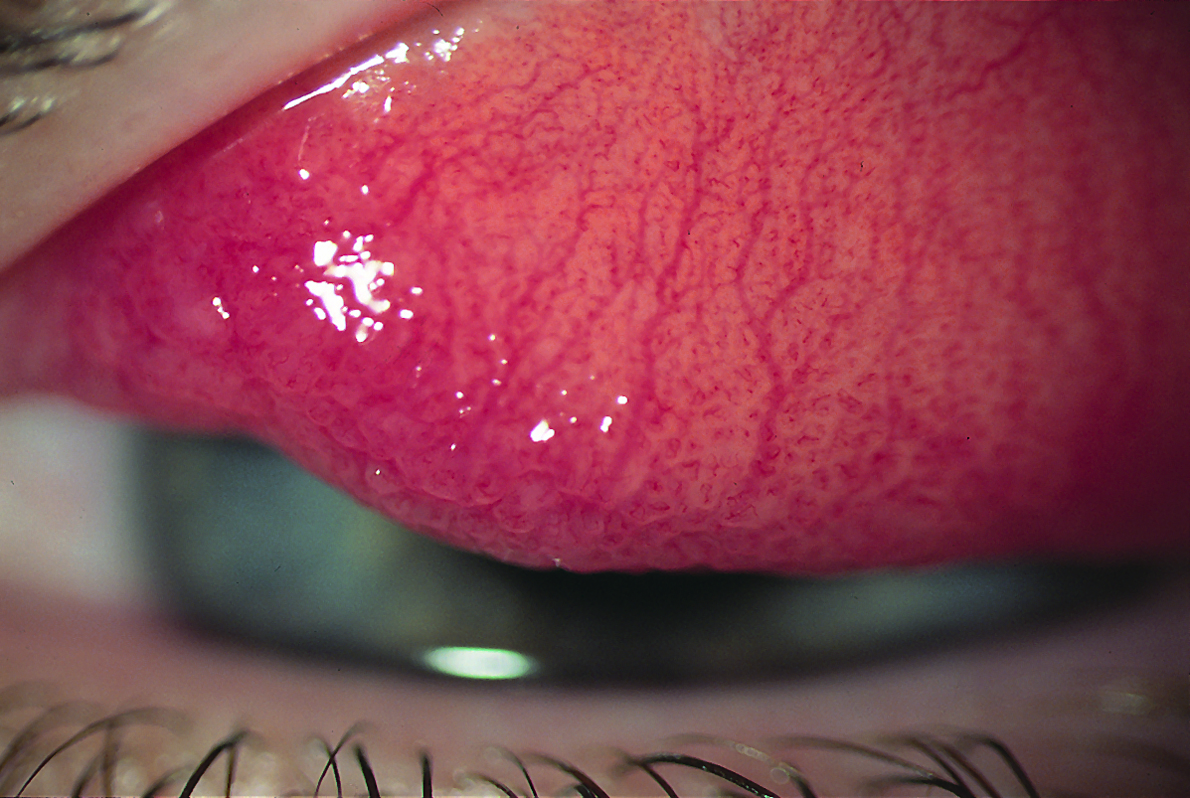 Figure 4: Localised and vascularised swellings, as a response to an allergen or mechanical insult from a high modulus contact lens, are described as papillae. Note the redder appearance of the small elevations in low grade papillary response
Figure 4: Localised and vascularised swellings, as a response to an allergen or mechanical insult from a high modulus contact lens, are described as papillae. Note the redder appearance of the small elevations in low grade papillary response Figure 5: Follicles, as seen in viral or chlamydial conjunctivitis, are aggregations of white blood cells and, as such, have a pale appearance
Figure 5: Follicles, as seen in viral or chlamydial conjunctivitis, are aggregations of white blood cells and, as such, have a pale appearance
The extent of the hyperaemia, follicular response and other signs are usually graded for recording purposes. In rare cases where the conjunctivitis is very severe, petechiae (small red spots caused by minor bleeding due to vessel rupture) or larger haemorrhaging becomes apparent. Though rare, this late stage conjunctivitis is usually described as haemorrhagic conjunctivitis.
One final and important point; because of both the proximity and the continuity of the conjunctiva and the cornea, conjunctivitis is often present alongside corneal signs of inflammation, a state of play which should be described as keratoconjunctivitis. The degree with which the cornea may respond to any cause of conjunctivitis varies with the nature of the infection and the severity and duration of the conjunctivitis. As will be mentioned again, a careful assessment of the cornea is always an important part of assessing conjunctivitis.
