Any discussion of prescribed prism, muscle imbalance and binocular vision is incomplete without an understanding of the underlying anatomy of the globe and orbit. This includes the complex nature of the relationship between the extraocular muscles, their origins, position and angle of insertion (where they attach to the globe), innervation, and the connective tissue structures within the orbit. It is also necessary to understand how the two eyes work together under normal circumstances to allow stereoscopic vision.
Ocular Anatomy
Looking at the intricate structures and precise functions of the eyes and associated tissues it is small wonder it was once thought that living things were divinely created rather than the product of hundreds of millions of years of evolution.
The structure of the globe and surrounding tissue enables smooth almost frictionless eye movements due to a complex and intricate arrangement of connective tissue that ‘suspends’ the globe within the orbital cavity (orbit). Orbital connective tissue and orbital fat lines, supports, covers and separates individual structures within the orbit facilitating these smooth movements referred to as rotations about differing axes notated as X, Y and Z (figure 1). Fick’s axes separate the globe into quadrants that intersect at the eye’s centre of rotation.1 In reality, the centre of rotation of the eye is not a fixed position due to compression of orbital fat allowing some translational movement. Yet, since the movement is just one or two millimetres,1 for the purpose of describing the actions of the extrinsic ocular muscles (EOMs), a fixed position is assumed some 13.5 to 14.0mm behind the corneal apex and 1.65mm nasally to the geometric centre of the globe.2
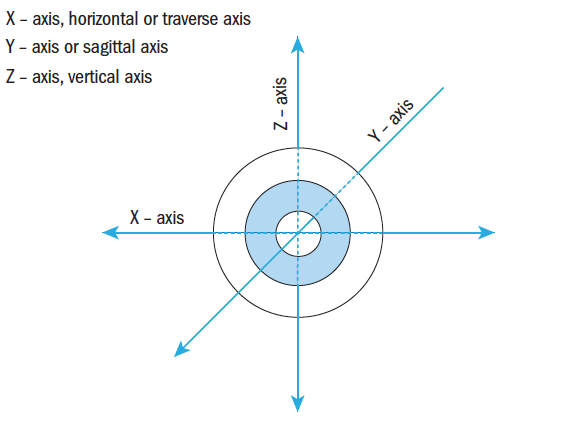 Figure 1: Fick’s Axes of Rotation
Figure 1: Fick’s Axes of Rotation
The orbit is shaped like pyramid, laid on its side with the eye looking out through an imaginary base, and the optic nerve leaving through the optic foramen at the apex of the pyramid. The orbit has four sides: roof, floor and lateral and medial walls, the medial walls of each orbit running almost parallel to each other (figure 2).
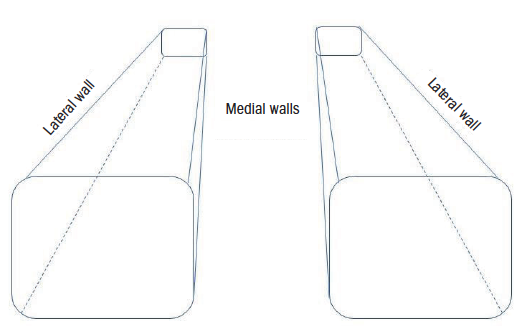 Figure 2: Representation of the orbital cavity
Figure 2: Representation of the orbital cavity
The orbit consists of seven bones (figure 3); the frontal, maxillary, zygomatic, sphenoid, ethmoid, palatine and lacrimal bones. The roof is comprised of the frontal bone; the floor made from the maxillary (upper jaw) bone, and the small palatine bone; the medial wall is made up of the ethmoid and the lacrimal bone; the lateral wall is made from the zygomatic and the sphenoid; the sphenoid also contributes small portions of the other three walls at the orbital apex.
