The first article in this series (see Optician 25.09.20) introduced the concept of the microbiome, its interaction with a number of our body’s physiological processes and how the maintenance of its balance has a significant impact on our wellbeing. Of particular interest was the fact that the largest proportion of the human microbiome is found in the gastrointestinal tract (GIT), which is also where a large percentage of our immune system is located. Studies show that our normal or commensal microbiota play a significant role in the regulation of inflammation and on immune mediated disease.1
A variety of immune mediated ocular disorders exist (see table 1). There are many immunological systemic diseases which can influence the onset and progression of ocular immune disease, such as arthropathies like ankylosing spondylitis and rheumatoid arthritis or multiple sclerosis. There is also increasing evidence that some further ocular diseases, such as age-related macular degeneration and choroidal melanoma, involve immunological processes in their progression and, therefore, an understanding of this may help in the development of prophylactic or therapeutic measures.2
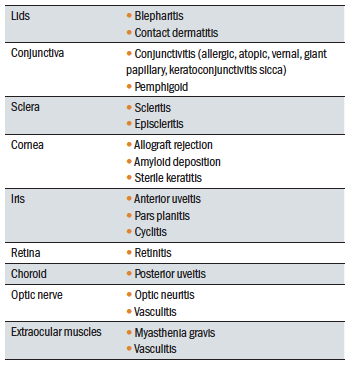 Table 1: Ocular immunological diseases
Table 1: Ocular immunological diseases
Complementopathy
There is the growing evidence linking inflammation with the pathogenesis of age-related macular degeneration (AMD).3-5 Of particular interest is the interaction of the various inflammatory processes implicated in AMD, including complement pathways, with the constituents of the gut microbiota.1,6,7
AMD is a multifactorial disease, and there is a wide range of influences which variously contribute to the disease pathogenesis, including ageing, genetics and environmental factors such as diet and light exposure.8,9 Exactly how these factors cause macular damage is not yet fully understood.10
Recent studies have shown that inflammatory response, and the complement pathway in particular, plays a ‘pivotal role in the pathogenesis of AMD’9,11-13 leading some to call AMD a ‘complementopathy’.14
A complementopathy has been usefully defined as a disorder where:
- Activation of the complement system is a driving factor in the disease pathophysiology
- Evidence that inhibition of complement disrupts or halts the pathogenic process of the disorder
The complement system is an essential part of the innate immune system that requires careful regulation to ensure responses are appropriately directed against harmful pathogens, while preventing collateral damage to normal host cells and
tissues.
There are three pathways of complement activation: the classical pathway, which is triggered by antibody or by direct binding of complement component C1q to the pathogen surface; the MB-lectin pathway, which is triggered by mannan-binding lectin, a normal serum constituent that binds some encapsulated bacteria; and the alternative pathway, which is triggered directly on pathogen surfaces. This is summarised in figure 1. 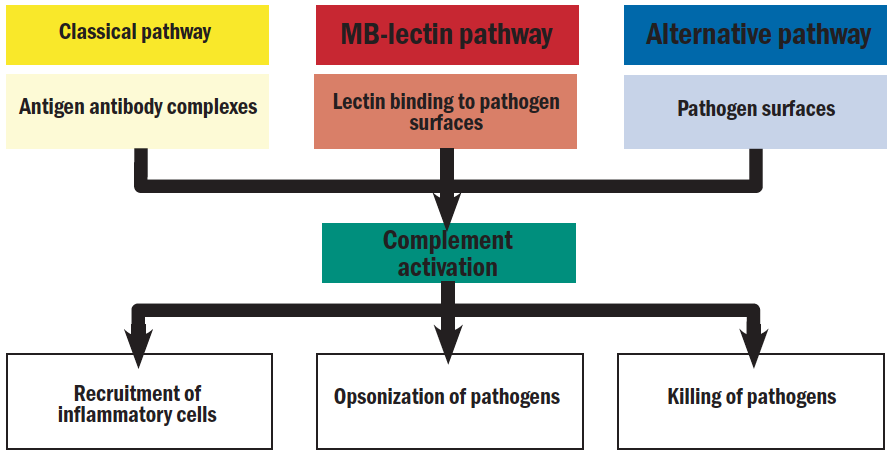 Figure 1: Schematic representation of the complement cascade15
Figure 1: Schematic representation of the complement cascade15
All of these pathways generate a crucial enzymatic activity that, in turn, generates the effector molecules of complement. This will be further discussed later in this article.
Age-related macular degeneration
Inflammation’s role in AMD includes the following;
- Detection of inflammatory molecules in drusen; this is considered by many to be the hallmark of early AMD11
- Presence of immune cells; macrophages, lymphocytes and mast cells are found in or adjacent to macular lesions16
- Polymorphisms of immune related genes10
This being the case, it is worth asking how and why does does a protective mechanism promote AMD?
