All too often, we concentrate on the side effects of systemic medication affecting the eyes of our patients, yet forget that the drugs we use for the diagnosis or treatment of ocular conditions may be equally problematic. This series concentrates on the systemic side effects of diagnostic and therapeutic ocular drugs.
Pharmacokinetic aspects
Pharmacokinetics is the term used to describe the time-dependent movement of a drug until it is eliminated from the body. This movement depends upon the mode and site of administration and how protected that ‘compartment’ is from the rest of the body. For example, in various areas of the body, barriers such as the blood-brain, the blood-placental, the blood-ocular and the blood-retinal, ensure a safe drug therapy.
However, when applying eye drops, it is known that more than 50% of the drug will be absorbed systemically, either via the
conjunctiva or through the lacrimal drainage system (figure 1). Other minor routes of absorption are via the skin around the eye, aqueous humour or the inner ocular tissues. Such a large ‘miss’ is also due to the fact that the volume of the available drop dispensers exceeds that of the conjunctival sac.1 Other factors contributing to the low bioavailability and exposure time of the eye drops with the ocular surface include:1
- Drug pH
- Drug osmolarity
- Lipophilicity/hydrophilicity of the active compound
- Concentration of the active compound
- Integrity of the barrier posed by scleral, conjunctival and corneal epithelium
- Level of lacrimation
- Extent of conjunctival hyperaemia, ocular inflammation, or other presenting signs of influence
 Figure 1: Over half of the drug applied topically passes systemically via the conjunctiva or lacrimal drainage system
Figure 1: Over half of the drug applied topically passes systemically via the conjunctiva or lacrimal drainage system
The absorption of topical drugs is via the conjunctiva and cornea. As the lacrimal fluid is drained from the conjunctival sac through the nasolacrimal duct, a large quantity of the drug may be absorbed systemically through the highly vascularised nasal and nasopharyngeal mucosa. It is interesting to note that, if absorbed via this route, drugs will bypass the liver and, therefore, will have a higher systemic toxicity due to the fact that this first step of being metabolised is not accomplished.1,2 When drugs are injected intravitreally, they are transported via the blood-retinal barrier. There is a further exit route from the vitreous humour via the anterior chamber.
Neonates and the elderly are most vulnerable to the side effects of ocular drugs. In neonates, all membranes are thinner and so drugs can pass more easily through the various barriers. Elderly patients, on the other hand, typically suffer from additional systemic disorders and the concomitant use of other drugs increases the risk of systemic toxicity from the ocular drug use.
We will now take a look at each of the groups of drugs commonly used in optometry and consider their potential side effects.
Cycloplegics and myrdriatics
Cycloplegics are used to induce paralysis of the ciliary muscle through an anticholinergic effect. They also induce mydriasis and the onset of this effect usually precedes cycloplegia. They are mostly used in children for refraction or for diagnosis of ocular motility problems, but they may also be used in poorly cooperative or impaired adults. They are sometimes used as an alternative for patching in the treatment of amblyopia (penalisation), in the treatment of uveitis for reducing ocular pain and prevention of posterior synechiae, in cases of corneal trauma, or post-operatively to reduce pain and prevent other complications. Mydriatics induce pupil dilation but also have a cycloplegic effect. The drugs in this category usually have a dual effect. Table 1 summarises the properties of cycloplegics used in eye care practice.
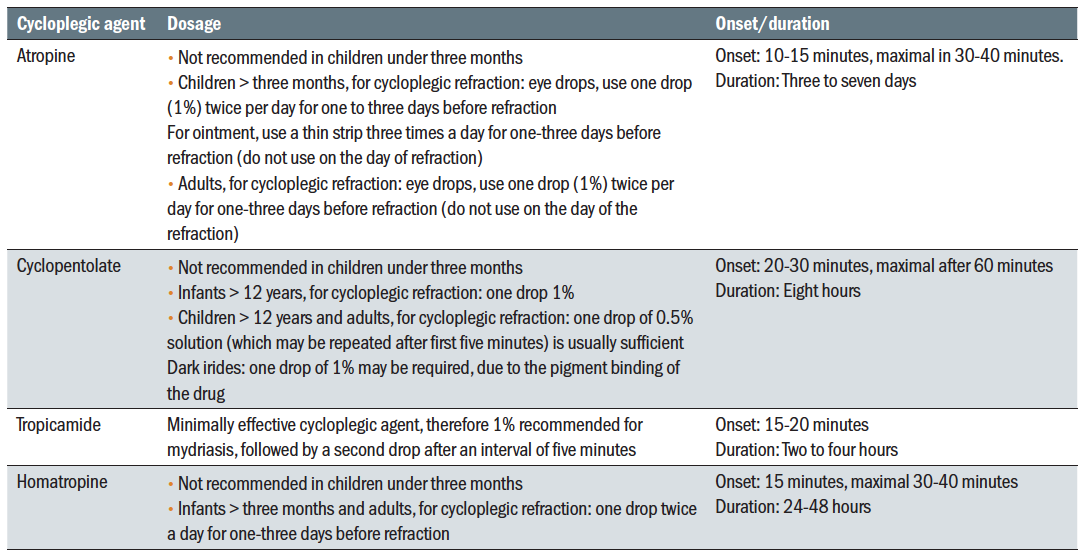 Table 1: Properties of cycloplegia agents
Table 1: Properties of cycloplegia agents
Atropine
Atropine is a competitive antagonist of acetylcholine and other muscarinic agonists. As a systemic drug, atropine can be used to treat some cardiac arrhythmias, asthma and heart block. It is also used before general anaesthesia or to treat some poisonings, especially with organophosphorus nerve agents. In optometry and ophthalmology, atropine is used for cycloplegia in very young convergent squinters who have no binocular vision or for penalisation in amblyopia therapy in older children that are intolerant to patching. Research into the use of a low dose atropine drop or spray for myopia management is ongoing.
Due to its significant toxicity, atropine is mostly used in the hospital settings and requires IP qualification for use by an optometrist. Indeed, after ophthalmic administration, the systemic bioavailability of atropine can be very high. For this reason, atropine is contraindicated in patients with a known hypersensitivity to the drug or to any belladonna alkaloids (active substances derived from the plant Atropa belladonna or deadly nightshade).