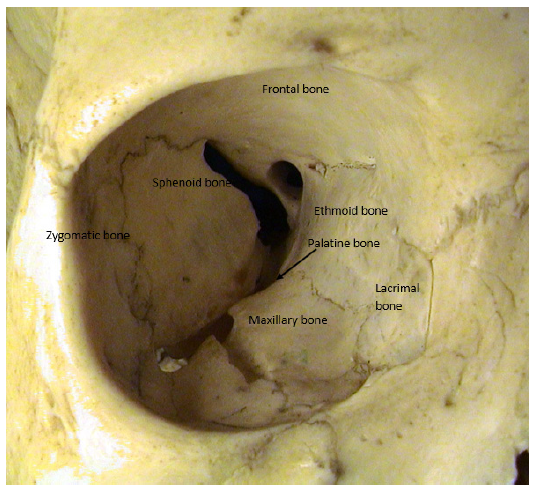 Figure 3: The orbital cavity
Figure 3: The orbital cavity
There are a number of connective tissue structures within the orbit with a wide variety of functions. The periorbita (orbital fascia) is a connective tissue membrane lining the orbital bones. It is mainly loosely attached although firm attachments are found at the orbital rim, suture lines (where bones meet) and at the edges of the foramina and fissures.1
The border of the anterior orbit is marked by the orbital septum (palpebral fascia) a connective tissue sheet extending from the orbital rim to the tarsal plate acts as a barrier to infection (preventing pre-septal cellulitis becoming the much more serious orbital cellulitis for example) and keeps the orbital fat in position (figure 4).3
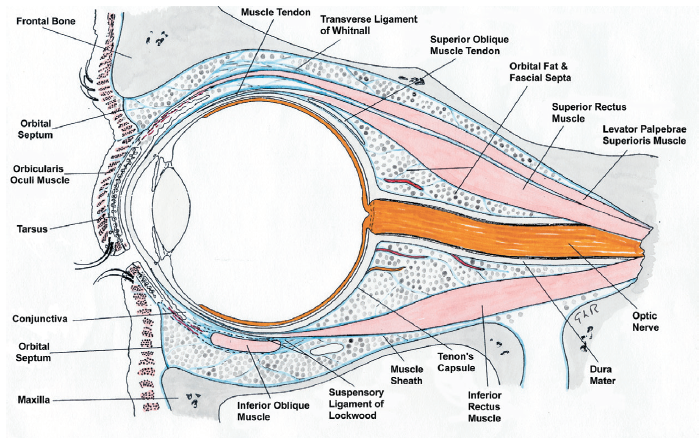 Figure 4: Cross-section of the orbit (drawn by Professor Gordon Ruskell)
Figure 4: Cross-section of the orbit (drawn by Professor Gordon Ruskell)
Tenon’s capsule (bulbar fascia) is a dense external vascular connective tissue sheath that runs from the optic nerve to the limbus where it merges with the conjunctiva and episclera.4 While the tendons of the oculorotatory muscles pass through the sheath to the sclera the sheath extends over the tendon in a sleeve like covering.5 Tenon’s capsule also offers a protective barrier against infections entering the globe.3 The lateral and medial check ligaments are fibrous connective tissue triangular extensions from the medial and lateral orbital walls to Tenon’s capsule and the lateral and medial rectus.2
Connective tissue (fascial) slings within the orbit help to support the globe and ocular structures. They include; the suspensory ligament of Lockwood formed from the inferior rectus and inferior oblique sheaths and Tenon’s capsule and the superior transverse ligament (Whitnall’s ligament) which extends from the trochlea to the lacrimal gland fascia supporting the orbital structures as well as the upper eyelid, and is also thought to act as a fulcrum.6
Orbital fat which is semi-liquid at body temperature fills the empty spaces or compartments within the orbit aiding movement as well as maintaining the position of the globe.3
The complex structure and design of the orbital connective tissue works well to keep infections out while allowing smooth ocular rotation and binocular vision through the actions of the six EOMs – four straight, rectus muscles and two oblique muscles.
Ocular Movements
The extrinsic ocular muscles move the eyes in different directions and the terms used to describe eye rotations/movements can be a little confusing for example monocular movements are called ductions and are expressed according to the direction of movement as follows:
- Upward = Supraduction
- Downward = Infraduction
- Inward = Adduction
- Outward = Abduction
Looking back at Fick’s axes (figure 1), it can be seen that supraduction and infraduction are rotations about the horizontal or transverse (X) axis and adduction and abduction are rotations about the vertical (Z) axis. Rotations about the sagittal (Y) axis produce cycloduction. If the 12 o’clock position moves nasally, this is cycloduction, and excycloduction describes the movement when the 12 o’clock position rotates temporally (outwards) towards the ear. It is worth noting that Fick’s axes are slightly confusing to remember as they do not conform with standard Cartesian coordinates used in other scientific disciplines where X marks the horizontal transverse (left to right) axis, Y the vertical and Z the horizontal sagittal (front to back) axis, unless the globe is viewed from above.
Movements are considered from the primary position which is a position that is also used when measuring spectacle lens centration and is defined in BS EN ISO 13666:2109.7 The visual axis is coincident with the sagittal axis when the eye is looking in the primary direction, looking straight ahead at a distant target at eye level. The extrinsic ocular muscles are said to be ‘in balance,’ so maintaining the globe in a central position within the orbit.1 Secondary positions of gaze are rotations about the vertical or horizontal axis and tertiary positions rotations about both X and Z axes.