Age is considered a risk factor for many neurodegenerative diseases, including cardiovascular disease, diabetes mellitus, malignancies and AMD.9,17 Although a significant percentage of the population show signs of AMD, (namely drusen of >125µ or soft drusen), only a small percentage develop features of late AMD (see figure 2 and 3). 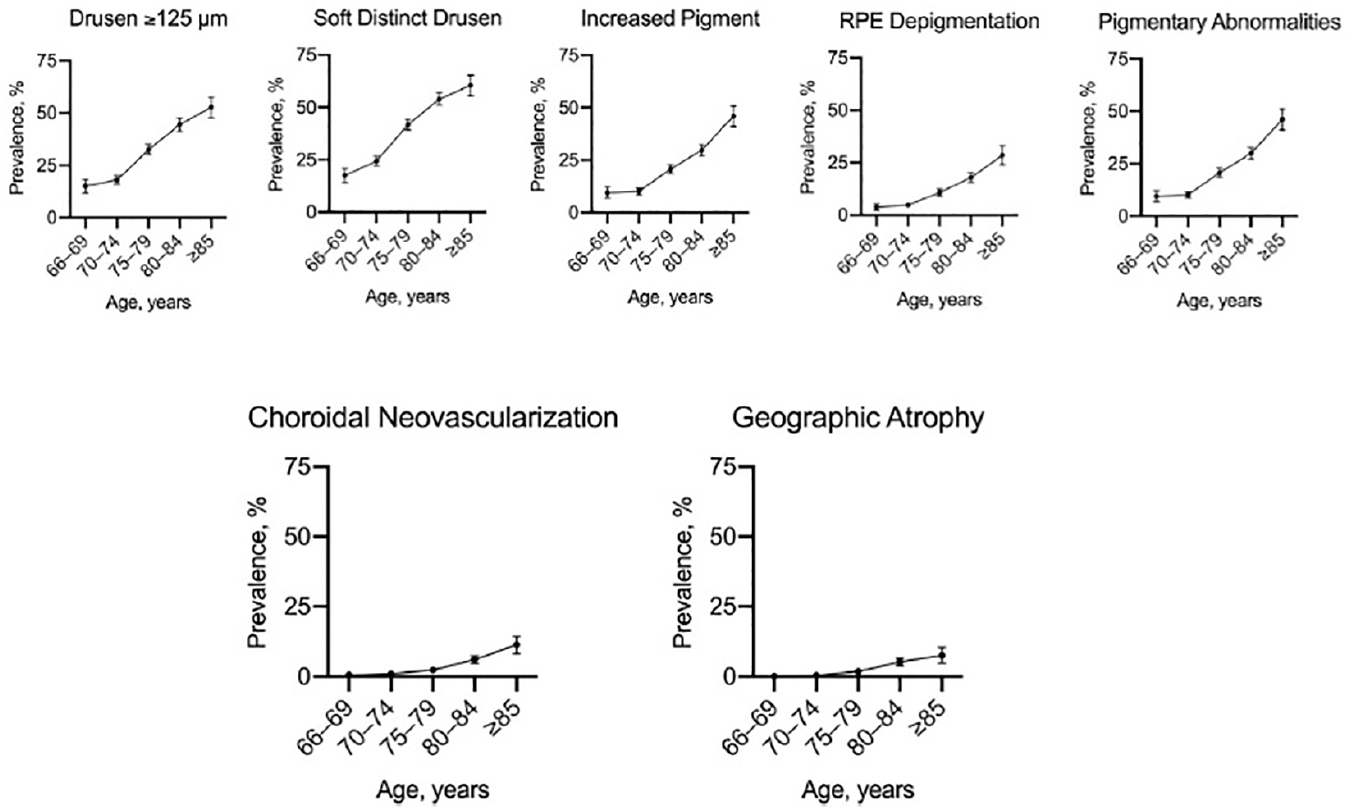 Figure 2: Prevalence of early features of AMD (top) and late features (bottom) as a function of age9
Figure 2: Prevalence of early features of AMD (top) and late features (bottom) as a function of age9
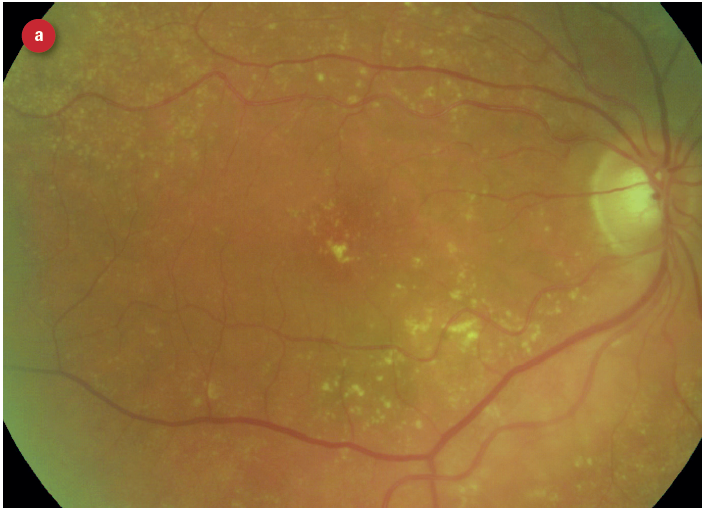
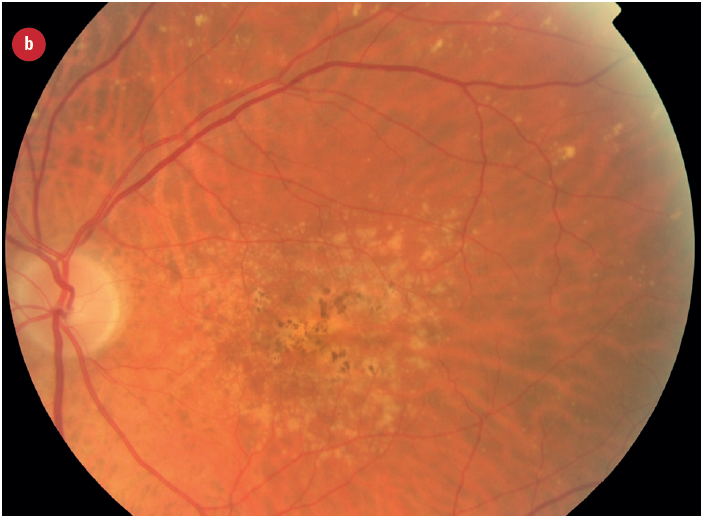
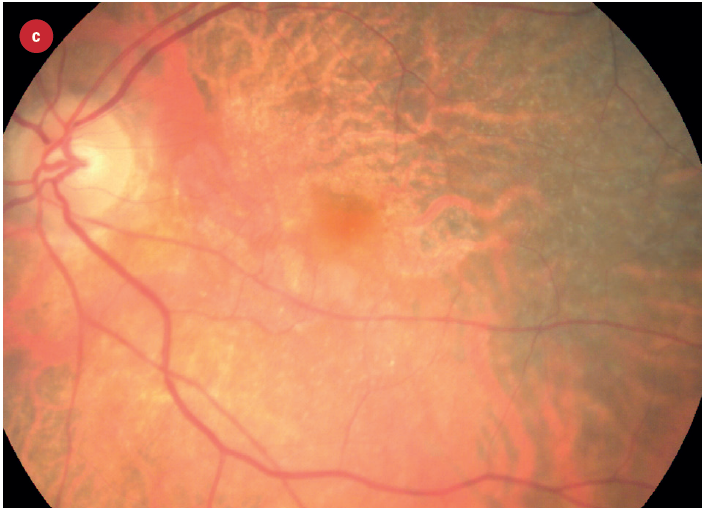
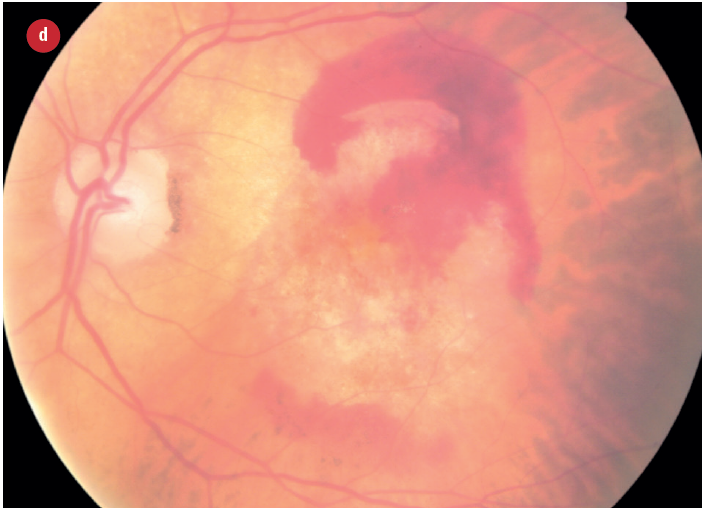 Figure 3: Appearance of early and late AMD changes; (a) drusen, (b) pigmentation, (c) geaographic atrophy, (d) choroidal neovascularisation with haemorrhage
Figure 3: Appearance of early and late AMD changes; (a) drusen, (b) pigmentation, (c) geaographic atrophy, (d) choroidal neovascularisation with haemorrhage
Ageing can be considered as a gradual build-up of tissue damage, resulting in the deterioration of an organs ability to function.9 A recurring feature of ageing tissues, and most if not all age-related disease, is chronic inflammation. This chronic low-grade, age-induced inflammation appears to occur in the absence of apparent infection and has also been described separately as ‘inflammageing’.18
Some of the possible mechanisms involved in inflammageing include the following:
- Reduced cell replication capability due to telomere shortening; a telomere is a region of repetitive nucleotide sequences at each end of a chromosome. It protects the end of the chromosome from deterioration or from fusion with neighbouring chromosomes. In humans, average telomere length declines from about 11 kilobases at birth to fewer than 4 kilobases in old age, with the average rate of decline being greater in men than in women, and this decline is thought to be integral to the ageing process.