Which viruses cause conjunctivitis?
There are many viruses that can cause conjunctivitis. Many of these have accompanying systemic effects that aid diagnosis. Examples include:
- Common cold ‘virus’; the most commonly implicated virus is a rhinovirus (some 30 to 80% of cases), a type of picornavirus with 99 known serotypes making immunity to all of them a lengthy process. Others affecting the respiratory tract and causing conjunctivitis include human coronaviruses (15%), influenza viruses (10 to 15%), adenoviruses (5%), and many others.
- Measles virus; sadly, back on the radar thanks to vaccine deniers.
- Varicella virus; conjunctivitis is common in chicken pox.
- Rubella; German measles is another viral infection that can cause conjunctivitis, but has most serious ocular complications in the gestating infant, such as congenital cataract.
- Mumps virus; along with measles and rubella, the likely re-acceptance of anti-viral disease vaccines post-pandemic, such as MMR, should make these causes of conjunctivitis increasingly rare.
- Molluscum contagiosum; one of the pox viruses, this infectious virus causes skin lesions which readily shed infective matter to cause a chronic follicular conjunctivitis. Most common in the very young, it has also been associated with HIV and other immunosuppressed states.
Localised viral conjunctivitis without or with minimal systemic manifestations usually results from adenoviruses (more about these shortly), but also may be caused by the following:
- Some enteroviruses; a group of RNA viruses (including those causing polio and hepatitis A) which typically occur in the gastrointestinal tract, but can cause a localised conjunctivitis.
- Herpes simplex virus; often causing corneal involvement with its characteristic dendritic ulceration, systemic involvement may be minimal.
Acute haemorrhagic conjunctivitis, a very severe conjunctivitis as previously mentioned, has a particular association with either one of two enteroviruses, enterovirus 70 and coxsackievirus A24. These were first identified in an outbreak in Ghana in 1969, and have spread worldwide since then, causing several epidemics.
The most common cause of viral conjunctivitis is, however, infection with adenovirus.
Adenoviral conjunctivitis
Unlike the many viruses that contain RNA strands, such as coronaviruses and rotaviruses, the adenoviruses contain DNA the exact detail of which define what individual serotype the adenovirus belongs to. Overall, there are nearly 60 serotypes so far identified that can infect humans, and these are categorised into seven groups (or species) described as A to G. So, for example, human adenovirus with serotype 14 would be recorded as HAdV-14. This belongs to species B (so is one of the HAdV-B viruses). Different serotypes and species are associated with different conditions as follows:
- Respiratory disease; mainly caused by species B and C
- Conjunctivitis; species B and D
- Gastroenteritis; species F (types 40 and 41) and G (type 52)
- Obesity or adipogenesis; A type 31, C type 5, and D types 9, 36, 37
Adenoviral conjunctivitis or keratoconjunctivitis is the most common acute viral infection of the external eye. It is highly contagious and is easily spread from systemic infection, via the tears, and so causes a secondary ocular infection. Direct ocular infection is from virus reaching the eye through direct contact (hands or optometric equipment) or via an aerosol spray emanating from an infected person (from a sneeze, or perhaps after non-contact tonometry). Incubation of the virus (during which it attacks host cells and replicates to levels that cause symptoms) takes typically between four to eight days. Once conjunctivitis presents, virus is shed and can re-infect for up to 12 further days.
Adenoviral conjunctivitis may present in one of three ways:
- Acute non-specific follicular conjunctivitis; ocular involvement without systemic viral disease symptoms.
- Pharyngoconjunctival fever (PCF); a follicular conjunctivitis presenting with systemic viral symptoms, typically of an upper respiratory tract infection. PCF is sometimes said to cause an alliterative triad of symptoms; follicles, fever and pharyngitis (sore throat).
- Epidemic keratoconjunctivitis (EKC); a highly contagious
follicular conjunctivitis along with systemic disease of varying severity (sometimes minor symptoms only) and nature, dependent upon which adenovirus is involved. Systemic disease may involve the gastrointestinal tract and/or respiratory system, and includes illnesses such as the common cold.