The rectus muscles (recti) originate from the Annulus of Zinn (or common annular tendon) and travel forward within the orbit to pass through Tenon’s capsule and insert into the sclera. The superior rectus makes an angle of 23º to the sagittal plane so, during contraction, causes supraduction but also adduction and incycloduction. The medial rectus and lateral rectus lines of action lie solely in the horizontal plane about the vertical axis, so producing adduction (medial rectus) and abduction (lateral rectus). The inferior rectus has the same angle as the superior rectus to the sagittal axis, so during contraction produces infraduction and adduction and excycloduction.
The superior oblique is inserted behind the Z axis at an angle to the sagittal Y axis of between 50 and 55º, producing infraduction, abduction and incycloduction. The inferior oblique is similarly inserted at an angle of 51º to the sagittal axis producing supraduction, abduction and excyclodution.
Muscle actions change once the eye moves away from the primary position. For example, when the eyes converge along the line of action of the oblique muscles, the principal action of the superior oblique is infraduction with no abduction.
The extrinsic ocular muscles never act alone, but work as either synergists with another muscle to aid a duction or an antagonist to oppose the duction. Sherrington’s Law states that while one muscle contracts, its antagonist is relaxed.8 For example, when the medial rectus contracts, the lateral rectus, the antagonist, is relaxed.
Secondary effects of muscles also cancel out. For example, consider supraduction. The superior rectus and the inferior oblique contract as synergists for this movement but the adduction (secondary action of the superior rectus), abduction (secondary action of the inferior oblique), incycloduction (tertiary action of the superior rectus) and excycloduction (tertiary action of the inferior oblique) cancel out.
The primary, secondary and tertiary actions of the muscles are shown in table 1 and figure 5.
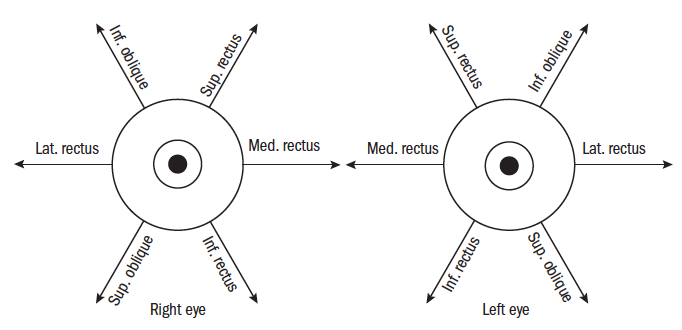 Figure 5: The extrinsic oculorotatory muscle actions and antagonists
Figure 5: The extrinsic oculorotatory muscle actions and antagonists
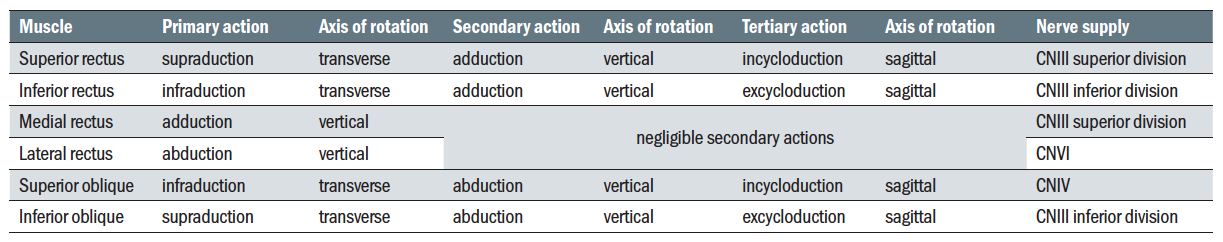 Table 1: Actions of the six oculorotatory muscles and nerve supply
Table 1: Actions of the six oculorotatory muscles and nerve supply
So far, monocular eye movements, or ductions, have been considered. Binocular movements are referred to as version and vergence movements and are summarised in tables 2 and 3
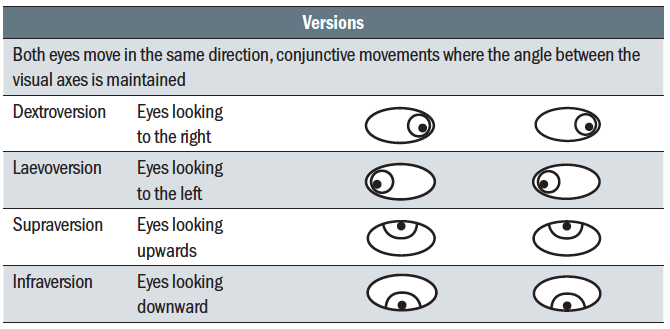 Table 2: Version eye movements
Table 2: Version eye movements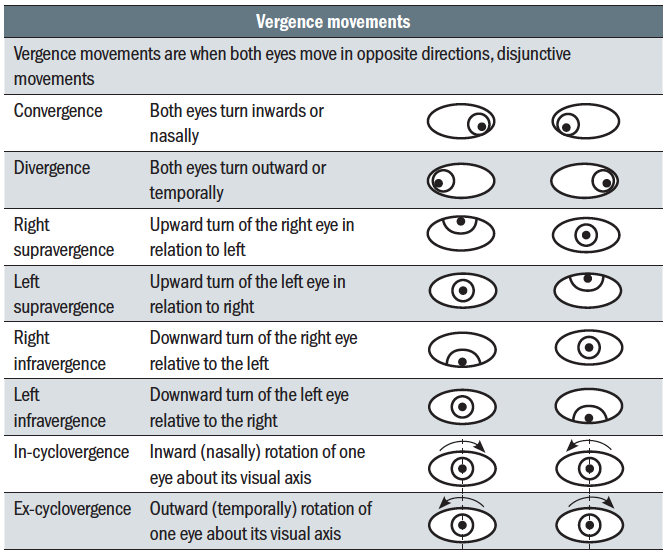 Table 3: Vergence eye movements
Table 3: Vergence eye movements
Versions (table 2) describe where the eyes move in the same direction such that the visual axes remain parallel; so-called conjunctive movements. Disjunctive movements, vergence movements (table 3), are ones where the visual axes cross and the eyes move in opposite directions.