- Increases in DNA mutations
- Increase production of faulty and damaged proteins resulting in inappropriate functioning
The retina has one of the highest metabolic turnovers in the body. From an evolutionary perspective, the metabolic demands are managed in adulthood, but due to longer life expectancy, may not have been put under stress in an ageing population until more modern times. Indeed, many age-related diseases may occur as a ‘side effect’ of either:
- Increase in late-acting mutations as one ages19
- Increase in mutations that aid fitness in the young, but have a negative effect of a higher rate of ageing in later life17,19
As a result of the increase in life expectancy and better treatments of infection (acknowledging obvious recent variations, of course), there appears to be a shift from acute communicable disease states to non-communicable disease states. The trigger of acute communicable disease potential in younger years appears to promote the genes that code for a more pro-inflammatory state in order to deal with potential of infection (figure 4).
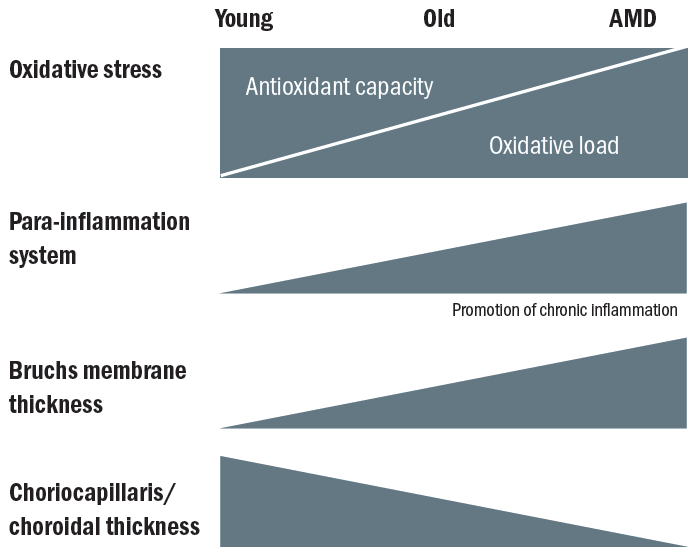 Figure 4: Natural retinal aging involves the shift towards greater oxidative stress and a pro-inflammatory environment. (Adapted from Ardeljan)20
Figure 4: Natural retinal aging involves the shift towards greater oxidative stress and a pro-inflammatory environment. (Adapted from Ardeljan)20
When considering AMD, there is strong evidence of genes coding for increased complement activity.21 While a pro-inflammatory state protects for infection, as one ages these inflammatory responses contribute also to development of chronic degenerative diseases. A number of degenerative diseases including AMD are associated with pro-inflammatory states, specifically dysfunctional complement activity and functional changes to both innate and adaptive immunity. Chronic inflammation is involved in most age-related degenerative conditions, including so-called immune privileged tissues such as the brain and retina.22
Immune privilege
Immune privilege is the mechanism whereby the body’s normal inflammatory immune response is restricted,23 thereby protecting certain tissues from the damage that might occur as part of the immune response. The eye is one of a few areas of the body with immune privilege. Other privileged sites include the brain, testes and placenta, and a foetus also has privileged protection.
In the retina, this protective mechanism reduces the risk of inflammation mediated retinal damage. This is achieved through a variety of ways.
- A physical barrier; the blood/retina barrier (BRB) includes tight junctions between vascular endothelial cells and the retinal pigment epithelial (RPE) cells. This limits the passage of cells and molecules passing freely from the circulation.
- Lack of a lymphatic system; this reduces the likelihood of antigens being detected
- Retinal immune regulatory system; the retina has its own unique immune regulatory system involving various neurones and RPE cells24,25 These cells can suppress pro-inflammatory signals26
It is important to point out that, were the retina to suffer an insult, it is still able to mount an immune response via local innate immune cells and the complement cascade. There are a number of immune cells residing in the retina, for example perivascular macrophages and microglial cells, which form an important part of retinal immune defence. The microglial cells appear to serve a dual role:27
- Surveillance; monitoring the surrounding microenvironment
- Engageing with the appropriate signals; becoming ‘active,’ changing shape and becoming phagocytic
In addition to these immune cells, a retinal complement regulatory system exists, forming an important part of retina innate immune defence. This is a version of the previously mentioned complement system. Upon stimulation, complement protein fragments can generate a variety of immune responses culminating in the formation of the membrane attack complex (MAC). The MAC is a highly potent molecule capable of killing cells by rupturing their surface. Complement is involved in a number of retinal diseases including diabetic retinopathy27 and AMD11 which lends weight to the view that the complement system plays an important part in retinal innate defence.10
Immune Response
So, age-related macular degeneration is an interplay of age and immune response as summarised in figure 5.