Beside blurred vision, atropine also have various systemic side effects ranging from drowsiness, dizziness and dry mouth, to myocardial infarction and anaphylaxis. Other possible systemic side effects include urinary retention, inhibition of sweating (anhidrosis) and constipation. Atropine also affects the normal cardiac rhythm, and may induce cardiac arrest. It is, therefore, important to establish whether a patient has any cardiac disease before administering the drug.
The quote ‘red as a beet, dry as a bone, hot as a hare, blind as a bat, mad as a hatter and full as a flask’ is commonly used in relation to atropine poisoning (figure 2). In this situation, the patient presents with:
- Red as a beet; face and trunk of body are bright red, also known as atropine flush
- Dry as a bone; dry mucous membranes and skin
- Hot as a hare; high fever
- Blind as a bat; dilated pupils
- Mad as a hatter; delirium, hallucinations, confusion, staccato speech, and picking at clothing and bedding
- Full as a flask; urinary retention
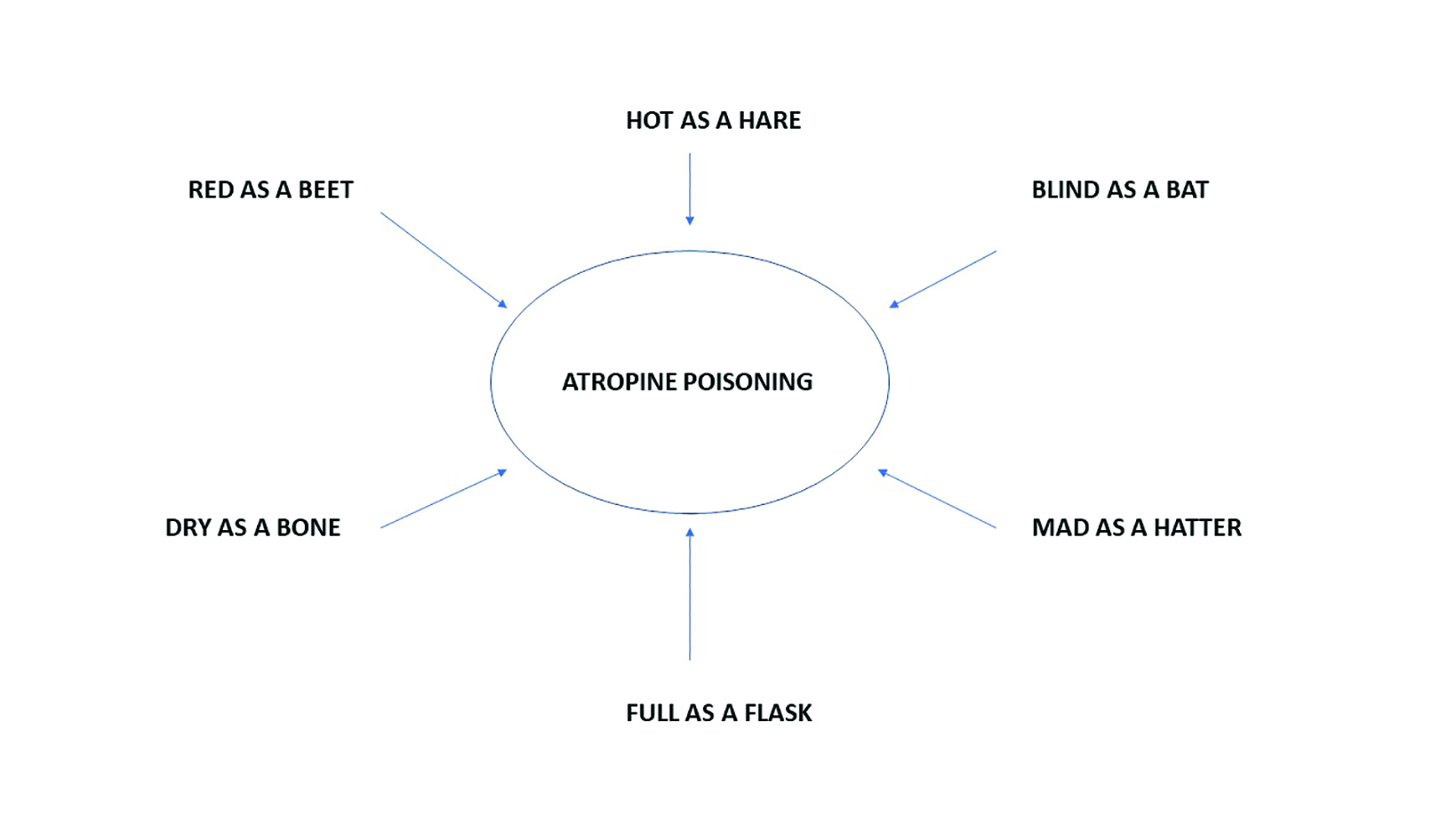 Figure 2: Features of atropine poisoning
Figure 2: Features of atropine poisoning
Other severe side effects, thankfully, are rare and only with the use of drops as opposed to ointment. Convulsions, coma and death have occurred after ocular instillation of atropine in infants and children. Accidental or intentional ingestion of atropine solution may also happen, and usually with disastrous consequences. Intentional poisoning with atropine solution has also been reported in a homicide case.3
The lethal dose of atropine is between 100mg and 200mg. Therefore, parents and guardians should be encouraged to keep all medications in a safe place that is inaccessible to children.4 In addition, carers and patients should also be advised to wash hands before and after instillation, and to remove all excess ointment with cotton wool. The drug should be stopped immediately if any local reaction occurs. In addition, the tube should be returned to the prescribing centre after the three-day application so that it may be disposed of safely.
Homatropine
Homatropine is a semi-synthetic antimuscarinic alkaloid rarely used in optometric practice. Homatropine is only about one-tenth as potent as atropine. The ocular effects of homatropine are more rapid in onset and of shorter duration than those of atropine (see table 1). Its systemic side effects are, however, very similar and the same precautions should be applied when using this drug.
