The recent lockdown to prevent the spread of the highly virulent coronavirus disease, Covid-19, effectively put a stop to eye care as we know it. The need to ensure patients with serious eye concerns were adequately cared for has shone a spotlight on the newly evolving role of teleoptometry, one which is likely to persist and increase in influence as restrictions ease and more general eye care services resume. The aim of this article is to describe the concept of teleoptometry: what is it, how is it undertaken, and what role might it play in the coming years?
What is teleoptometry?
Telemedicine is a general term that describes the practice of assessing a patient’s health and managing any disease without the need for the clinician and the patient being in the same location. The prefix ‘tele-’ is from the Greek for ‘far off’ or ‘at a distance’ and may be applied to any interaction between two remote parties. Telephones allow sound interaction at distance and television, images visible from far off (‘television’ a term famously predicted never to catch on by Churchill due to its unwieldy mix of Greek and Latin roots). Teleoptometry therefore relates to the provision of optometric care when the optometrist is remote from the patient.
Is teleoptometry new?
No. With the rapid development of digital technology and networks, health workers have been able, for some years, to communicate with patients remotely, to use the latest smart
technologies to gather data from them, to analyse the results and to send back appropriate advice or treatment plans. Until the recent crisis, telemedicine has tended to be most useful in accessing remote or disparate populations who previously had little access to healthcare.
A good example of this has been the development of the PEEK system for assessing vision and eye health in areas of the world with poor health resource (figure 1). The inventor of the system, Dr Andrew Bastawrous, was first inspired to develop a smartphone-based retinal imaging system and a range of smartphone app-based vision checks when discovering how, in countries where only a small percentage of the population could access healthcare, the vast majority had use of a mobile phone network.
 Figure 1: The PEEK system; a good example of telemedicine in action before the pandemic
Figure 1: The PEEK system; a good example of telemedicine in action before the pandemic
And in better resourced countries, modern media have encouraged a greater interest in personal health and this has inspired many apps and devices designed to offer greater individual ownership of health status, both for good and for bad.
Remote Consultation
An immediate consequence of the coronavirus pandemic and subsequent lock was the sudden need to be able to correspond, whether with family, friends or patients, and the rapid adoption of the various video conferencing platforms allowing this. Technophobe and technophile alike have found software such as Zoom, Skype, Microsoft teams, Google Hangout or even FaceTime easy to use and more involving than a simple telephone call. Add to this the ability of some software to include various metrics and to complement the call with documents, data, images and so on, and videoconferencing became the obvious route to maintain contact with patients once primary care facilities were closed for all but emergency care provision.
Guidance for appropriate remote consultation has now been established, and readers should already be familiar with the advice given by the GOC,1 the College of Optometrists (CoO)2 and the Association of Optometrists,3 the AOP also producing a useful video guide (viewable at https://youtu.be/9tz0Z1yiMLI).
It is worth remembering the Information Commissioner’s Office has stated that practitioners need to consider the same kinds of security measures for remote consultation that would apply to normal in-practice interactions. If a personal device is used to conduct the consultation, any information from patient consultation must be transferred to the appropriate record system and then deleted from the device, including back-up data.
With this in mind, many practitioners may prefer to use a dedicated software platform for remote consultation and information transfer. Examples of such include the system from Optimed which, as well as helping set up a remote video triage service (figure 2), also allows the clinician to share a range of animations to help inform and reassure those not requiring contact appointments. The Australian Oculo platform is another, a cloud-based clinical communications network designed to support teleoptometry and allow video consultation and the secure, instant transfer of clinical imaging, referrals, and other clinical correspondence between healthcare professionals.
 Figure 2: Videoconferencing from Optimed
Figure 2: Videoconferencing from Optimed
Teleoptometry Today
At the time of writing, direct contact between people is to be avoided when possible, routine eye examinations have been suspended in all parts of the UK and procedures put in place to continue urgent or emergency and essential eye care. Remote communication (telephone or video) is required to triage those that might warrant face-to-face eye care assessment, to offer reassurance and advice on non-urgent concerns, or to identify those at risk of Covid-19 and give appropriate instruction about testing, isolation or treatment.
As restrictions lift, the need to avoid a second spike in infection in the absence of confirmed immunity means that our profession is likely to continue operating a sort of hybrid teleoptometry
service for some time yet. There will likely be two options available, each with provisos:
- Face-to-face interaction; adapted to minimise infection risk
- Remote assessment; where possible and appropriate.
Both require some considerable thought if eye care is to be both safe and of the highest standard, so a few thoughts on each option.