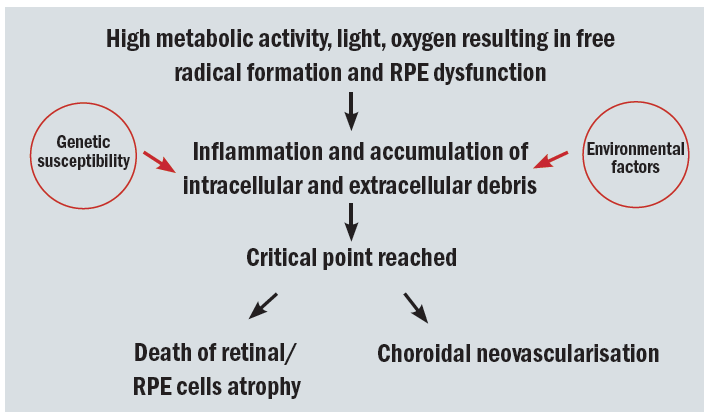 Figure 5: The ageing retina and progression to age-related macular degeneration
Figure 5: The ageing retina and progression to age-related macular degeneration
The primary role of the immune system is to protect the host from external (exogenous, such as infection) or internal (endogenous) insult. It consists of a number of systems specialised to detect and to effect a response to pathogens, thereby maintaining a degree of homeostasis. The immune system also monitors and responds to more general noxious stress conditions where the cell functioning is altered.
Dysfunction of the immune system can lead to an overall low-grade chronic inflammation.10 This contributes to many diseases not traditionally considered as inflammatory, including the following:
- Obesity28
- Atherosclerosis29
- Neurodegenerative disorders30
Chronic inflammation is involved in most age-related degenerative conditions, including those affecting immune privileged tissues such as the brain and retina.10
There are two key mechanisms for immunity:
- Innate immunity; The innate system responds rapidly to infection or insult in a non-specific manner, but does not offer lasting protection. The pathogen carries a specific ‘identification tag’; the pathogen associated molecular pattern or PAMP. This PAMP is recognised by receptors, known as pattern recognition receptors or PRRs, found within the innate immune system. This then triggers a variety of responses aimed at eliminating the pathogen. PAMPS are not carried by our own cells, thereby allowing the immune system to recognise self from non-self.
- Adaptive immunity; the adaptive immune response takes longer to initiate. It is designed to target specific foreign antigens and produce a response that amplifies the innate response and destroy the pathogen more efficiently. As part of this adaptive response, the system logs, or remembers this particular antigen, allowing for a more rapid response upon future exposure to the same antigen.
These are summarised in table 2 and figure 6.
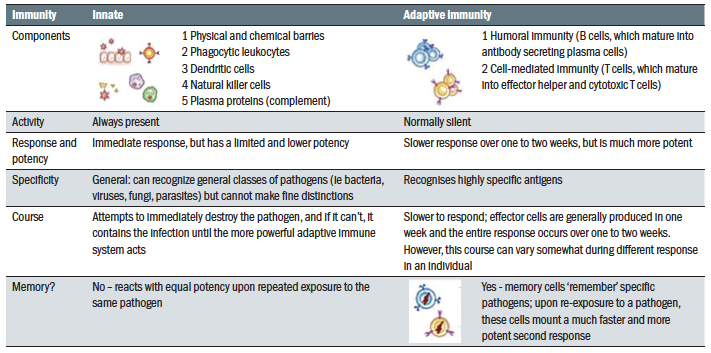 Table 2: Innate versus adaptive immunity
Table 2: Innate versus adaptive immunity
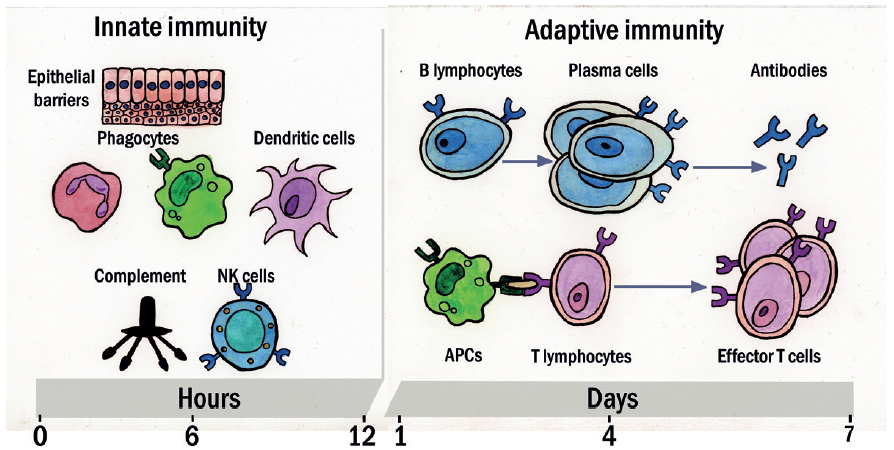 Figure 6: Innate and adaptive immunity time line. The mechanisms of innate immunity provide the initial defence against infections. Adaptive immune responses develop later and require the activation of lymphocytes. The kinetics of the innate and adaptive immune responses are approximations and may vary in different infections. (Key: NK = natural killer; APC = antigen-presenting cells)
Figure 6: Innate and adaptive immunity time line. The mechanisms of innate immunity provide the initial defence against infections. Adaptive immune responses develop later and require the activation of lymphocytes. The kinetics of the innate and adaptive immune responses are approximations and may vary in different infections. (Key: NK = natural killer; APC = antigen-presenting cells)
The mechanisms are involved and covered in sufficient detail elsewhere,26 but I will now offer a brief overview to clarify the relationship with AMD.
The innate system can sense and mount an immune response of lower magnitude to restore homeostasis, also called para-inflammation.26,31 The ageing retina is associated with oxidative and metabolic stress which can damage retinal cells. To counter this, a para-inflammatory response can repair the damaged cells, thereby maintaining retinal homeostasis.10 The degree of the para-inflammatory response varies depending upon the degree of stress the cell or tissue is under. If the tissue dysfunction is present for sustained periods, the para-inflammation can become chronic and can contribute to further disease progression.31
As part of its privileged immunity status, as previously described, the cells of the retinal immune system consists of microglia, perivascular macrophages and some dendritic cells. These can detect and respond to damage in the ageing retina and this response is coupled with an upregulation of the complement system.
There appears to be a balance here. If the retinal stress is greater than repair capacity of the immune system, tissue damage occurs. Likewise, if the para-inflammatory response of the RPE cells, the innate immune system or the systemic immune system becomes dysfunctional, the hallmarks of chronic inflammation appears and contribute to tissue damage. The level of inflammageing is more significant in AMD patients than age-matched controls.10 This may be due to genetic predisposition or related to lifestyle factors, such as smoking, high fat diets and so on. This is considered further in the last part of this series.