Cyclopentolate
Cyclopentolate is a synthetic anti-cholinergic drug. Cyclopentolate 0.1% is used as mydriatic, while 0.5% and 1% strengths are used for cycloplegia. Cyclopentolate is the main cycloplegic drug used in optometric practice due to its safety. However, systemic adverse effects have been reported to occur in up to 10% of patients after repeated instillation of 1% strength. These can include the following:5
- Tachycardia; increased heart rate
- Restlessness
- Hallucinations
- Psychosis
- Hyperactivity
- Seizures
- Incoherent speech
- Ataxia
Such effects are especially likely in children. The central nervous system (CNS) toxicity of this drug is due to its anticholinergic action, causing stimulation of the medulla and cerebral centres. They occur within 20 to 30 minutes of administration and subside within four to six hours with no permanent sequelae and with the patients having no recollection of the hallucinations.6 Atropine-like systemic side effects can also occur. In addition, patients can present with rashes, headaches, nausea and wheeze or even severe anaphylactic reactions.
Although some of these effects occur after multiple instillations and are dose dependent, it is not unheard of that severe reactions occur after the first use of this drug. Therefore, as with any other drug, close observation of the patient after instillation of cyclopentolate is highly recommended and early recognition of systemic toxicity is important for prompt management.
Tropicamide
Tropicamide is an antimuscarinic drug that is mainly used as mydriatic and only occasionally used as a cycloplegic in optometric practice. Its cycloplegic effect is weak and transitory (see table 1). It is available as either 1.0% or 0.5% solution, in unit dose Minims (figure 3). Its mydriatic effect occurs within around 15 minutes and recovery is complete around five hours after instillation. Highly pigmented irides often need a higher dose by using a higher concentration, repeated instillations, or both.  Figure 3: Tropicamide in a unit dose Minim
Figure 3: Tropicamide in a unit dose Minim
Its cycloplegic effect occurs after 20 minutes and recovery complete after some three hours from instillation.
Tropicamide’s potential systemic side effects are due to its antimuscarinic properties and can include the following:
- Dry mouth
- Tachycardia
- Headache
- Nausea
- Vomiting
- Pallor (generalised)
- Allergic reactions
- Muscular rigidity
These adverse responses are, however, very rare.7
Scopolamine
Scopolamine is derived from the plants of Datura stramonium (commonly known as Jimson weed), Scopolia carniolica, and Hyoscyamus niger (or henbane, figure 4). As a systemic medication, scopolamine is mainly used to treat motion sickness and postoperative nausea and vomiting. It can also be used as a drug, and it sometimes known as the Devil’s Breath because can be blown into a victim’s face or to spike food or drinks to disable someone.  Figure 4: Henbane
Figure 4: Henbane
It is available as ophthalmic solution and is used to induce mydriasis and cycloplegia. Used in this formulation, systemic side effects can include flushed skin, tachycardia, agitation, and confusion; similar to other antimuscarinic drugs.
Phenylephrine
Phenylephrine, an alpha-1 adrenoceptor agonist, is the only sympathomimetic mydriatic available to optometrists and is produced in Minims (2.5% and 10%). It can be used on its own as mydriatic, or in combination with tropicamide for an enhanced dilation with reduced light reflex response.
Maximum dilation occurs around one hour from instillation and recovery lasts for three to four hours. Phenylephrine is
generally contraindicated in patients suffering from cardiac disease, hypertension, thyrotoxicosis and long-standing insulin dependent diabetes mellitus. It is also contraindicated in patients taking certain medications, such as monoamine oxidase (MAO) inhibitors, tricyclic antidepressants and anti-hypertensive agents (including beta-blockers).
Systemic side effects of phenylephrine can include contact dermatitis, significant increases in blood pressure, cardiac arrhythmias, and even myocardial infarction when used in high concentration. The use of phenylephrine 10% solution is forbidden for children and the elderly because of the increased risks of systemic toxicity.1
Topical anaesthetics
Using topical anaesthetic (TAs) is part of the normal daily routine in any optometric practice. They are used for routine examination assessments such as contact tonometry, pachymetry and gonioscopy, as well as occasionally in contact lens work. Sometimes, TAs are used in optometric practice to enhance the time course and depth of cycloplegia or to facilitate mydriasis in individuals with high levels of pigmentation. Some practitioners use a TA before testing tear volume with the Schirmer test, which relies upon paper strips being placed in the lower fornices (figure 5). They may also be used as an aid in investigative techniques where a sustained fixation is required and where eye blinking can affect the quality of data collection.