Modified Face-to-Face Consultation
The general rule is to only arrange a face-to-face assessment if it is clearly in the patient’s best interests. Such consultations will need to minimise the risk of infection and so some basic rules will be observed for some time yet.4 As well as the importance of social distancing and appropriate personal protective equipment, practitioners will need to keep contact time to a minimum. This is where some aspects of teleoptometry encroach upon practices because as much data as possible should be obtained prior to consultation, either via online form-filling, phone or video calls. This should be detailed enough to require minimal confirmation on attendance at the practice, and consultation should be as quick as possible and involve the minimum of conversation.
Also, there may be some changes to the testing itself. For example, non-contact tonometry is no longer advised as it may produce a local aerosol from the tear pool. Rebound tonometry is a useful option for routine assessment, while Goldmann (and indeed any other slit-lamp based technique) will be carried out behind a face shield (figure 3). There may well be some renewed interest in single use contact viewing lenses, such as the gonioscopy lenses from Sensor Medical (figure 4).
 Figure 3: No talking at the slit-lamp, and breath shields help minimise virus spread
Figure 3: No talking at the slit-lamp, and breath shields help minimise virus spread
 Figure 4: Single use contact viewing lenses are available
Figure 4: Single use contact viewing lenses are available
Tests which rapidly, but accurately, capture data, and allow further consideration after the consultation would be preferable to longer testing times. So, expect more onus upon automated refraction, imaging and functional testing. Results can be followed up and explained later. Also, instruments that perform multiple testing with a minimum of operator input might be attractive, such as the Visionix Vx 120 (figure 5) or the Essilor Medica 700+.  Figure 5: Instruments capable of several different tests in one brief sitting, such as the Visionix 120, will help minimise testing time
Figure 5: Instruments capable of several different tests in one brief sitting, such as the Visionix 120, will help minimise testing time
A similar approach would seem sensible for dispensing too. Tablet-based measurements from a distance, or dedicated digital consoles capable of accurate measurements without the need for proximity, such as the Essilor Visioffice or the Zeiss Analysis systems, will help, while imaging systems that allow frame choice without the need for handling of display frames will also reduce infection risk. Indeed, digital display of frame and lens wear might be made available online for home use either prior to or after the consultation.
Taking this approach one step further, there is some increasing interest in certain tests operated remotely, or being operated in practice by a suitably trained technician for the results to be