The role of complement
The complement system forms part of both the innate and the adaptive immune system,32 playing an important role in both surveillance and homeostasis in the eye.33 Complement consist of over 30 proteins which play an important role in enhancing the host immune response. When activated, a cascade of enzyme reactions is initiated that enhances the destruction and elimination of microbes and damaged cells. An inappropriate activation or a deficiency in the process underpins the mechanisms behind many diseases processes.34
There are three complement pathways as outlined in figure 1, and each consists of a tightly regulated network of 30 proteins and regulators circulating in the systemic circulation that play an important role in host defence and inflammation in what is often described as the complement cascade (figure 7).
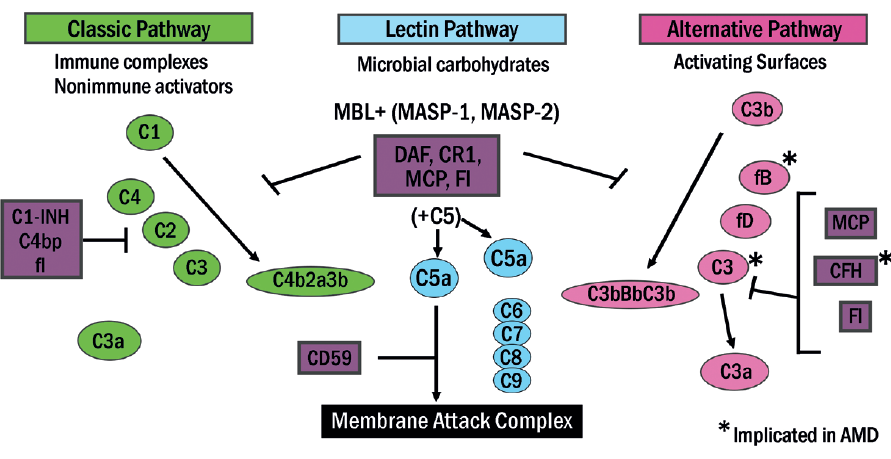 Figure 7: The complement cascade
Figure 7: The complement cascade
Of the three complement pathways, it is the third (the alternative pathway) that is linked with AMD.35 This pathway requires the activation of a specific protein fragment found on the host surface (C3b). The alternative pathway is continuously activated due to the presence of foreign invaders as well as the presence of damaged self-tissues. Regardless of the particular activation component, all three pathways lead to enzymatic cleavage of complement component C3. The three main consequences of this complement activation are:
- Opsonization of pathogens; connecting them to antibodies, complements, or other proteins so they can then be attached to cell surface receptors on phagocytes and natural killer cells
- Recruitment of inflammatory cells
- Direct killing of pathogens15
This is summarised in figure 8.
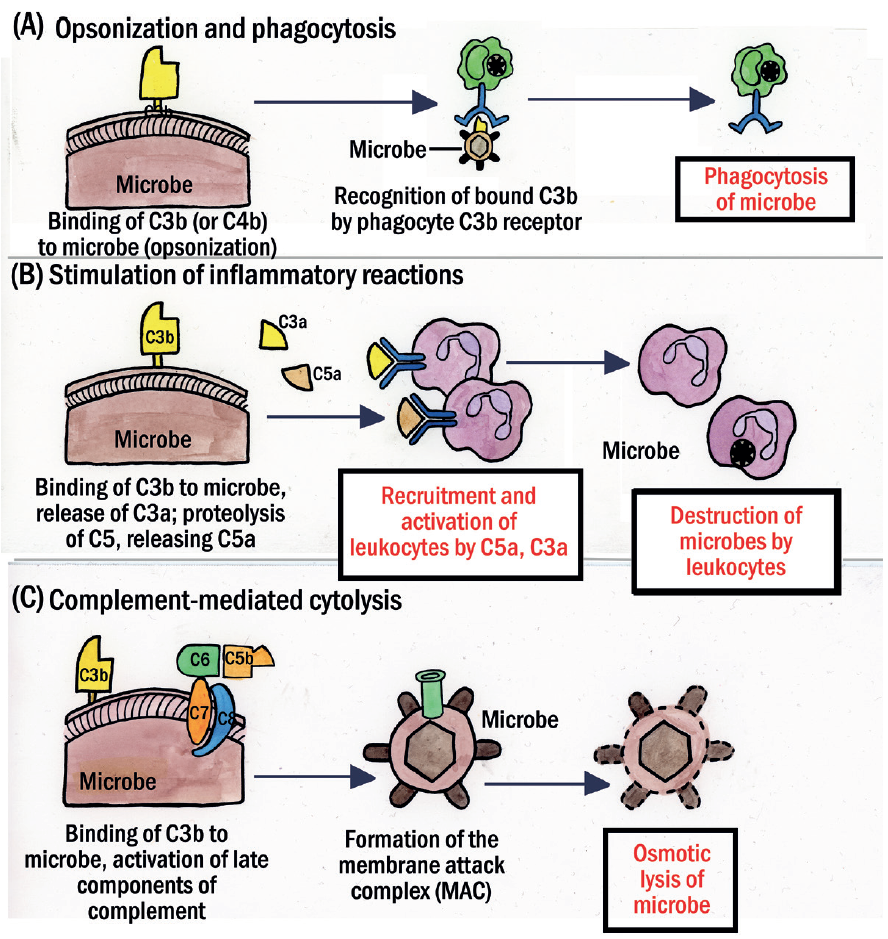 Figure 8: The major functions of complement. (A) Complement fragment C3b on the surface of microbes (or other cells) promotes phagocytosis (also called opsonization). (B) C3a and C5a proteolytic fragments increase vascular permeability and cause vasodilation by releasing histamine from mast cells; C5a is also chemotactic and enhances leukocyte binding to endothelium (shown here is a neutrophil), while stimulating leukotriene synthesis and the production of reactive oxygen species. (C) The C5b–C9 complex forms a membrane attack complex (MAC) that punches holes in microbes (and other cells) leading to osmotic rupture
Figure 8: The major functions of complement. (A) Complement fragment C3b on the surface of microbes (or other cells) promotes phagocytosis (also called opsonization). (B) C3a and C5a proteolytic fragments increase vascular permeability and cause vasodilation by releasing histamine from mast cells; C5a is also chemotactic and enhances leukocyte binding to endothelium (shown here is a neutrophil), while stimulating leukotriene synthesis and the production of reactive oxygen species. (C) The C5b–C9 complex forms a membrane attack complex (MAC) that punches holes in microbes (and other cells) leading to osmotic rupture
Given that complement activation products have the potential to initiate damage against the body’s own tissues, ineffective clearance may result in deposits of complement products appearing in host tissues, thereby promoting host tissue damage.36 Clearly, some regulation of the complement pathway is required and this happens in the form of a number of regulatory proteins, found either bound to the various membranes or as soluble
proteins.9
Complement regulatory protein factor H (CFH) is one such soluble protein that can also bind onto the glycosaminoglycans on the host surface and suppress this activation pathway. Polymorphism in CFH can confer a greater than five-fold increased risk of developing AMD and is present in approximately 30% of people of European descent.37 The fact that CFH has been found in drusen38 suggests further evidence that dysregulation of the complement system could drive ocular inflammation and contribute towards AMD (figure 9).