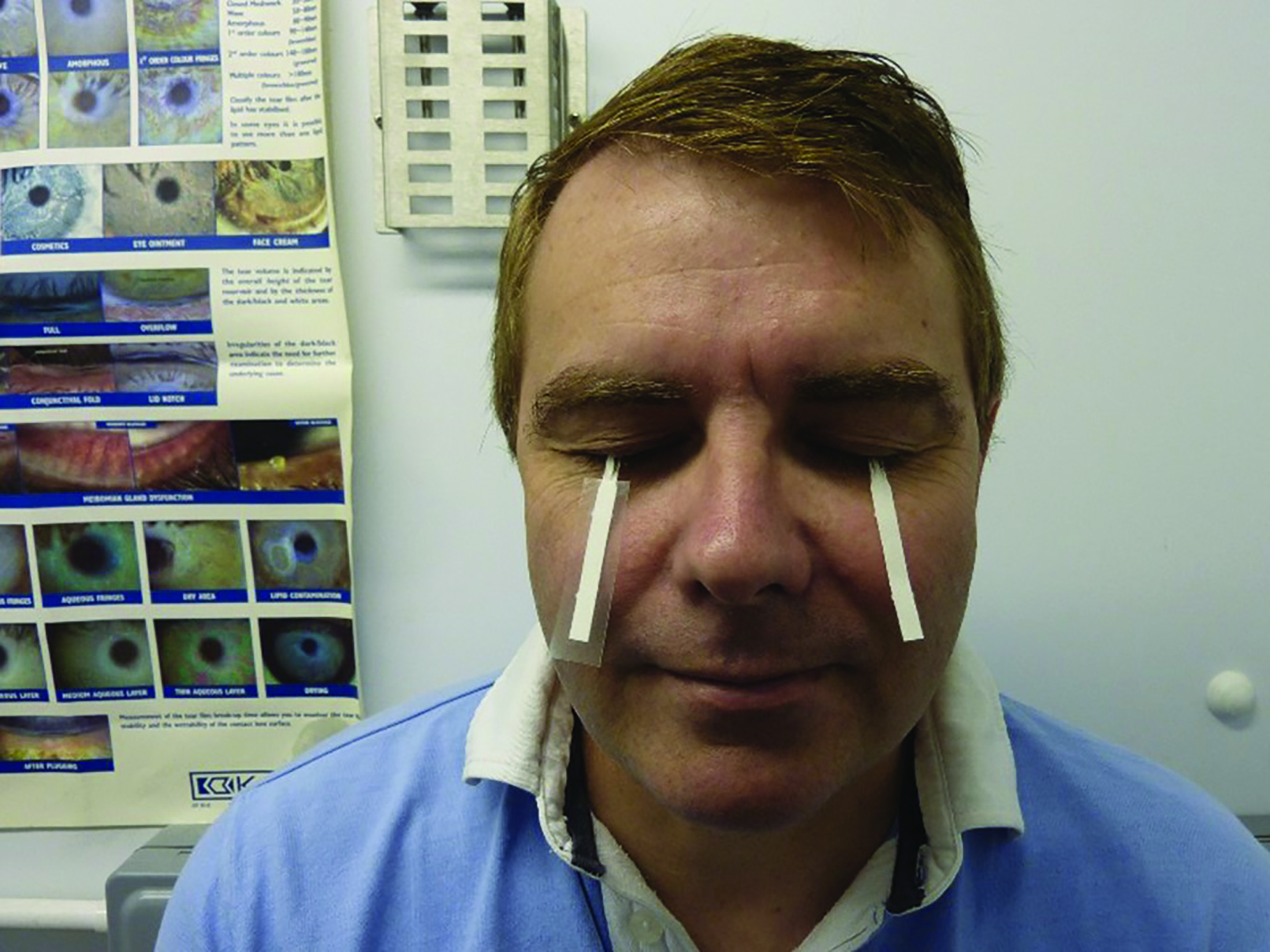 Figure 5: The Schirmer test
Figure 5: The Schirmer test
The onset of their effect is very rapid (maximum of 30 seconds) and the full recovery is expected after 20 to 30 minutes from the instillation.
Proxymetacaine HCl 0.5%
Proxymetacaine HCl is a very commonly used TA in optometric practice as it is very well tolerated by the patients. Having a pH close to that of the ocular surface, it stings less. However, it needs to be refrigerated for storage. Proxymetacaine should be avoided in patients with known allergies to the product or in those suffering from cardiac disease or hyperthyroidism. It has also been reported to cause contact dermatitis. Cases of seizures after topical administration of this drug have also been reported.8
Oxybuprocaine 0.4%
Oxybuprocaine (often known as benoxinate) is mainly used for contact tonometry when it is applied alongside a saline
moistened fluorescein paper strip. There are no documented systemic side effects after topical administration of this drug.
Lidocaine
As a diagnostic drug, this anaesthetic is not available in isolation but is found in combination, but is available in Minims in combination with fluorescein. It is not very well tolerated, tending to cause significant stinging, but is useful if patients are sensitive to other TAs. Lidocaine can also be used in injections of 2% solution for local anaesthesia by those qualified to undertake the procedure. At these low concentrations, there are no known systemic side effects.
Tetracaine HCl
This TA is not commonly used in practice. In the hospital eye service, it is sometimes used before minor surgical procedures.
Diagnostic dyes
Topical diagnostic dyes are mostly used to assess superficial ocular surface damage in various ocular conditions (figure 6), in contact lens fitting, for the assessment of the dynamics of tear fragmentation over time and for contact tonometry (figure 7). In addition, diagnostic dyes, given systemically by injection, can also be used in assessing retinal and choroidal circulation in posterior eye pathologies such as AMD and diabetic retinopathy. Table 2 summarises the properties of those dyes used topically in
optometry.