transmitted to the clinician who can then interpret and respond to them remotely. An example of this is available for the Revo OCT instruments. As initial positioning, focusing and data capture is fully automated, there need only be an auxiliary staff member (suitably attired as in figure 6) present and the results can be viewed remotely via any suitable remote desk top software that is compatible with Windows 10.
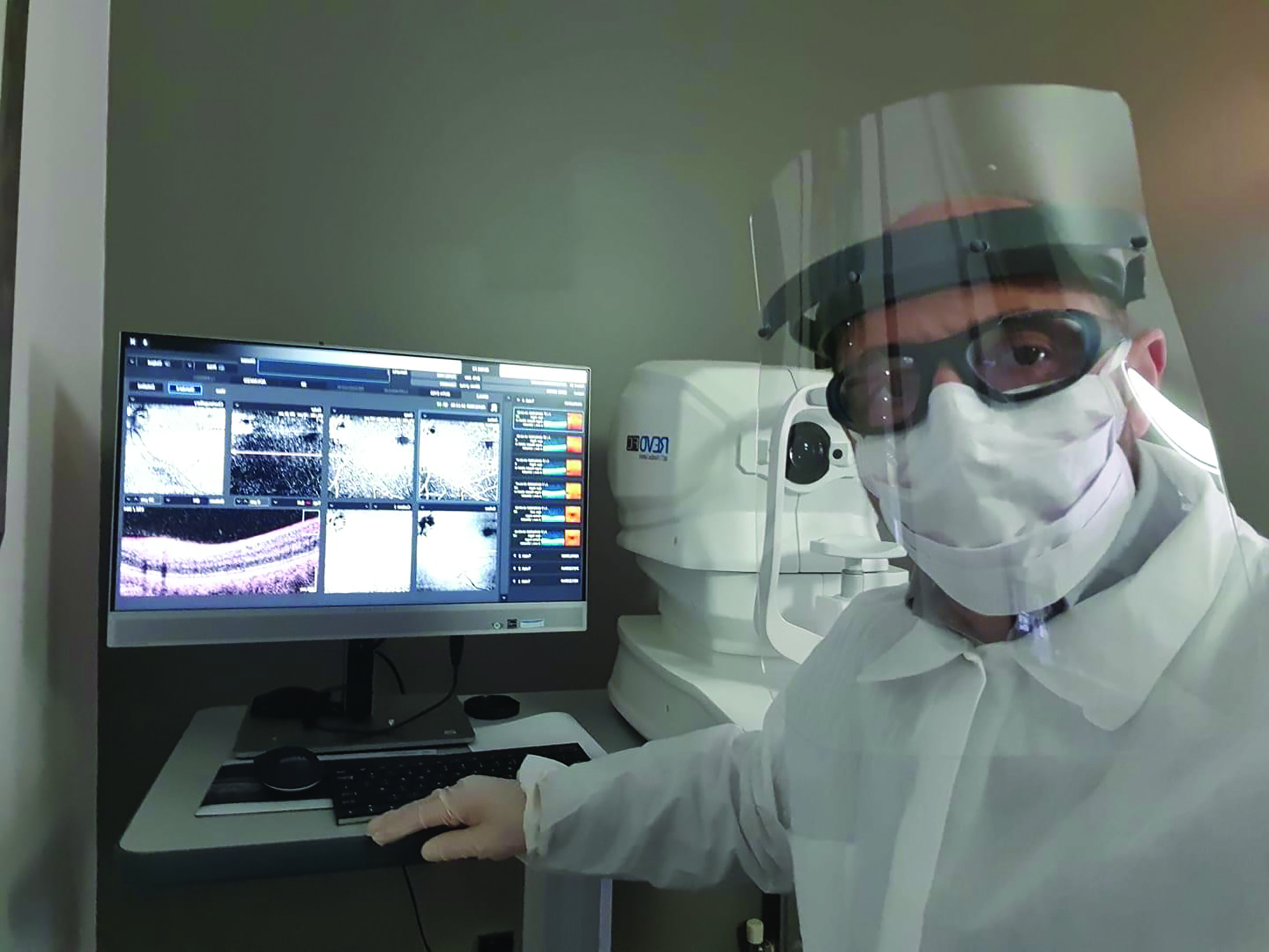 Figure 6: Automatic operation of instruments such as the Revo OCT allows testing and remote data interpretation, with only a support staff member required to guide the patient, so minimising contact risk
Figure 6: Automatic operation of instruments such as the Revo OCT allows testing and remote data interpretation, with only a support staff member required to guide the patient, so minimising contact risk
Remote Testing
True teleoptometry would include remote testing of a patient, with results being securely transferred to the clinician, without the need for attendance at a clinic or with any support staff involvement. In terms of infection risk reduction, this obviously is a very attractive concept. But is it possible? Well, in a recent review by Saleem et al, it was concluded that ‘home-based remote monitoring is still relatively premature and not widely available.’5 Their preference for now was the aforementioned minimally staffed clinic sending results to a remote clinician. Indeed, a sort of ‘half-way house’ approach is proving useful.
The DigitalOptometrics teleoptometry eye exam process begins with what they describe as ‘the Tablet Kiosk-Patient Experience.’ In essence this is a sort of vision screening kiosk, where a patient inputs their initial data, history and symptoms and so on, then undergoes autorefraction, non-contact tonometry, has a retinal photo taken, a slit-lamp based video recorded of the anterior eye, and finally a remotely operated real-time video-based subjective refraction is completed. Results, including where further concerns need addressing, are then presented. In just over a year since its launch, the DigitalOptometrics system has completed over 20,000 exams and was set up at over 150 locations by the end of 2019.
That said, new techniques for patients to self-test or for remote testing are springing up all the time offering options for most of the key parts of an eye examination. Here are some examples:
- Acuity testing: most of us have already sent out acuity charts, such as the 3m self-printable version from the CoO, or perhaps an Amsler grid to patients. Apps offering these abound and are reasonably accurate. It is always useful to check first the likely size of printout and compare with an original version, however, as printer settings can affect final print size significantly. One automated option is the Eyecare Live test, which requires a patient to download an app onto their smartphone and use a laptop to access a website (figure 7). Once linked, the patient moves the required distance from the computer screen and then identifies the sequence of Landolt C targets until no longer possible, at which point their acuity is recorded. Such a system appears to have good repeatability.