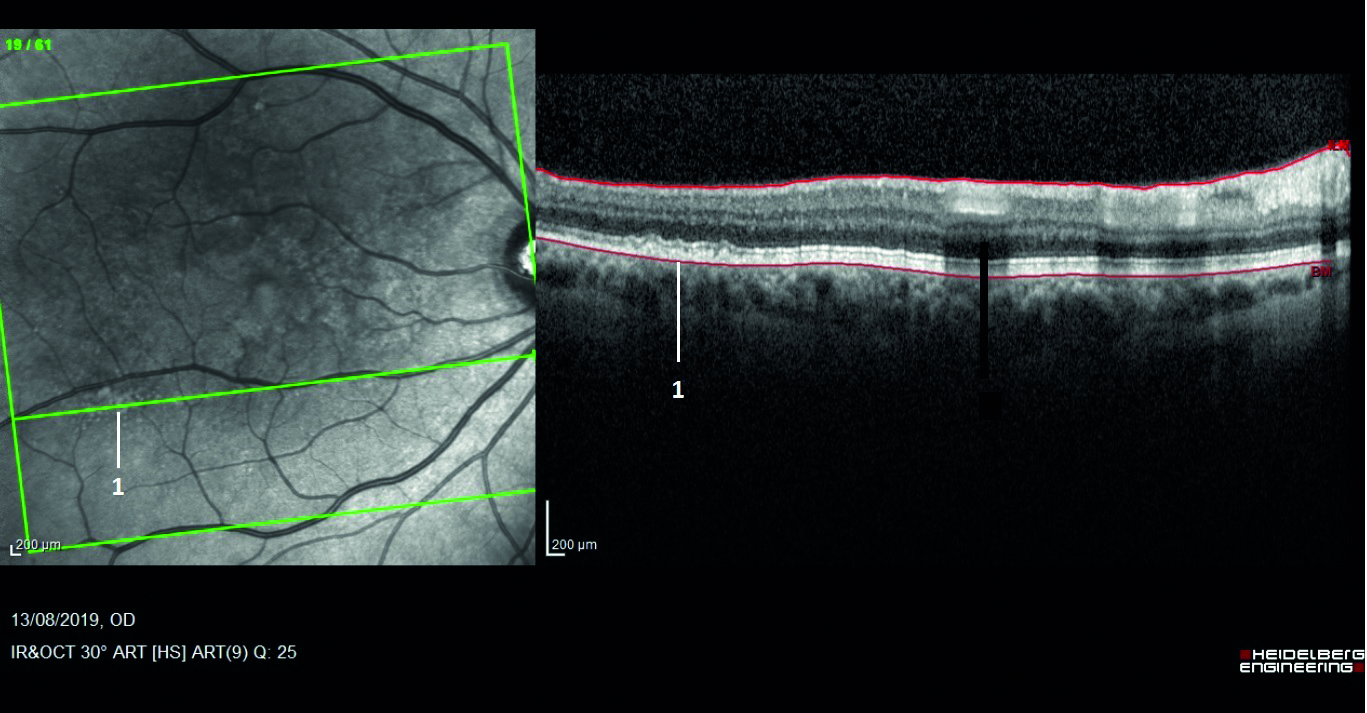 Figure 9: Drusen (as labelled 1 here) are found to contain complement regulatory protein
Figure 9: Drusen (as labelled 1 here) are found to contain complement regulatory protein
CFH
CFH is produced in the liver and consists of 20 units whose main function is to inhibit the activation of the alternative complement pathway (figure 10).4,39 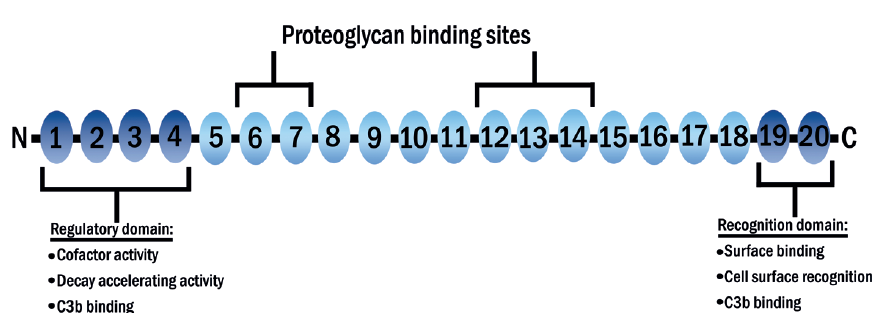 Figure 10: The structure of CFH. There are 20 complement control protein units (CCPs). Unit 7 is prone to polymorphism which represents a major genetic risk factor for AMD
Figure 10: The structure of CFH. There are 20 complement control protein units (CCPs). Unit 7 is prone to polymorphism which represents a major genetic risk factor for AMD
The normal structure of the seventh unit (402Y) includes the amino acid tyrosine. However, it is susceptible to polymorphism (existing in multiple forms due to changes in its alleles – an allele is a variant form of a gene) whereby the tyrosine is replaced by genes coding for histidine resulting in an altered structure. This altered structure is known as 402H.39,40 This alteration (described as Y402H polymorphism) prevents CFH from binding onto the surface of Bruch’s membrane correctly,41 thereby impairing its ability to suppress C3b and so resulting in complement activation.39,42
It is estimated that over 30% of the population having European descent are carriers of at least one of the risk alleles. This will increase the risk of developing visually compromising, late stage AMD by 2.3 to 5.2 times.12,43,44 It is suggested that this is responsible for over 40% of AMD cases.43 The implications of this for clinical practice will be discussed in the next part of this series.
Genetics
Genes code for a variety of processes. There are a number of genes that code for the alternative complement pathway known to be involved in AMD. Indeed, AMD is considered to be one of the best genetically defined complex disorders.45
The CFH gene is one of the most studied. When polymorphisms of this gene occur, there is an elevated risk of developing AMD.46 Many other genes have also been linked with AMD.45 However, it is estimated that over 50% the heritability of AMD is due to variations involving the age-related maculopathy susceptibility 2 (ARMS2) gene and CFH.45 The precise function of the ARMS2 gene is yet to be clarified, though some form of impact upon complement regulation has been suggested.47 The impact of CFH gene, however, is far better understood as previously described.