Binocular single vision
Binocular single vision (BSV) is important as it improves visual acuity, contrast sensitivity, provides depth perception and is shown to aid visual motor skills.9 In evolutionary terms, it is an attribute that has contributed to humans being ‘top of the food chain’ predators to all other species. BSV is the ability of the eyes to work together comfortably, the brain receiving almost identical images from each eye that are combined (fused) into a single stereoscopic image.
In ophthalmic dispensing, understanding the importance of BSV is essential because incorrectly centred spectacles, anisometropic prescriptions, and uncorrected ametropia may lead to a breakdown in binocularity.
Optometrists, as part of routine eye tests, not only assess the refractive status of each eye individually but also have to achieve a working balance between the eyes enabling them to function together comfortably.
Rowe explains Worth’s classification of BSV as having three parts; simultaneous perception combined with superimposition, fusion (sensory and motor) and stereoscopic vision.10
- Simultaneous perception; both eyes need to be able to receive images one from each eye at the same time. Superimposition is when the two images received by simultaneous perception are formed on corresponding retinal areas which then project into space at the same position.
- Fusion; where images received from each eye are able to be fused into a single visual perception;
- Sensory fusion; similar images from each eye perceived as one single image.
- Motor fusion; also known as fusional vergence, this is the ability to maintain sensory fusion during differing vergence movements.
- Stereoscopic vision; the perception of depth due to detection of binocular disparity. Although the brain fuses the two slightly different images from the right and left eye the differences allow judgement of depth and distance.
Sensory fusion requires normal retinal correspondence, where light from any object enters both eyes to stimulate both foveae and corresponding or matching points on each retina such that similar or concordant images stimulate a single binocular image. These image points projected into space fall on a line known as the horopter (figure 6). Objects whose images fall on the horopter are able to be fused. Image points not exactly falling on the horopter can potentially still form a single binocular image providing they fall within Panum’s fusional area, a projection of retinal points with ‘retinal disparity’ meaning they almost correspond sitting very near to but on either side of the horopter (figure 6). 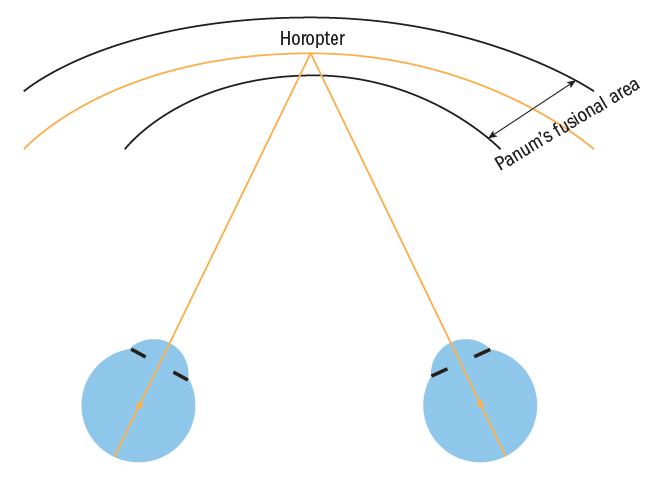 Figure 6: Panum’s fusional area and the horopter
Figure 6: Panum’s fusional area and the horopter
Retinal disparity of images outside Panum’s area stimulates motor fusion. There is no stimulus if the images fall on the horopter. Panum’s area is considered to be an ellipse broader in the horizontal than the vertical regions,11 approximately six to 10 minutes of arc horizontally arc at the fovea, widening towards the periphery to about 30 to 40 minutes of arc at 12° from the fovea.10,12
Within the limits of Panum’s area is the amplitude of motor fusion or fusional reserves. As discussed later, the fusional reserves dictate the amount of vergence that is possible in order to maintain a single image. As convergence is an important part of our vision, the EOMs have much greater horizontal vergence capability and hence the horizontal fusional reserves are much greater than vertical, and hence vertical prismatic disparity is much harder to maintain a single image through.