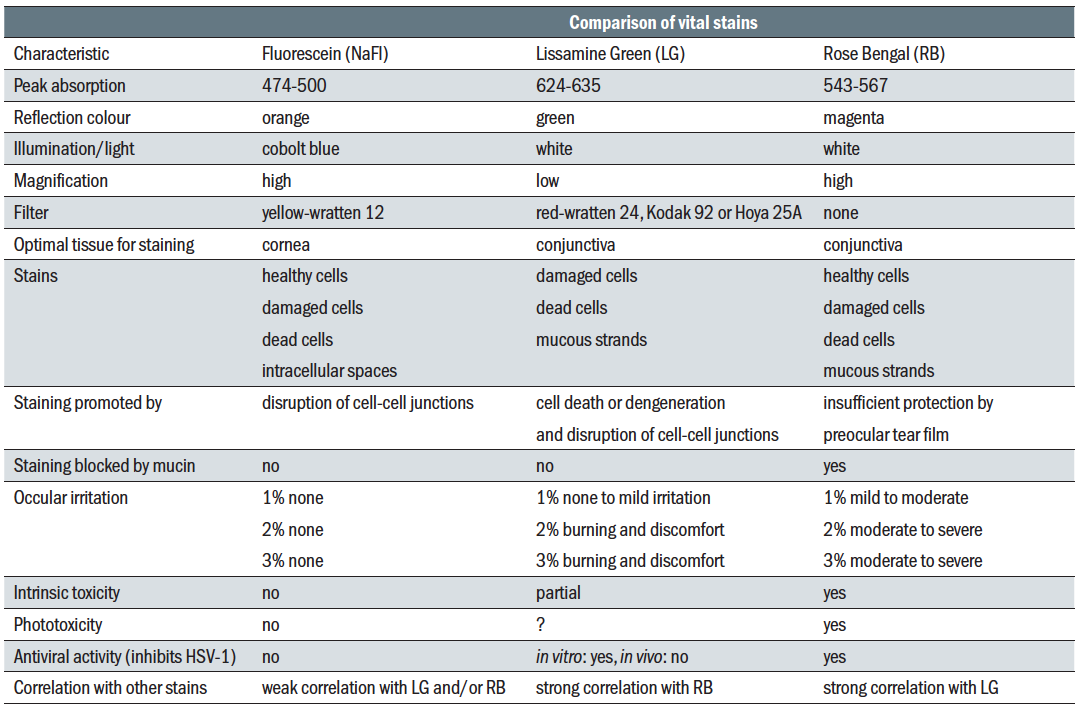 Table 2: Dyes used in eye care
Table 2: Dyes used in eye care
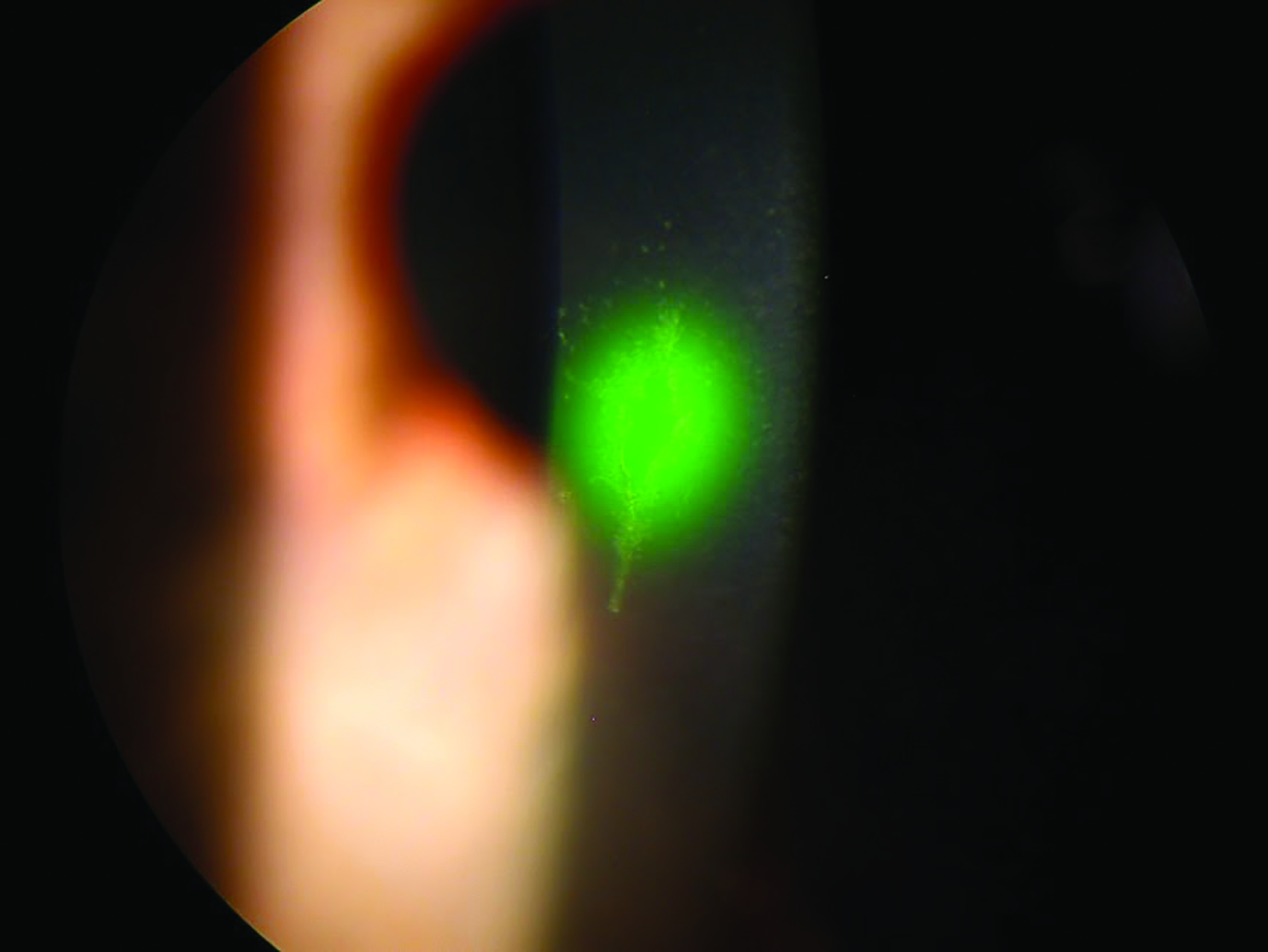 Figure 6: Fluorescein will fluoresce under blue light when taken up by damaged cells
Figure 6: Fluorescein will fluoresce under blue light when taken up by damaged cells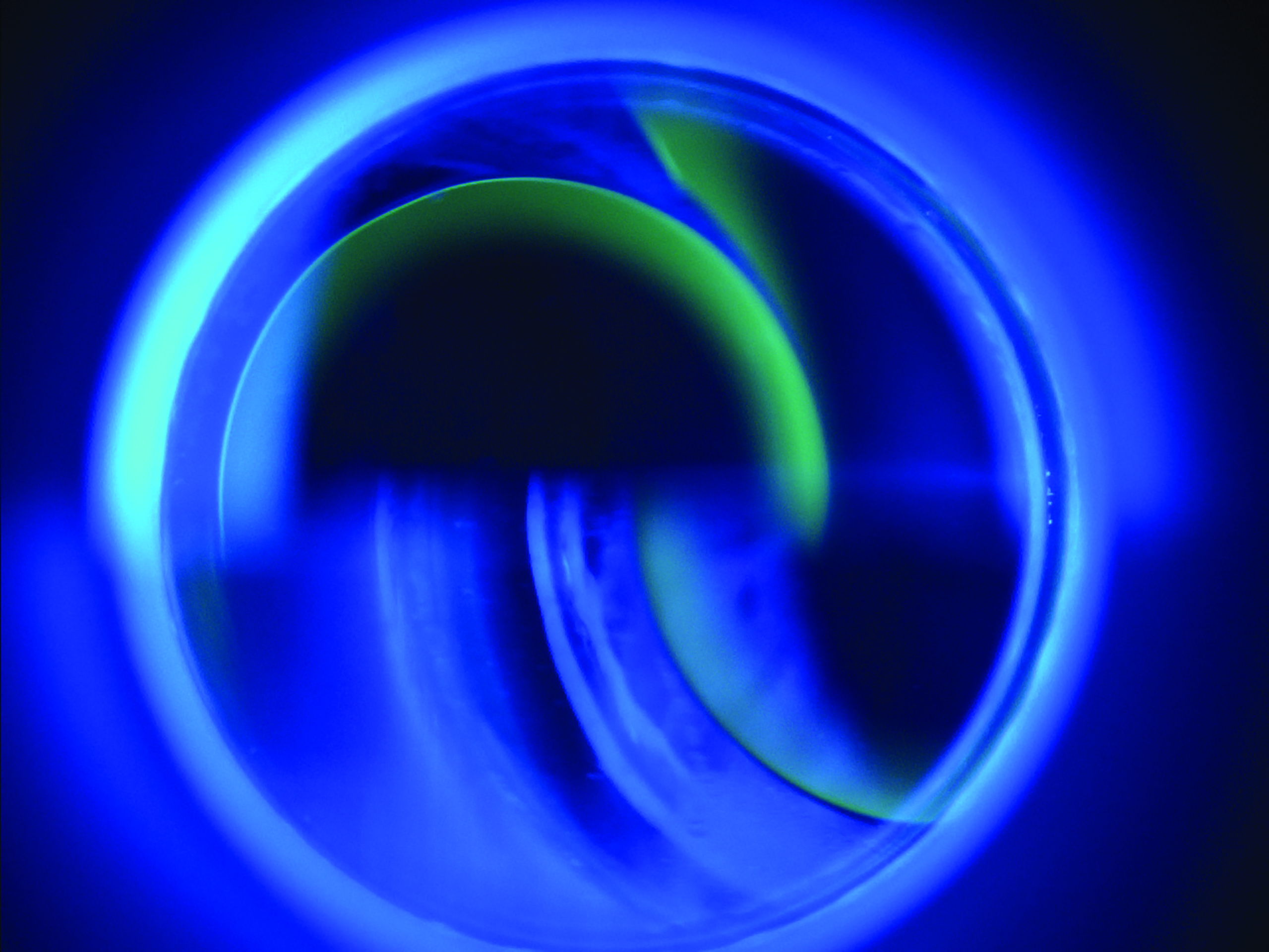 Figure 7: Fluorescein pools around the edge of the applanated area during contact tonometry
Figure 7: Fluorescein pools around the edge of the applanated area during contact tonometry
Fluorescein sodium
Fluorescein sodium (FS) has the specific property of fluorescence when exposed to short wavelength (blue) light. It is mainly used for contact tonometry, but is also widely used for checking the integrity of the ocular surface, to assess the fit of contact lenses, for assessing the tear break up time and to demonstrate the patency of the lacrimal drainage system; a patent drainage should be confirmed by instilled fluorescein in the eye being revealed in the nostril. Instillation is usually via a saline-wetted strip (figure 8).
 Figure 8: Fluorescein ready for instillation
Figure 8: Fluorescein ready for instillation
As a 10% solution, it is used intravenously (IV) for retinal diagnosis, a technique known as fluorescein angiography or FA (figure 9).