While Y402H polymorphism is the best-known genetic risk factor for AMD, over 52 further genetic variants have also been described.48 Not only have genetic variants in the complement system been associated with AMD, but systemic complement activation levels have also been found to be elevated in AMD. It has even been suggested ‘that AMD is a systemic disease with local disease manifestation at the ageing macula.’9,35,49
Conclusion
As we have seen, AMD is a disease with inflammation strongly interwoven into its pathogenesis. This apparent ‘upregulation’ of the complement system in AMD patients seems to form part of the pathogenesis of AMD. This is demonstrated by the presence of complement within drusen regardless of a known genetic link.49,50
The gut microbiota appear to regulate the immune response51 and, in cases of dysbiosis, may contribute to an overactivation of the complement cascade even in those without a genetic link, resulting in AMD.1,51
Table 3 offers an overview of the genetic and environmental influences upon AMD presentation.
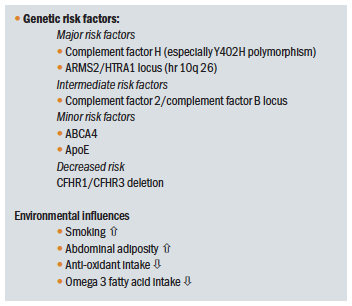 Table 3: Predisposing factors for AMD other than ageing
Table 3: Predisposing factors for AMD other than ageing
We will look at what this all means for the busy clinician ‘at the coalface’ in the next article.
- Dr Rohit Narayan is a therapeutic optometrist and Clinical Scientist at Aston University.
Acknowledgement
Figures 6, 7, 8 and 10 courtesy of Kitty Harvey
References
- Lin, P., 2018. The role of the intestinal microbiome in ocular inflammatory disease. Current Opinion in Ophthalmology, 29(3), pp.261-266
- Song J, Huang YF, Zhang WJ, Chen XF, Guo YM. Ocular diseases: immunological and molecular mechanisms. International Journal of Ophthalmology, 2016; 9 (5):780-788
- Parmeggiani, F., Romano, M.R., Costagliola, C., Semeraro, F., Incorvaia, C., D’Angelo, S., Perri, P., De Palma, P., De Nadai, K. and Sebastiani, A., Mechanism of inflammation in age-related macular degeneration. Mediators in Inflammation. 2012; 2012: 546786.
- Kauppinen, A., Paterno, J.J., Blasiak, J., Salminen, A. and Kaarniranta, K., 2016. Inflammation and its role in age-related macular degeneration. Cellular and Molecular Life Sciences, 73(9), pp.1765-1786
- Wu, J. and Sun, X., 2019. Complement system and age-related macular degeneration: drugs and challenges. Drug design, development and therapy, 13, p.2413
- Kiang, L., McClintic, S., Saleh, M., Metea, C., Mitio, K., Asquith, M., Martin, T.M., Klein, M.L., Karstens, L. and Lin, P., 2017. The gut microbiome in advanced age-related macular degeneration. Investigative Ophthalmology & Visual Science, 58(8), pp.5739-5739
- Baim AC et al. The microbiome and ophthalmic disease. Experimental Biology and Medicine, vol. 244,6 (2019): 419-429.
- Coleman, H.R., Chan, C.C., Ferris III, F.L. and Chew, E.Y., 2008. Age-related macular degeneration. The Lancet, 372 (9652), pp.1835-1845
- Rozing, M.P., Durhuus, J.A., Nielsen, M.K., Subhi, Y., Kirkwood, T.B., Westendorp, R.G. and Sørensen, T.L., 2019. Age-related macular degeneration: A two-level model hypothesis. Progress in Retinal and Eye Research, 2020 May;76:100825
- Chen, M. and Xu, H., 2015. Parainflammation, chronic inflammation, and age-related macular degeneration. Journal of Leukocyte Biology, 98(5), pp.713-725
- Anderson, D.H., Radeke, M.J., Gallo, N.B., Chapin, E.A., Johnson, P.T., Curletti, C.R., Hancox, L.S., Hu, J., Ebright, J.N., Malek, G. and Hauser, M.A., 2010. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Progress in retinal and eye research, 29(2), pp.95-112
- Park, D.H., Connor, K.M. and Lambris, J.D., 2019. The challenges and promise of complement therapeutics for ocular diseases. Frontiers in Immunology, 10, p.1007
- Wu J, Sun X. Complement system and age-related macular degeneration: drugs and challenges. Drug Design, Development and Therapy. 2019 Jul 19; 13: 2413-2425
- Baines, A. C., & Brodsky, R. A. (2017). Complementopathies. Blood reviews, 31(4), 213–223
- Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. The complement system and innate immunity
- Penfold, P.L., Killingsworth, M.C. and Sarks, S.H., 1985. Senile macular degeneration: the involvement of immunocompetent cells. Graefe’s Archive for Clinical and Experimental Ophthalmology, 223(2), pp.69-76
- Niccoli, T. and Partridge, L., 2012. Ageing as a risk factor for disease. Current Biology, 22(17), pp.R741-R752
- Franceschi, C. and Campisi, J., 2014. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences, 69 (Suppl_1), pp.S4-S9
- Luu, J. and Palczewski, K., 2018. Human aging and disease: lessons from age-related macular degeneration. Proceedings of the National Academy of Sciences, 115(12), pp.2866-2872
- Ardeljan, D., & Chan, C. C. (2013). Aging is not a disease: distinguishing age-related macular degeneration from aging. Progress in retinal and eye research, 37, 68-89
- Paun, C.C., Lechanteur, Y.T., Groenewoud, J.M., Altay, L., Schick, T., Daha, M.R., Fauser, S., Hoyng, C.B., den Hollander, A.I. and de Jong, E.K., 2016. A novel complotype combination associates with age-related macular degeneration and high complement activation levels in vivo. Scientific Reports, 6, p.26568
- Chen, M., Muckersie, E., Robertson, M., Forrester, J.V. and Xu, H., 2008. Up-regulation of complement factor B in retinal pigment epithelial cells is accompanied by complement activation in the aged retina. Experimental Eye Research, 87(6), pp.543-550
- www.aao.org/eye-health/tips-prevention/eye-immune-privilege
- Streilein, J.W., Ma, N., Wenkel, H., Ng, T.F. and Zamiri, P., 2002. Immunobiology and privilege of neuronal retina and pigment epithelium transplants. Vision Research, 42(4), pp.487-495