The fusion reflex means the eyes are constantly moving, correcting any alignment errors by a combination of version and vergence movements (see table 2 and 3), maintaining stable fusion requires effort and depends upon having sufficient fusional reserves. Fusional reserves vary considerably between patients and are affected by health, general fatigue which affects all motor skills, medication, alcohol, uncorrected ametropia and induced prism which can displace retinal images off the horopter or even outside Panum’s area.
When both eyes are uncovered and fixating upon an object, the eyes are considered to be associated. By occluding one eye, the fusion reflex is disrupted and the eyes are said to be disassociated. If when either eye is occluded there is no deviation of the visual axis observed, both remain fixated on the object; this is known as orthophoria.
Heterophoria, often shortened to phoria, is when the eyes are disassociated and the visual axis of the eye under cover deviates. Classification is based upon the movement of the eye under cover (table 4). Heterophoria is also known as a latent (present but not visible) deviation as it not seen (is not manifest) when the eyes are associated under conditions not conducive to maintaining this position (for example, poor lighting, duration, fatigue, alcohol). 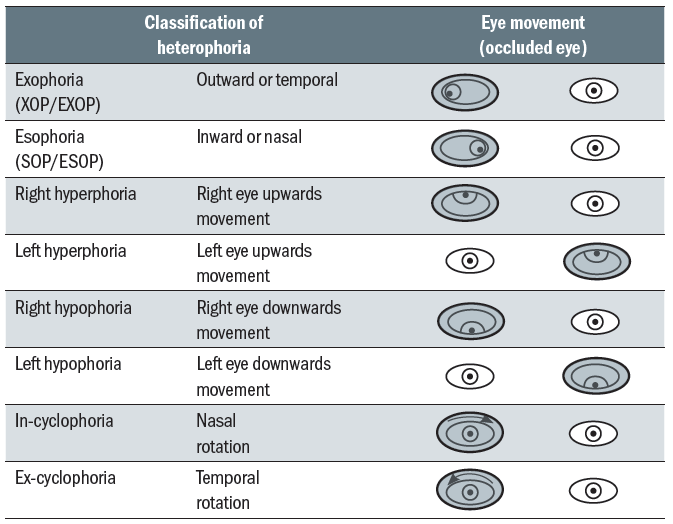 Table 4: Classification of heterophoria
Table 4: Classification of heterophoria
Any misalignment of the eyes is usually overcome to maintain BSV by motor fusion, initiating and maintaining version and vergence movements by the required amount as available from the fusional reserves.13 Demands upon fusional reserves can change through for example an increase in close work, use of tablets, computers or smartphones. These near vision tasks are often performed at very short working distances putting increased demands on fusional reserves and potentially meaning that the reserves are unable to overcome demand. If between one-third and two-thirds of the total vergence amplitude (fusional reserve) is used usually the visual system is capable of maintaining the demands placed upon it.14,15
The most important feature of heterophoria is whether it is compensated (asymptomatic) or decompensated which would almost always be accompanied by symptoms such as headaches, aching eyes, focusing difficulties and intermittent diplopia.
Fusional reserves vary considerably between patients and, as previously mentioned, may be affected by health, general fatigue – affecting all motor skills, prescribed and recreational drugs, alcohol, uncorrected ametropia and induced prism.10
Fusional reserves or fusional amplitudes are usually measured using prisms recording blur point, break point – where diplopia occurs then recovery – reducing the prism until single vision is restored.
- Positive fusional reserves; maximum the eyes can converge, measured with base out prism
- Negative fusional reserves; maximum the eyes can diverge, measured using base in prism
- Vertical fusional reserves; the eye’s ability to maintain BSV when vertical prism is introduced
Fusional reserves differ in the vertical and horizontal with vertical reserves typically only about 2 to 4∆,16 horizontal positive fusional reserves (distance) about 20∆ and negative reserves about 6 to 8∆.12
Richardson and Firth17 show that even small amounts of induced vertical prism not only impact on vertical reserves but also reduce horizontal reserves so any spectacle differential vertical prismatic effects whether due to inaccurate centration of poorly made spectacles or anisometropic prescriptions should be avoided wherever possible. There is very little current evidence on physiological tolerance of vertical prism but Tunnacliffe and Andrews suggest that even 1∆ may be too great.18 The authors concur with this and have found it easy to replicate in training sessions where registrants used prism bars or plano prisms to determine their own fusional reserves. This shows that though almost all people can fuse 1∆, many will experience considerable discomfort when doing so, and 2∆ of vertical differential prismatic effect causes double vision in a significant proportion of people (around half) and is uncomfortable for almost everyone. Subjects felt their eyes were being ‘pulled’ and further complain that the exercise induces a variety of symptoms over the following 24 hours including headache, asthenopia and migraine.