- Refraction: a number of online or smartphone-based refraction systems exist, but not without controversy. The Visibly system, for example, was recalled by the FDA in the US after pressure from optometrists. Their, in my view, justifiable worries over the lack of clinician input and heavy reliance on patient self-adjustment outweighed accusations of protectionism. The debate still rages and now extends to the UK.6 That said, a validation study of a web-based refraction and visual acuity test called Easee BV showed excellent agreement with conventional subjective refraction.7 However, other systems, such as the smartphone-based refraction and lensmeter system, the EyeNetra (figure 8), have been found to err by half a dioptre or more.8 Where there is remote control over online targets by the clinician, such as with the COMPlog test chart, results seem much better.9
- Tonometry: the iCare Home rebound tonometer is designed for use by the patient and is able to relay the results taken to a server which allows the remotely located clinician to analyse IOP patterns (figure 9). I admit that my patients found this tricky to use, but with practice it does serve a useful purpose. Similarly, the Sensimed Triggerfish contact lens system is not completely patient-friendly, and is rather expensive to boot.
- Visual fields: There are a number of visual fields tests for remote use which appear to show consistency of results. The Peristat home-based visual field test exhibits a reasonable receiver operating characteristic curve when compared to the Humphrey perimeter, without the need of specialised equipment (figure 10).10 Several apps for fields testing, such as the visualFields easy app, the MRF Lite (figure 11) and the ForSee preferential hyperacuity app for central field distortion all show reasonable repeatability.