Predisposing factors that increase the risk of the disease include:
- History of a recent cold or other upper respiratory tract
infection - Poor hygiene
- Crowded living conditions
- Recent direct contact with another infected person
- Recent contact with virus being carried on another medium, for example an ECP’s hands or a non-sterilised or replaced tonometer prism
Symptoms of conjunctivitis typically present as:
- Redness
- Discomfort, usually described as burning or grittiness
- Watering
Eyelids may be reported as being stuck together in the morning and have to be bathed open. Initially, the condition is often unilateral, becoming bilateral within days when the first eye usually remains more significantly affected. Transiently blurred vision is usually due to excessive tearing, but can be due to central cornea involvement. Depending on the extent of systemic infection involvement, many patients may complain of a generalised malaise, or aches and pains.
Signs of the conjunctivitis are:
- Watery discharge
- Conjunctival hyperaemia
- Follicles on palpebral conjunctiva, especially upper and lower fornix
- Pre-auricular lymphadenopathy which may be tender (not present in every case)
The cornea may be involved to varying degrees, affecting around 30% of those with PCF and anything up to 80% of people with EKC. The classic corneal presentation follows a characteristic course, and again the extent of the progression along the course will vary significantly:
- Days 1 to 7; the development of fine pinpoint epithelial erosions that do not stain and may only be visible on retroillumination.
- Days 7 to 12; the presence of a focal or diffuse superficial punctate epitheliopathy that stains with fluorescein.
- Days 12 to 17; subepithelial keratitis may be seen, sometimes with the development of stromal infiltrates (figure 6). These can persist for years and may, in rare cases, cause a reduction in visual acuity.
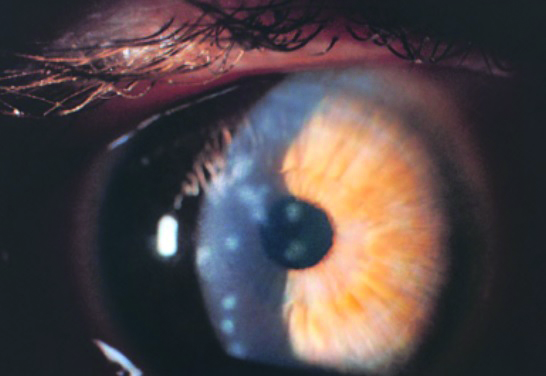 Figure 6: The development of subepithelial (stromal) infiltrates in the cornea may occur after around two weeks from initial conjunctivitis, and in some cases may persist for many months or even years
Figure 6: The development of subepithelial (stromal) infiltrates in the cornea may occur after around two weeks from initial conjunctivitis, and in some cases may persist for many months or even years
Petechial and haemorrhagic signs, or in some very severe case, the formation of a pseudomembrane, are rare and might indicate that the cause is another virus.
Management of adenoviral conjunctivitis usually involves the following:
- Reassurance; viral conjunctivitis usually resolves on its own, typically within two weeks but may be longer in severe cases, and there is no effective treatment. Antibiotics are obviously inappropriate, and antiviral medications have not been shown to have any effect. It is of value to reassure the patient of the self-limiting nature of the disease and to give a timescale. This is especially helpful if the disease turns out to be more chronic and persistent as the patient will know when to report again.
- Advice should be given about the contagious nature of the condition throughout its course and the importance of avoiding direct contact or contact via shared surfaces, such as towels and bed linen. If the patient works in a crowded environment, for example a school or college, they might be advised to let someone at the institution know of the nature of their condition.
- For the same reasons, the ECP must thoroughly disinfect all surfaces that have come into proximity or contact with the patient before seeing the next patient.
- Many patients find the regular use of a preservative-free ocular lubricant is helpful in reducing the symptom of grittiness.
For a summary of key points relating to viral conjunctivitis, see box.
SARS-CoV-2 and Viral Conjunctivitis
As Covid-19 took hold in China, early studies of the disease seemed to suggest that, despite anecdotal evidence to the contrary, viral conjunctivitis was not a sign of infection. For example, one study of 81 patients with coronavirus disease declared, ‘There wasn’t any novel coronavirus related conjunctivitis.’1
One widely held view was that people suffering Covid-19 were much more likely to be susceptible to infection from other, more common viruses acting opportunistically. Most typically, as a cause of a viral conjunctivitis, these would be an adenovirus. But the question remained, could the SARS-CoV-2 itself cause a follicular conjunctivitis? The more that was known about the coronavirus, the better researchers were able to compare with adenovirus. Here are a few significant differences between the two:2
- Human adenovirus is an extremely common cause of viral conjunctivitis, responsible for up to 90% of cases. Patients confirmed as having Covid-19 only rarely present with
conjunctivitis. - A coronavirus is quite different in structure from adenoviruses. All CoVs contain RNA as their genetic material. This is surrounded by a protein shell called a nucleocapsid. Like other CoVs, SARS-CoV-2 is an enveloped virus, meaning its nucleocapsid is surrounded by a lipid bilayer. SARS-CoV-2 has three proteins which are anchored into and protrude from the envelope, including the spike protein so familiar from images and which give the coronaviruses their name (figure 7).