It is noteworthy that subjects with the ability to fuse more than 2∆ of vertical differential prism typically had significant ametropia. This makes perfect sense as they have likely adapted to intermittent differential prism caused by even slight misalignment of their spectacles. Take for example a -10.00DS myope who had knocked their spectacles out of alignment such that they were twisted, with one lens raised and the other lowered, each by 1mm relative to pupil centre. The differential would be 2mm, and they would routinely experience a 2Δ differential prismatic effect but which they seem able to adapt to.
When BSV breaks down diplopia occurs. However, pathological diplopia should not be confused with physiological diplopia which is normal and can be easily demonstrated showing that a subject has the ability to use both eyes without suppression of one eye (figure 7). If an object at point X is fixated the object at point Y will appear double as the points Y’L and Y’R fall on non-corresponding retinal points causing heteronymous (crossed) diplopia, the image seen by the right eye appearing on the left, when the right eye is closed the left image disappears. If the near object Y is fixated the distant object X will appear double, this time X’L and X’R fall on non-corresponding points causing homonymous (uncrossed) diplopia, the image seen by the right eye appearing on the right, when the right eye is closed the right image disappears. 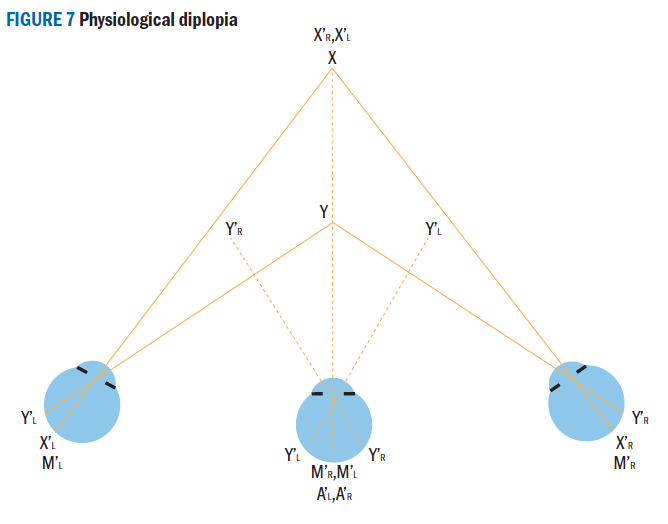 Figure 7: Physiological diplopia
Figure 7: Physiological diplopia
Heterotropia or manifest (obviously visible) deviation, is when only one eye fixates the object when both eyes are uncovered, the deviation or misalignment is unchanged whether the eye is covered or uncovered (associated or disassociated), however the strabismic deviating eye will likely take up fixation if the ‘straight’ eye is now covered, and the non-strabismic eye would itself turn under cover.
Students often struggle to appreciate the level of movement that might be witnessed when performing a cover test, and also that sufficient time (four to five seconds) must be allowed for the uncovered eye to take up fixation if it is able to. To gain an idea of how much movement a deviation of say 2∆ would cause, it should be born in mind that the definition of the prism dioptre is a lateral displacement of 1cm at a distance of 1m. 2∆ is a displacement of 12cm at a the standard testing distance of 6m, and by asking the patient to alternate their view between two points around 12cm apart (such as the first and last letter on the 6/12 line for example on many Snellen charts) the amount of movement can be witnessed.