- Image capture and viewing: In his recent review, Saleem suggests that ‘Remotely controlled slit lamp devices, non-mydriatic fundus cameras and OCT machines may become more available in public areas.’5 Plans for OCT distribution in local health centres throughout the UK have been mooted for many years. Until then, this area of assessment is still reliant on practitioner and patient proximity to some extent. That said, high quality image and video capture with portable devices is increasingly easy to achieve.
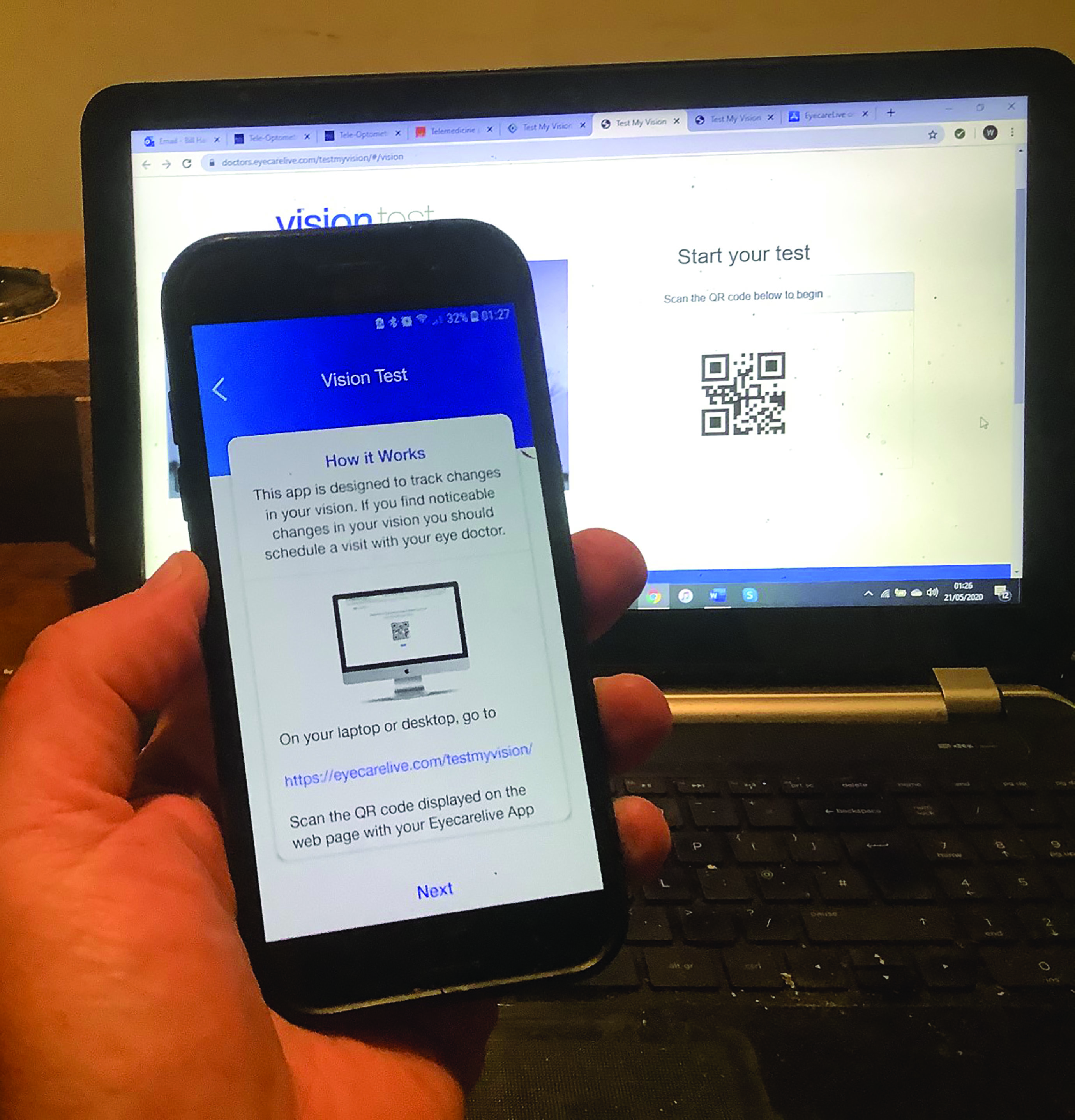 Figure 7: The Eyecare Live test requires smartphone and laptop used in conjunction
Figure 7: The Eyecare Live test requires smartphone and laptop used in conjunction Figure 8: Smartphone-based EyeNetra system for refraction and lens measurement
Figure 8: Smartphone-based EyeNetra system for refraction and lens measurement Figure 9: The iCare Home rebound tonometer is for patient use at home
Figure 9: The iCare Home rebound tonometer is for patient use at home
 Figure 10: The screen-based Peristat uses blind spot targeting to helps control fixation while the patient identifies a sequence of dimmer peripheral targets
Figure 10: The screen-based Peristat uses blind spot targeting to helps control fixation while the patient identifies a sequence of dimmer peripheral targets
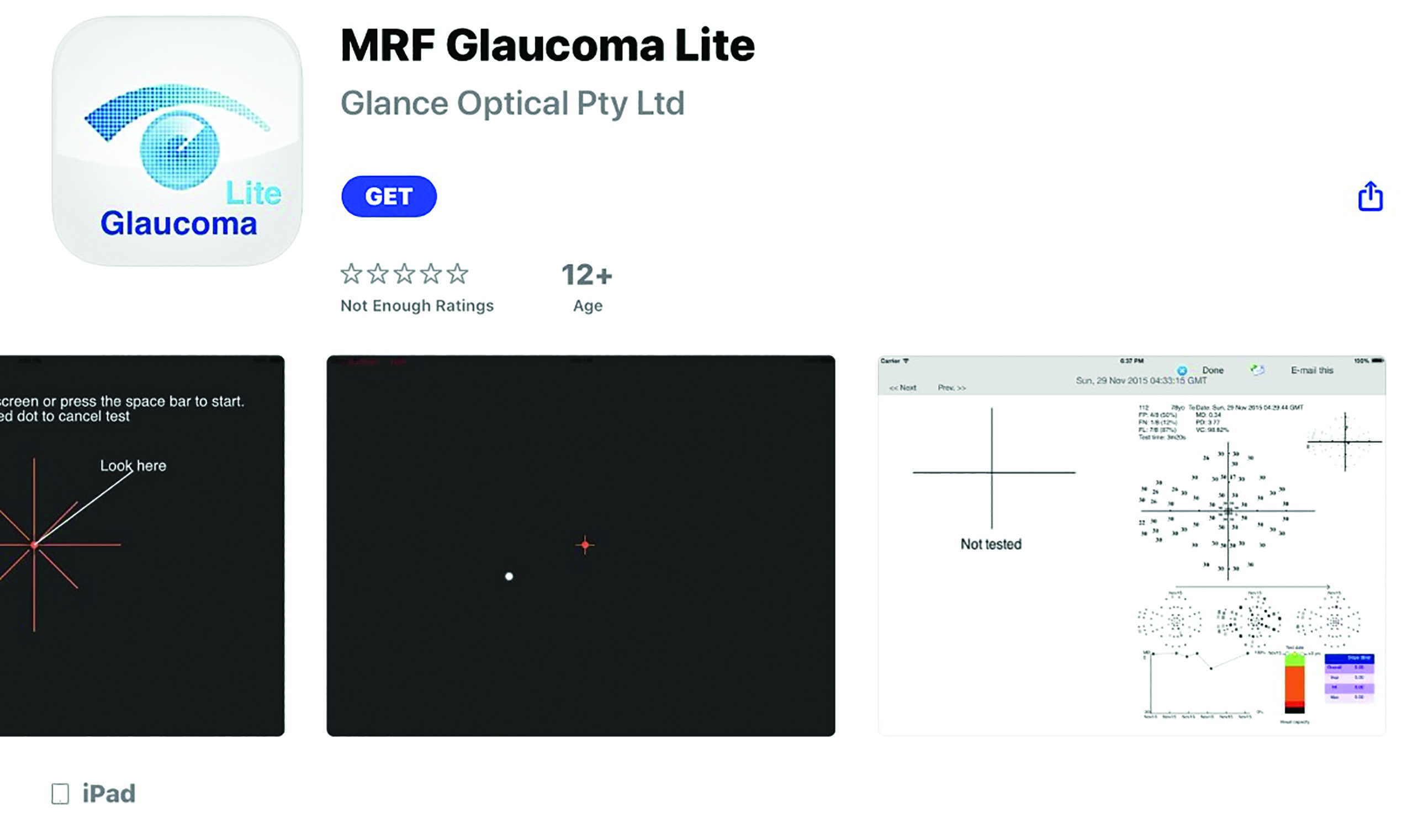 Figure 11: The MRF Lite is an app for fields assessment
Figure 11: The MRF Lite is an app for fields assessment
And finally, a note on contact lens fitting. In a recent review, Nagra M et al reinforce the challenge of remote slit-lamp viewing and conclude that, though much contact lens work can be carried out remotely, such as patient education and support, ‘the feasibility of lens fitting apps is likely to be limited by difficulties in visualising lenses, particularly soft lenses, against the non-uniform background of the ocular surface, without the magnification and illumination benefits provided by a slit lamp. This will be followed by a complementary pipeline of remote and mobile technology products.’11
All Round Platforms