- Adenoviruses, on the other hand, are non-enveloped and so have no lipid bilayer. They do, however, incorporate a large set of proteins and a long DNA molecule (four times longer than HIV genome) that make the virion relatively big, about 90nm in diameter (figure 8). The capsid has an icosahedral shape with rounded edges. The trimeric spikes that are located at each vertex interact with the cellular receptors to make the entry of the virus possible.
- The SARS-CoV-2 coronavirus uses its well-known spike proteins to bind to angiotensin-converting enzyme 2 (ACE2) receptors on human cells to gain entry. Evidence of ACE2 can be found in respiratory and intestinal tracts, renal, cardiac and immune cells. It has also been demonstrated in aqueous humour and retina, but not in conjunctiva or cornea. This is one reason why ocular impact might be minimal.
- Adenoviruses bind to a wide range of receptor sites, including those found in mucous membranes such as the ocular surface, respiratory, gastrointestinal and genitourinary tracts. Without the lipid bilayer, they do not readily spread throughout the host because they cause lysis (or splitting) of the host cells they invade (unlike SARS-CoV-2). Figures 9 and 10 show the different ways the two viruses infect host cells. However, this does make them likely to trigger a much stronger immune response in their immediate vicinity and viral spread without symptoms is highly unlikely.
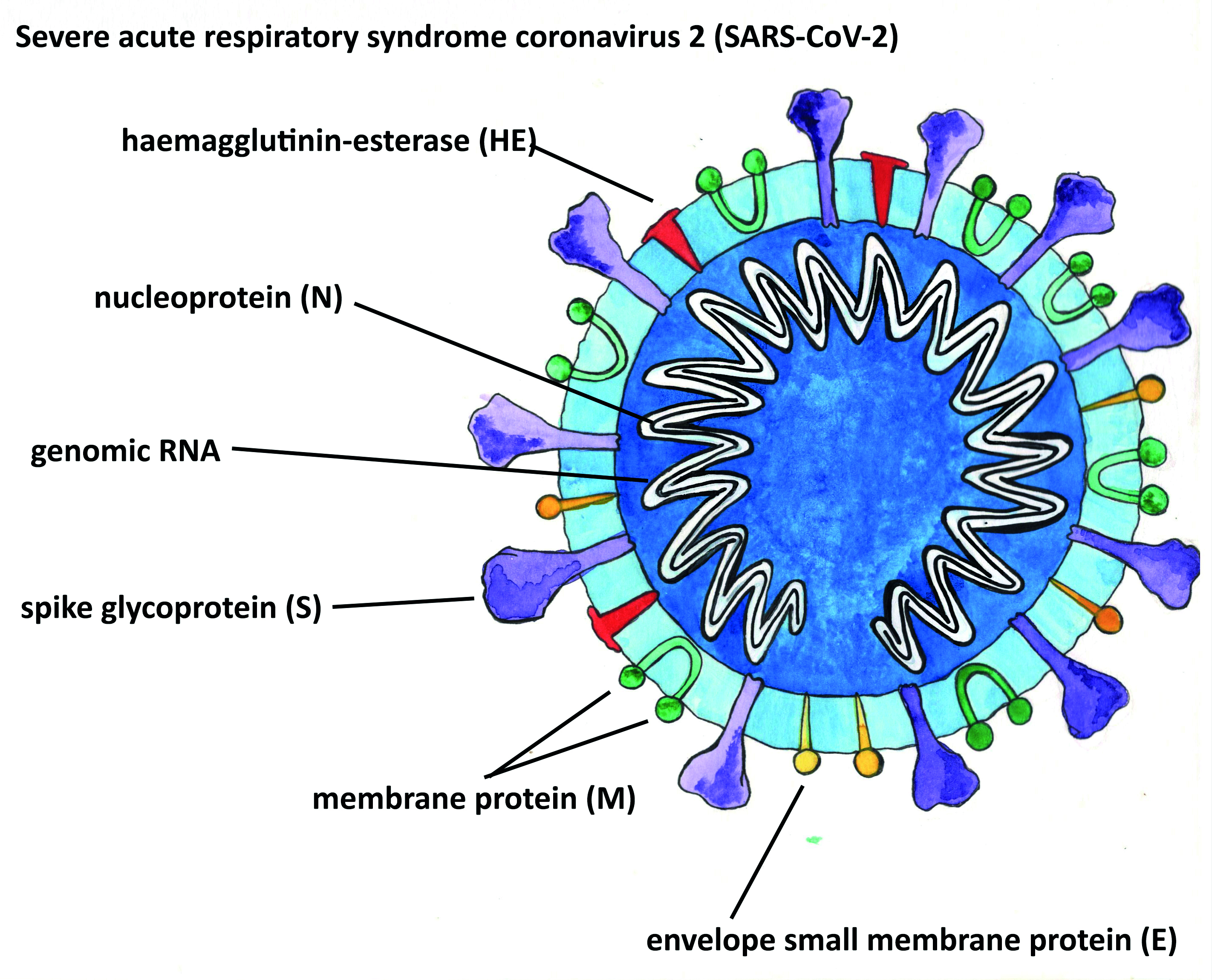 Figure 7: Structure of coronavirus. Note the presence of RNA within the protein shell (image courtesy of Kitty Harvey)
Figure 7: Structure of coronavirus. Note the presence of RNA within the protein shell (image courtesy of Kitty Harvey)