Classification of heterotropia is consistent with heterophoria (table 5) the heterotropia is recorded according to the eye that unilaterally deviates or as an alternating strabismus. A further classification is made depending on whether the deviation is concomitant or incomitant. 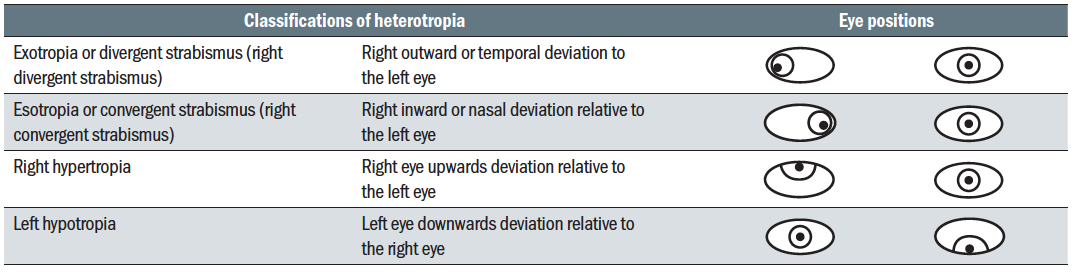 Table 5: Classification of heterotropia
Table 5: Classification of heterotropia
Concomitant is when the angle of deviation remains constant in all directions of gaze. Incomitant is when the angle of deviation varies indicating a partial failure (paresis) or total failure (paralysis) of one or more of the extraocular muscles. The cranial nerves including those which control the extrinsic ocular muscles are shown in table 6. 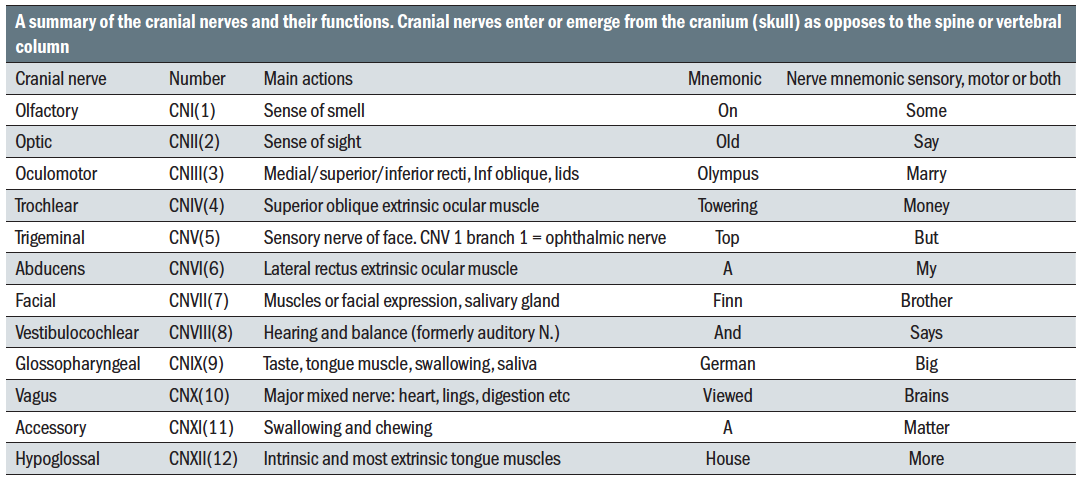 Table 6: A summary of the cranial nerves and their functions
Table 6: A summary of the cranial nerves and their functions
Heterotropia may also be classified according to the amount of deviation between the visual axes, and here the different emphasis between the professions of optometry/optics and ophthalmology/orthoptics are evident. Orthoptists refer to a deviation between the eyes of less than 10∆ as a microtropia, indicating that they perceive it to be small, whereas in primary care optometric practice prescribed prism of any size is seen relatively infrequently, and prisms as large as 10∆ are perhaps an annual event for most practitioners.
That said, a failure to prescribe even small prisms where they are required may leave a patient with symptoms of unnecessary discomfort. More important is a failure to take into account the interrelationship between accommodation and convergence or to recognise the certainty of induced prism in certain kinds of prescription or as a result of poor quality dispensing, which will be the topic of the next article.
A Final Note
It is important to use the correct terminology when considering binocular vision. Table 7 summarises most of the relevant terms, prefixes and suffixes that need to be used when considering the tipic and when recording information.
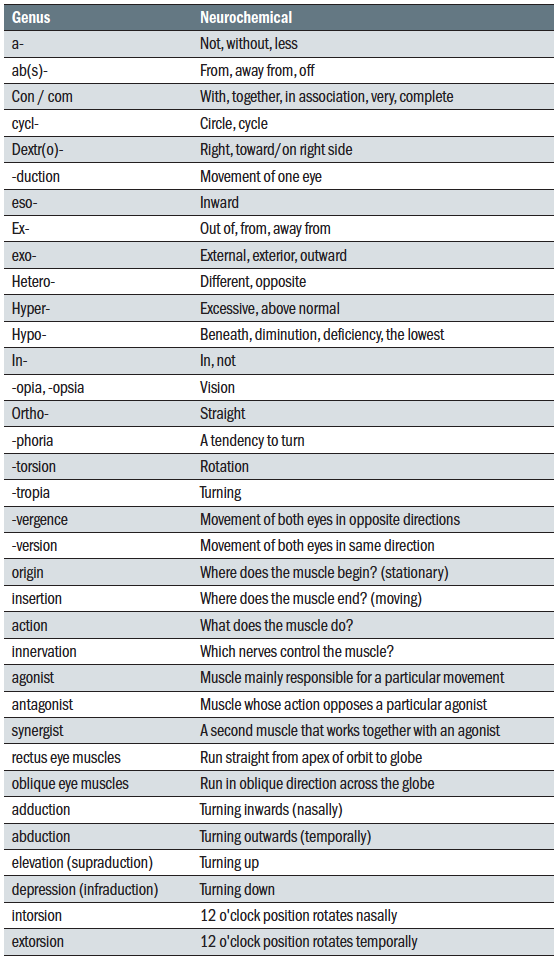 Table 7: Medical terms, prefixes, and suffixes relevant to binocular vision
Table 7: Medical terms, prefixes, and suffixes relevant to binocular vision
Peter Black MBA FBDO FEAOO is senior lecturer in ophthalmic dispensing at the University of Central Lancashire, Preston, and is a practical examiner, practice assessor, exam script marker, and past president of the Association of British Dispensing Opticians.