Teleoptometry is very much in evolution, and as might be expected from any discipline steeped in digital technology, progress is rapid. The UK is home to two designers of digital platforms worth keeping an eye on. The EYOTO group is based in Birmingham and has links with Aston University. They promise that the ‘first product in our teleoptometry platform, launching late 2020, will allow an ECP to conduct an eye examination from a remote location – a different room, city, or continent.’
Meanwhile in Bristol, TED talk-renowned research optometrist, Dr Stephanie Campbell has established a reputation for her visionary approach to the future of optometry, and the company she helped set up, Okko Health, has garnered considerable funding for its development of a home monitoring technology system.
A Note About Apps
Individual downloadable programmes for use on tablets and smartphone make it easy for individuals to complete often complex tasks, hopefully with a degree of accuracy and usually with full engagement. The popularity and versatility of apps, combined with a widespread obsession with matters of health, has led to a massive increase in the number of health-related apps currently available. On the app store alone at the start of 2020, IoS apps numbered some 45,478, so the total including Android apps is likely to be much higher.
That said, and bearing in mind that such apps open to direct use by the public might have significant influence over somebody’s wellbeing, their production and design is not without regulation. This very month, the Medical Device Regulation (EU) 2017/745 is adopted in Europe which broadens the definition of any app to be defined as Class IIa, rather than the less restricted Class I as most were previously. The regulatory change requires medical app developers to have a qualified quality manager, central product registration on the EUDAMED database and a continued quality review when the software is launched and in use – forcing the less serious to exit the market.12 Software designed for use in diagnosis, prevention, monitoring, prediction, prognosis, treatment or alleviation of disease have to carry the CE mark.