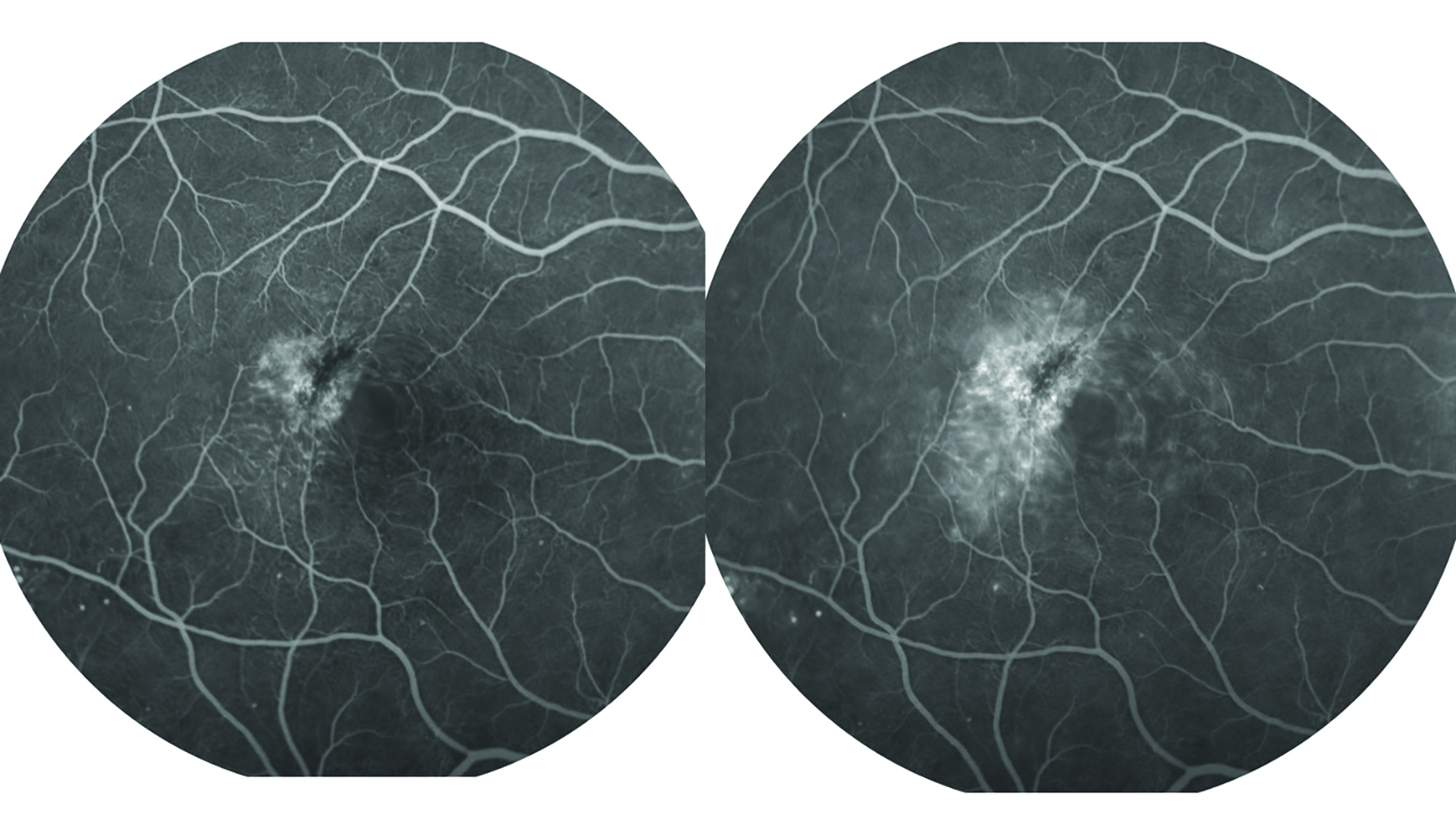 Figure 9: Fluorescein angiography. Serial photography of the retina as fluorescein passes through the vasculature helps assess vessel integrity
Figure 9: Fluorescein angiography. Serial photography of the retina as fluorescein passes through the vasculature helps assess vessel integrity
The use of this dye triggers few side effects. Incidence of adverse reactions increases if the patients have a history of allergy or if they suffer from diabetes or systemic arterial hypertension. Reports of anaphylactic reactions following the topical administration of FS to the eye are rare but potentially fatal.9 Most common side effects after its IV use include nausea, vomiting, flushing, itching or urticaria. The dye passes out via urine over the next few hours.
It has been reported that the most likely cause of a life-threatening medical emergency in outpatient ophthalmology clinics is the administration of intravenous fluorescein 10% during retinal angiographic investigations.9 Indeed, laryngeal oedema, bronchospasm, myocardial infarction, cardiac arrest and tonic-clonic seizure and death were reported but with an extreme rare occurrence. The development of non-invasive OCT angiography may reduce the dependence upon the invasive FA technique.