- Wenkel, H. and Streilein, J.W., 2000. Evidence that retinal pigment epithelium functions as an immune-privileged tissue. Investigative Ophthalmology & Visual Science, 41(11), pp.3467-3473
- Xu, H., Chen, M. and Forrester, J.V., 2009. Para-inflammation in the aging retina. Progress in Retinal and Eye Research, 28(5), pp.348-368
- Yang, Y., Lu, H.L., Zhang, J., Yu, H.Y., Wang, H.W., Zhang, M.X. and Cianflone, K., 2006. Relationships among acylation stimulating protein, adiponectin and complement C3 in lean vs obese type 2 diabetes. International Journal of Obesity, 30(3), pp.439-446
- Kanneganti, T., Dixit, V. Immunological complications of obesity. Nature Immunology, 2012, 13, 707–712
- Hansson, G., Hermansson, A. The immune system in atherosclerosis. Nature Immunology, 2011, 12, 204–212
- Stephenson, J., Nutma, E., van der Valk, P. and Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology, 2018, 154: 204-219
- Medzhitov, R. Origin and physiological roles of inflammation. Nature, 2008, 454 (7203), pp.428-435
- Dunkelberger, J.R. and Song, W.C. Complement and its role in innate and adaptive immune responses. Cell Research, 2010, 20(1), pp.34-50
- Anderson, D.H., Mullins, R.F., Hageman, G.S. and Johnson, L.V. A role for local inflammation in the formation of drusen in the aging eye. American Journal of Ophthalmology, 2002, 134(3), pp.411-431
- Sarma, J.V. and Ward, P.A The complement system. Cell and Tissue Research, 2011 343(1), pp.227-235
- Lynch, A.M., Mandava, N., Patnaik, J.L., Frazer-Abel, A.A., Wagner, B.D., Palestine, A.G., Mathias, M.T., Siringo, F.S., Cathcart, J.N. and Holers, V.M. Systemic activation of the complement system in patients with advanced age-related macular degeneration. European Journal of Ophthalmology, 2019, 1120672119857896
- Sarma JV, Ward PA. The complement system. Cell Tissue Research, 2011 Jan; 343(1):227-35
- Langford-Smith A, Keenan TD, Clark SJ, Bishop PN, Day AJ. The role of complement in age-related macular degeneration: heparan sulphate, a ZIP code for complement factor H? Journal of Innate Immunology. 2014; 6(4): 407-16
- Hageman, G.S., Luthert, P.J., Chong, N.V., Johnson, L.V., Anderson, D.H. and Mullins, R.F. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Progress in retinal and eye research, 2001, 20(6), pp.705-732
- Maugeri A, Barchitta M, Mazzone MG, Giuliano F, Agodi A. Complement System and Age-Related Macular Degeneration: Implications of Gene-Environment Interaction for Preventive and Personalized Medicine. Biomedicine Research International. 2018 Aug 26; 2018: 7532507
- Day, A.J., Clark, S.J. and Bishop, P.N. Understanding the molecular basis of age-related macular degeneration and how the identification of new mechanisms may aid the development of novel therapies. Expert Review of Ophthalmology, 2011, 6(2), pp.123-128
- Clark, S. J., Perveen, R., Hakobyan, S., Morgan, B. P., Sim, R. B., Bishop, P. N., & Day, A. J. Impaired binding of the age-related macular degeneration-associated complement factor H 402H allotype to Bruch’s membrane in human retina. The Journal of Biological Chemistry, 2010, 285(39), 30192–30202
- Prosser, B. E., Johnson, S., Roversi, P., Herbert, A. P., Blaum, B. S., Tyrrell, J., Jowitt, T. A., Clark, S. J., Tarelli, E., Uhrín, D., Barlow, P. N., Sim, R. B., Day, A. J., & Lea, S. M. Structural basis for complement factor H linked age-related macular degeneration. The Journal of Experimental Medicine, 2007, 204(10), 2277–2283
- Haines, J.L., Hauser, M.A., Schmidt, S., Scott, W.K., Olson, L.M., Gallins, P., Spencer, K.L., Kwan, S.Y., Noureddine, M., Gilbert, J.R. and Schnetz-Boutaud, N. Complement factor H variant increases the risk of age-related macular degeneration. Science, 2005, 308(5720), pp.419-421
- Sofat, R., Casas, J.P., Webster, A.R., Bird, A.C., Mann, S.S., Yates, J.R., Moore, A.T., Sepp, T., Cipriani, V., Bunce, C. and Khan, J.C. Complement factor H genetic variant and age-related macular degeneration: effect size, modifiers and relationship to disease subtype. International Journal of Epidemiology, 2012 41(1), pp.250-262
- DeAngelis, M.M., Owen, L.A., Morrison, M.A., Morgan, D.J., Li, M., Shakoor, A., Vitale, A., Iyengar, S., Stambolian, D., Kim, I.K. and Farrer, L.A. Genetics of age-related macular degeneration (AMD). Human Molecular Genetics, 2017, 26(R1), pp R45-R50
- Edwards, A.O., Ritter, R., Abel, K.J., Manning, A., Panhuysen, C. and Farrer, L.A. Complement factor H polymorphism and age-related macular degeneration. Science, 2005, 308 (5720), pp.421-424
- Micklisch, S., Lin, Y., Jacob, S., Karlstetter, M., Dannhausen, K., Dasari, P., von der Heide, M., Dahse, H.M., Schmölz, L., Grassmann, F. and Alene, M. Age-related macular degeneration associated polymorphism rs10490924 in ARMS2 results in deficiency of a complement activator. Journal of Neuroinflammation, 2017, 14(1), p.4
- Fritsche, L.G., Igl, W., Bailey, J.N.C., Grassmann, F., Sengupta, S., Bragg-Gresham, J.L., Burdon, K.P., Hebbring, S.J., Wen, C., Gorski, M. and Kim, I.K., 2016. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nature Genetics, 2016, 48(2), pp.134-143
- Scholl, H.P., Issa, P.C., Walier, M., Janzer, S., Pollok-Kopp, B., Börncke, F., Fritsche, L.G., Chong, N.V., Fimmers, R., Wienker, T. and Holz, F.G. Systemic complement activation in age-related macular degeneration. PloS one, 2008, 3(7), p.e2593
- Lin, P. Importance of the intestinal microbiota in ocular inflammatory diseases: A review. Clinical & Experimental Ophthalmology, 2019, 47(3), pp.418-422
- Wen, X., Hu, X., Miao, L., Ge, X., Deng, Y., Bible, P.W. and Wei, L. Epigenetics, microbiota, and intraocular inflammation: New paradigms of immune regulation in the eye. Progress in Retinal and Eye Research, 2018, 64, pp.84-95