Tina Arbon Black BSc (Hons) FBDO CL is director of accredited CET provider Orbita Black Limited, an ABDO practical examiner, practice assessor and exam script marker, and a distance learning tutor for ABDO College.
References
- Remington, L A. Clinical Anatomy and Physiology of the Visual System. 3rd. St Louis, USA : Elsevier, 2012. ISBN 978-1-4377-1926-0.
- Pipe, D M and Rapley, L J. Ocular Anatomy & Histology. 3rd. Kent : Association of British Dispensing Opticians, 2008. ISBN 0-900099-22-4.
- Orbital Anatomy for the Surgeon. Turvey, T A and Golden, B A. 4, s.l. : Oral and Maxillofacial Surgery Clinics of North America, 2012, Vol. 24, pp. 525-536.
- Anatomy of The Human Orbit. Wilkinson, M J. 4, s.l. : Operative Techniques in Otolaryngology Head and Neck Surgery, 2018, Vol. 29, pp. 186-192.
- Extraocular Connective Tissues: A Role In Human Eye Movements? McClung, J R, Dimitrova, D M and Goldberg, S J. 1, s.l. : Investigative Ophthalmology & Visual Science, 2006, Investigative Ophthalmology & Visual Science, Vol. 47, pp. 202-205.
- The Anatomy of Whitnall Ligament. Codere, F, Tucker, N A and Renaldi, B. 12, s.l. : Ophthalmology, 1995, Ophthalmology, Vol. 102, pp. 2016-2019.
- British Standards Institution. BS EN ISO 13666:2019. Ophthalmic Optics. Spectacle Lens. Vocabulary. . London : British Standards Institution, 2019.
- Sherrington, C S. Further Experimental Notes on the Correlation of Action of Antagonistic Muscles. Further Experimental Notes on the Correlation of Action of Antagonistic Muscles. s.l. : Journal of Physiology, 6 10, 1893.
- The Effect of Degrading Bimacular Single Vision on Fine Visuomotor Skill Task Performance. Piano, M E and O’Connor, A R. 13, s.l. : Investigative Ophthalmology and Visual Science, 12 2013, Vol. 54, pp. 8204-8213.
- Rowe, F J. Clinical Orthoptics. West Sussex : Wiley-Blackwell, 2012. ISBN 978-1-4443-3934-5.
- Spatiotemporal Properties of Panum’s Fusional Area. Schor, C C and Tyler, C W. s.l. : Vision Research, 1980, Vol. 21, pp. 683-692.
- Von Noorden, G K and Campos, E C. Binocular Vision and Ocular Motility. Theory and Management of Strabismus. 6th. s.l. : Mosby, 2002.
- Repeatability and Agreement in The Measurement of Horizontal fusional Reserves. Antona, B, et al. 5, s.l. : Journal of College of Optometrists Ophthalmic & Physiological Optics, 2008, Vol. 28, pp. 475-491.
- Elliott, D B. Clinical Procedures in Primary Eye Care. s.l. : Elsevier, 2021. ISBN 9780702077890.
- Validity of Diagnostic Criteria and Case Analysis in Binocular Vision Disorders. Sheedy, J E and Saladin, J J. 7, s.l. : American Journal of Optometry & Physiological Optics, 7 1977, American Journal of Optometry & Physiological Optics, Vol. 54, pp. 474-478.
- Tunnacliffe, A H. Introduction To Visual Optics. s.l. : Association of British Dispensing Opticians, 1993. ISBN 0 90099283.
- The Effects of Induced vertical Divergence on Horizontal Fusional Amplitudes. Richardson, G A and Firth, A Y. s.l. : British and Irish Orthoptic Journal, 2009, British and Irish Orthoptic Journal, Vol. 6, pp. 71-74.
- The Effect of Vertical Differential Prism on the Binocular Contrast Sensitivity Function. Tunnacliffe, A H and Williams, A T. 4, s.l. : Ophthalmic & Physiological Optics, January 28, 1985, Ophthalmic & Physiological Optics, Vol. 5, pp. 417-424.