Rose Bengal
Rose Bengal is a right red stain which is adsorbed by compromised epithelial cells, mucus and fibrous tissue. This dye is very rarely used in the optometric practice in the assessment of dry eye and does sting significantly on instillation. There are no known systemic side effects associated with this drug.
Lissamine green
Lissamine green has similar staining characteristics to Rose Bengal, but is better tolerated by patients. In addition, it is more specific to staining devitalised cells than Rose Bengal and is increasingly used for assessment of dry eye disease (figure 10). There are no known systemic side effects associated with this drug.
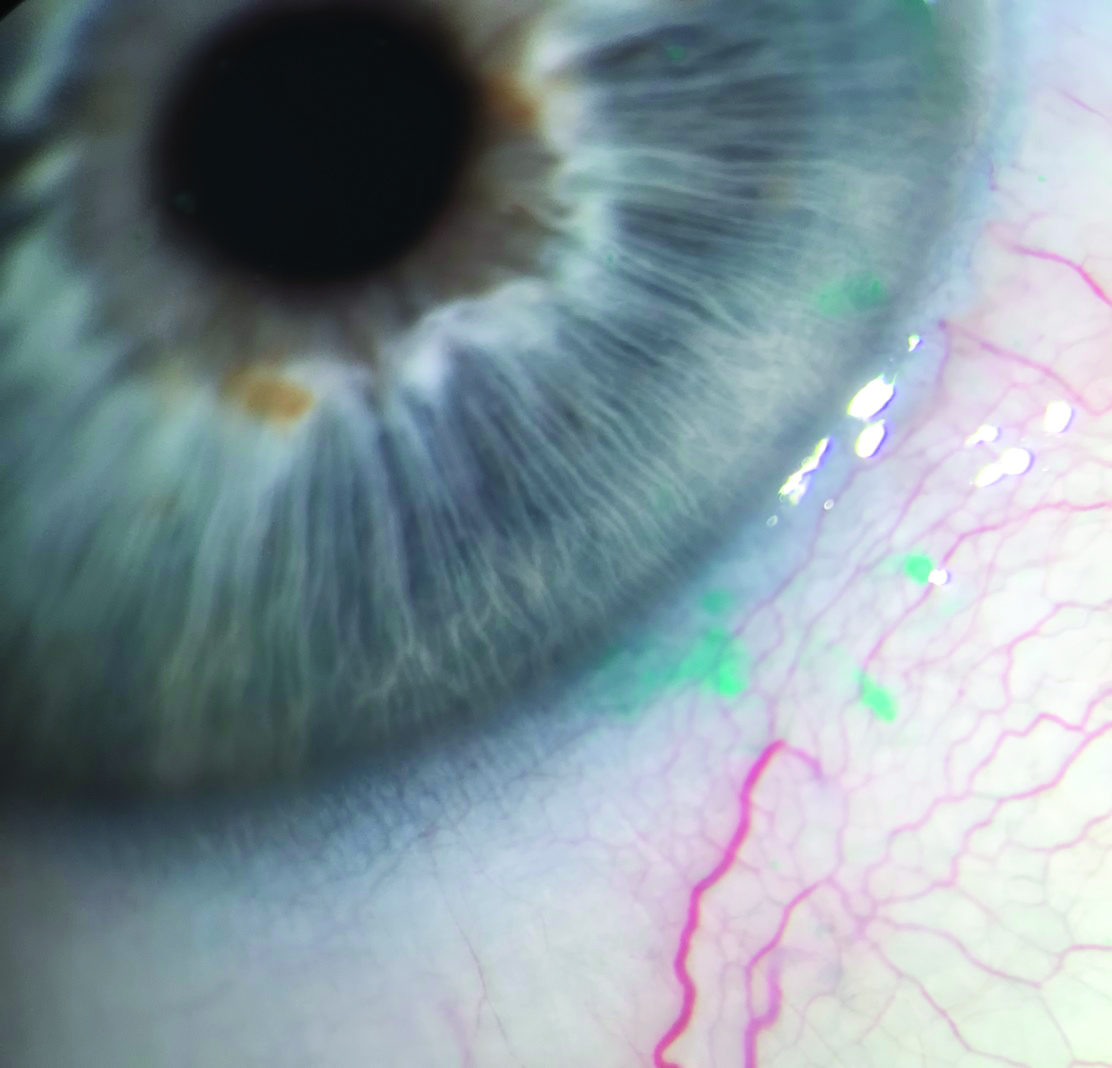 Figure 10: Lissamine green staining
Figure 10: Lissamine green staining
Indocyanine green
Indocyanine green (ICG) is a medical dye used in tests to help determine the cardiac output, liver function, and blood flow in the liver. It is also used for contrast rapid sequence angiography for retinal disease diagnostic procedures, especially where more detail of choroidal circulation is required (figure 11). Its use has been associated with various side