Figure 8: Structure of adenovirus . Note the presence of 1) trimeric spikes, 2) penton protein shell with no lipid layer and 3) a DNA core strand (image courtesy of Kitty Harvey)

Figure 9: Note release of the replicated virus without lysis of the host cell (image courtesy of Kitty Harvey)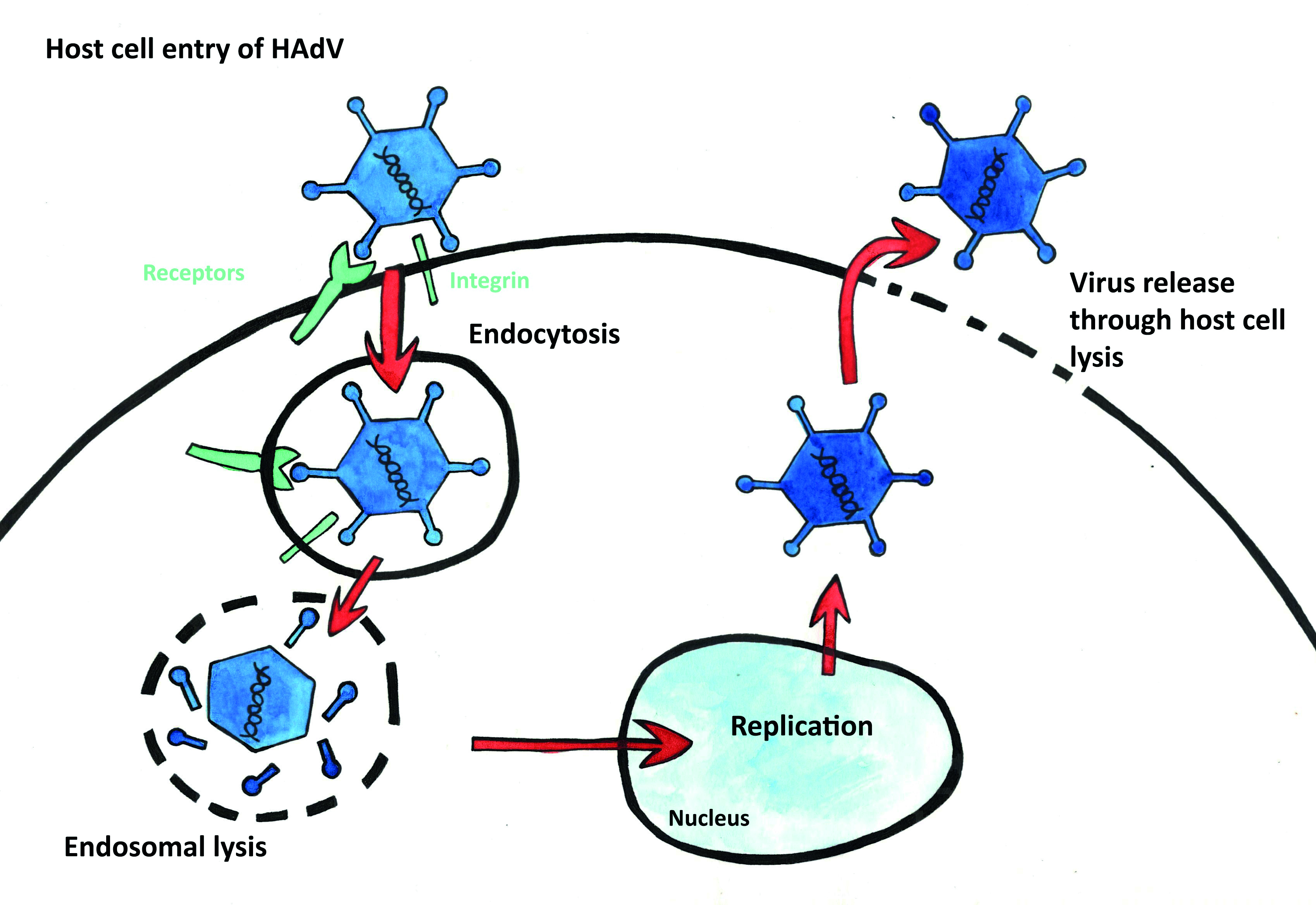
Figure 10: HAdV may attach to a range of host cell receptors. Virus release relies on lysis of the host cell. (image courtesy of Kitty Harvey)
So, what has been established about the SARS-CoV-2 virus and eye disease. A team from Canada reported a case where a 29-year-old, otherwise healthy woman presented to the emergency eye clinic with a 1-day history of right eye conjunctivitis, photophobia, and clear watery discharge from the right eye.3 As they stated in conclusion: ‘To the best of our knowledge, this is the first reported case of Covid-19 (caused by the SARS-CoV-2 virus) presenting with keratoconjunctivitis as the main symptom.’
Estimates of conjunctivitis in Covid-19 patients vary, from around 1% to a more significant 4.68% of sufferers with conjunctival congestion and 11.8% with ocular discomfort.4 The higher numbers are reported when the studies are undertaken by eye specialists. So far, all the main studies are from China, and we know there appears to be ethnicity influence over the expression of Covid-19. More recently, a case of pseudomembranous, haemorrhagic conjunctivitis has been associated with coronavirus infection.5
A very recent study found one-third of patients with Covid-19 had ocular manifestations consistent with viral conjunctivitis,6 but the presence of the virus in the conjunctival sac was very low. This suggests that, while it is possible to transmit the disease via the eyes, eyes are not the main transmission routes. In general, most studies are moving towards the view that, while SARS-CoV-2 may cause a follicular conjunctivitis, the conjunctiva is not a favoured target, and furthermore, transmission of infection via the eye is not a favoured route for transmission.7