As we have seen, some apps exist as functioning tools, or instruments for measuring or testing. Further examples of these include a colour vision testing app, a Hess chart app or an electronic Amsler app (figure 12). Others, such as the reasonably well-known Dry Eye Toolkit app, act instead as a diagnostic tool.  Figure 12: Many apps assist in testing and measurement
Figure 12: Many apps assist in testing and measurement
For diagnosis, there is a distinct difference between those apps that collate input data and predict and outcome, and those that make a clinical decision. The former act in many ways like the risk calculators that have been around for years (figure 13), and have proven performance in predicting the likelihood of conditions like glaucoma and AMD.13,14 Those that instead offer a diagnosis or a management plan tend to have a more variable level of success when compared with clinicians.15,16 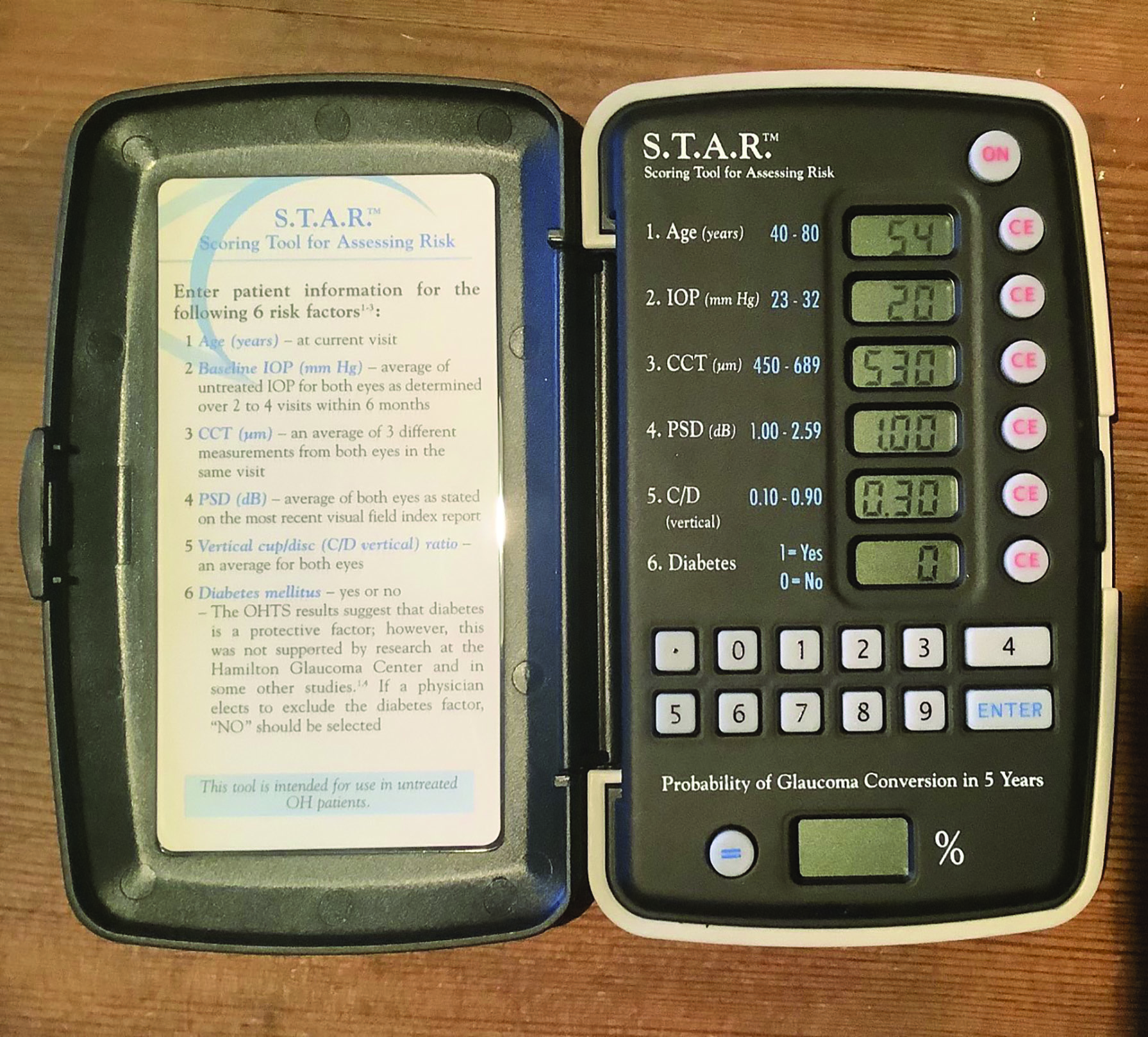 Figure 13: The STAR calculator. An old example of machine learning
Figure 13: The STAR calculator. An old example of machine learning
Artificial Intelligence and Machine Learning
Much publicity has been given to AI. In most cases, what is described as artificial intelligence is actually machine learning, whereby a computer assimilates increasing amounts of data and so is able to refine responses or decisions with increasing levels of accuracy. In many ways this is no different to the normative databases that we have known for years in fields screeners, and are to be found in current OCT software.
An algorithm is a set of instructions that a computer may follow, but does not necessarily require machine learning, such as a flow chart for use in managing a virtual patient. Examples of where an algorithm does use machine learning is the application of Bayes theorem and, with greater complexity, the Google Mind/Moorfields/UCL interpretation of OCT data. Bayes theorem involves updating existing beliefs with new data to modify a belief. Application to eye disease would first involve establishing initial beliefs – how common one or more outcomes are. Examples of outcomes might include specific eye conditions or urgency of referral. Pre-test odds may be derived for these initial beliefs. Objective new information may include presenting history, signs and test result data. Diagnostic value of these may then be expressed in terms of likelihood ratios which can be used to improve efficiency of assessment by identifying which have the greatest diagnostic value.