effects, similar to those reported after the use of IV fluorescein. In order to minimise the occurrence of these reactions, the practitioner should fully assess the patient’s history for allergies and systemic pathologies before considering using this drug for diagnostic proposes. 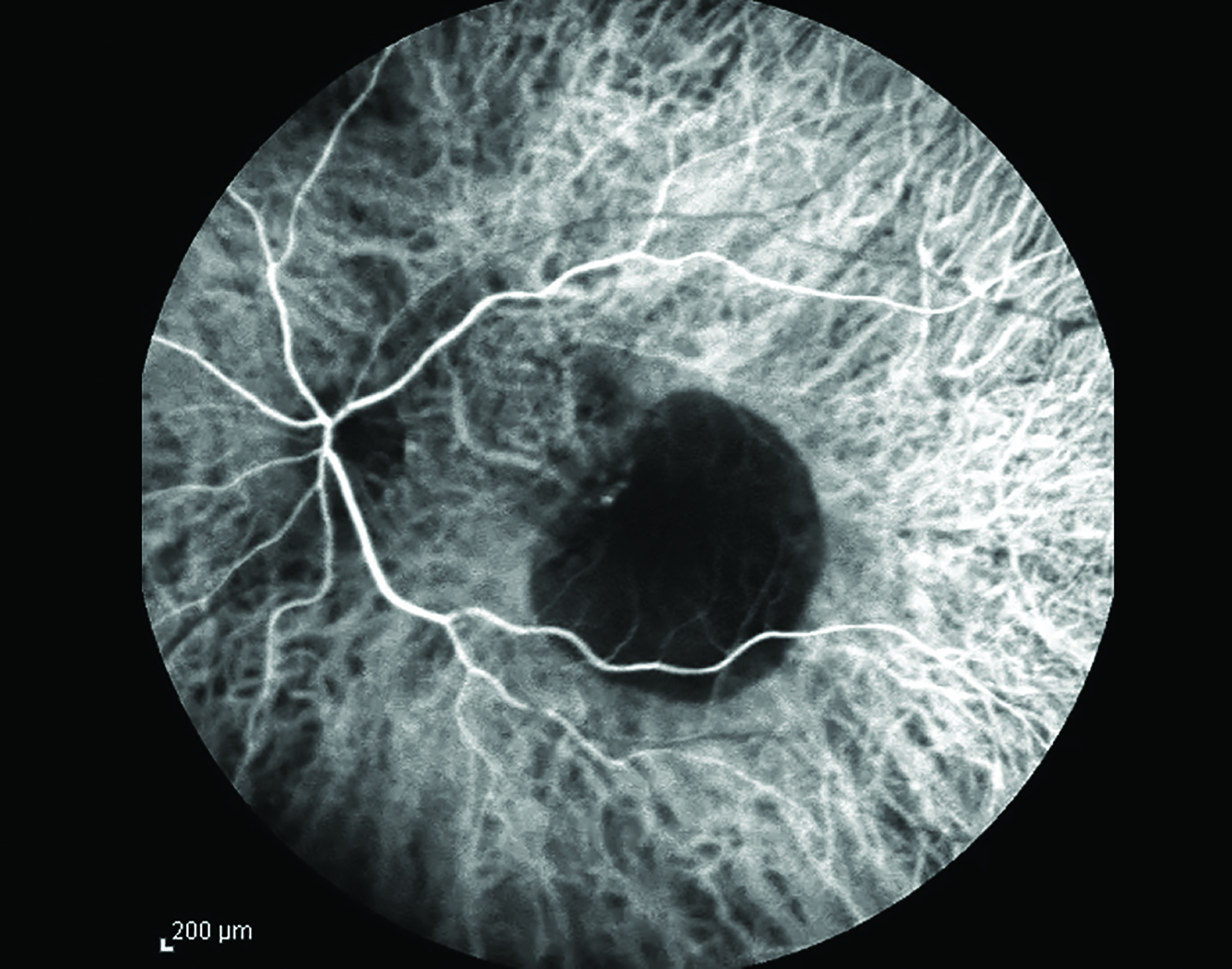 Figure 11: ICG angiography image
Figure 11: ICG angiography image
Dr Doina Gherghel is an academic ophthalmologist with a special interest in inter-professional learning for
optometrists.
- The second part of this series will refer to the systemic side effects of ocular therapeutic drugs.
References
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5153265/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5725525/
- Lisa V Stottlemyer, Alan Polnariev, in https://www.sciencedirect.com/bookseries/side-effects-of-drugs-annual, 2016
- https://www.ncbi.nlm.nih.gov/books/NBK534798/
- (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4921337/#:~:text=Adverse%20effects%20are%20seen%20in,ataxia%20%5B2%2C%203%5D.
- https://indianpediatrics.net/march2000/march-329-331.htm
- https://www.ncbi.nlm.nih.gov/books/NBK541069/
- https://pubmed.ncbi.nlm.nih.gov/2362113/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2837668/