The advice to ECPs, therefore, is likely to remain:
- Consider recommending Covid testing for any relevant systemic symptoms, including anosmia.
- Viral conjunctivitis rarely requires direct medical intervention, and the systemic implications of any presentation and their appropriate management far outweigh consideration of a conjunctivitis in isolation.
- The presence of the virus in the fornices and the potential, however low, risk of aerosol and direct contact transmission that is part and parcel of eye examination means that adequate personal protective equipment remains essential for ECPs.

References
- Lan Q et al. Chinese Journal of Ophthalmology, 2020, 56. https://doi.org/10.3760/cma.j.cn112142-20200322-00213
- Meng-Jou Chen et al. Precaution and Prevention of Coronavirus Disease 2019 (COVID-19) Infection in the Eye. Journal of the Chinese Medical Association. https://doi.org/10.1097/JCMA.0000000000000334
- Cheema M et al. https://doi.org/10.1016/j.jcjo.2020.03.003
- Chen L et al. https://doi.org/10.1101/2020.03.12.20034678
- Navela V. Haemorrhagic conjunctivitis with pseudomembranous related to SARS-CoV-2. https://doi.org/10.1016/j.ajoc.2020.100735
- Ping W et al. Characteristics of Ocular Findings of Patients With Coronavirus Disease
- 2019 (Covid-19) in Hubei Province, China. https://doi.org/10.1001/jamaophthalmol.2020.1291
- Guo M et al. https://doi.org/10.1002/jmv.25856
- For regular updates on all research relating to Covid-19, go to www.ncbi.nlm.nih.gov/research/coronavirus.