In an excellent study by Gurney et al,13 a Bayesian algorithm was used to decide on referral or otherwise of possible glaucoma patients, and was found to perform comparably with a group of experienced glaucoma specialist optometrists. This underlines the strength of large data sets, but still does not imply any human-like intellectual process.
The London-based AI firm DeepMind, acquired by Google in 2014, has worked with Moorfields Eye Hospital to develop algorithms for a system that can analyse retinal scans and spot symptoms of sight-threatening eye diseases, such as early detection of diabetic retinopathy and age-related macular degeneration. Results demonstrate a performance in making a referral recommendation that reaches or exceeds that of experts for a range of sight-threatening retinal diseases after training on only 14,884 scans.14 More recently still, a team have developed a system capable of predicting the progression to a wet form of AMD better than five out of the six experts against which it was compared.15 Studies continue to accumulate suggesting the same pattern, including improved prediction of cardiovascular risk factors from retinal images,16 prediction of late stage AMD17 and comparable consistency of retinopathy grading with experienced graders.18
Conclusion
We should embrace the evolution of ever more accurate, easy to use and accessible technologies in supporting our eye care profession. Ultimately, machines, apps and software will always out-perform any human in terms of amount of data and information they can assimilate, the subtlety of pattern variation they might detect, the accuracy of prediction based on analysis of a data set, or the ability to detect minor changes in a large screening operation. But so what? As we move towards a post-Covid, increasingly tech-driven future, as long as patients are human, then skilled interpreters and communicators of findings will always be needed if the end result is to be successful.
References
- www.optical.org/filemanager/root/site_assets/publications/covid_19/High-level-principles-for-remote-prescribing_.pdf
- www.college-optometrists.org/the-college/media-hub/news-listing/remote-consultations-during-covid-19-pandemic.html
- www.aop.org.uk/advice-and-support/clinical/clinical-governance/remote-consultations
- Meeting eye health needs and preventing vision impairments during Covid 19: A framework for primary eye care providers. FODO, published: 17/05/2020
- Saleem SM, Pasquale LR, Sidoti PA, Tsai JC, Virtual Ophthalmology: Telemedicine in a Covid-19 Era, American Journal of Ophthalmology (2020), doi:https://doi.org/10.1016/j.ajo.2020.04.029
- www.opticianonline.net/features/in-focus-us-spat-hints-at-whats-to-come-for-uk-optics
- Wisse, R et al. Validation of an independent web-based tool for measuring visual acuity and refractive error. J Med Internet Res 21 (11) (2019) p.e14808.
- Jeganathan, S et al. Accuracy of a Smartphone-Based Autorefractor Compared to Gold-Standard Refraction. Optometry and Vision Science, 95 (12) (2018) 1135
- Srinivasan, K et al. Efficacy of a remote based computerised visual acuity measurement. British Journal of Ophthalmology, 96 (7) (2012) 987–990
- 10. Tsapakis S et al. Home-based visual field test for glaucoma screening comparison with Humphrey perimeter. Clinical Ophthalmology, 2018;12:2597-2606
- www.eyoto.com/tele-optometry.aspx
- Campbell S. The Newcomer’s Guide to Digital Health. https://medium.com/@stephanie_98002/the-newcomers-guide-to-digital-health-1be5e4dd5060
- Gurney AC et al. Application of Bayes’ to the prediction of referral decisions made by specialist optometrists in relation to chronic open angle glaucoma. Eye, https://doi.org/10.1038/s41433-018-0023-5
- De Faux J et al. Clinically applicable deep learning for diagnosis and referral in retinal disease. Nature Medicine. 2018; 24:1342-1350
- Yim, J et al. Predicting conversion to wet age-related macular degeneration using deep learning. Nat Med (2020). https://doi.org/10.1038/s41591-020-0867-7
- Thakur A et al. Predicting glaucoma prior to its onset using deep learning. Ophthalmology Glaucoma doi: 10.1016/j.ogla.2020.04.012
- Poplin R et al. Prediction of cardiovascular risk factors from retinal fundus photographs via deep learning. Nature Biomedical Engineering. https://doi.org/10.1038/s41551-018-0195-0
- Yan Q et al. Deep-learning-based prediction of late age-related macular degeneration progression. Nature Machine Intelligence, https://doi.org/10.1038/s42256-020-0154-9
- Arcadu F et al. Deep learning algorithm predicts diabetic retinopathy progression in individual patients Digital Medicine. https://doi.org/10.1038/s41746-019-0172-3
