A patient’s’ quality of life can significantly deteriorate due to ocular discomfort. In fact, suffering from severe dry eye disease (DED) has been shown to have a similar impact on quality of life to that of moderate to severe angina.1 DED can affect up to 50% of the population, with peaks of up to 75% in certain populations2 and in particular in populations older than 50 years.3
Following the latest Tear Film and Ocular Surface Society Dry Eye WorkShop II (TFOS DEWS 2) report in 2017, researchers defined DED as ‘a multifactorial disease of the ocular surface characterised by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles’.
Background
DED can play an important role in the perceived benefit of ocular surgery where the aim is to modify the refractive power of the eye. This can be achieved by either of the following:
- Modifying the cornea (keratorefractive surgery)
- Replacing the crystalline lens with a new artificial intraocular lens (IOL) either due to its opacification (cataract surgery) or by inserting a multifocal IOL designed to give the benefit of clear all round vision (refractive lens exchange surgery).4
Naturally, no surgical procedure is totally risk-free and despite the safety and efficacy of contemporary corneal and IOL surgical treatments, some patients may develop surgically-induced dry eye which could interfere with the perceived success of the procedures.5,6 But why might patients experience DED, even when the procedure is technically successful?
In both types of refractive eye surgeries, DED could occur as a temporary post-operative complication due to the impact of the procedures on the ocular surface. In corneal refractive procedures, the laser ablation which aims to reshape the cornea can target both the epithelium and the stroma layers of the cornea: the epithelium is responsible for producing and secreting important growth factor to help corneal nerve trophism while the mid-stroma houses the subbasal corneal nerves structure. The transection of these nerves has been associated with a reduction in tear functions due to diminution in corneal sensitivity.7,8
In IOL procedures, a surgical incision of approximately 1.5-3mm is required for the phacoemulsification probe and a small incision is created to facilitate the capsulorhexis (figure 1). Therefore, a physical impact on the corneal nerves can occur. Additionally, one of the most recognised risk factors for developing DED is age,2 and the changes to the proteins of the crystalline lens leading to lens opacification is also driven by age.9
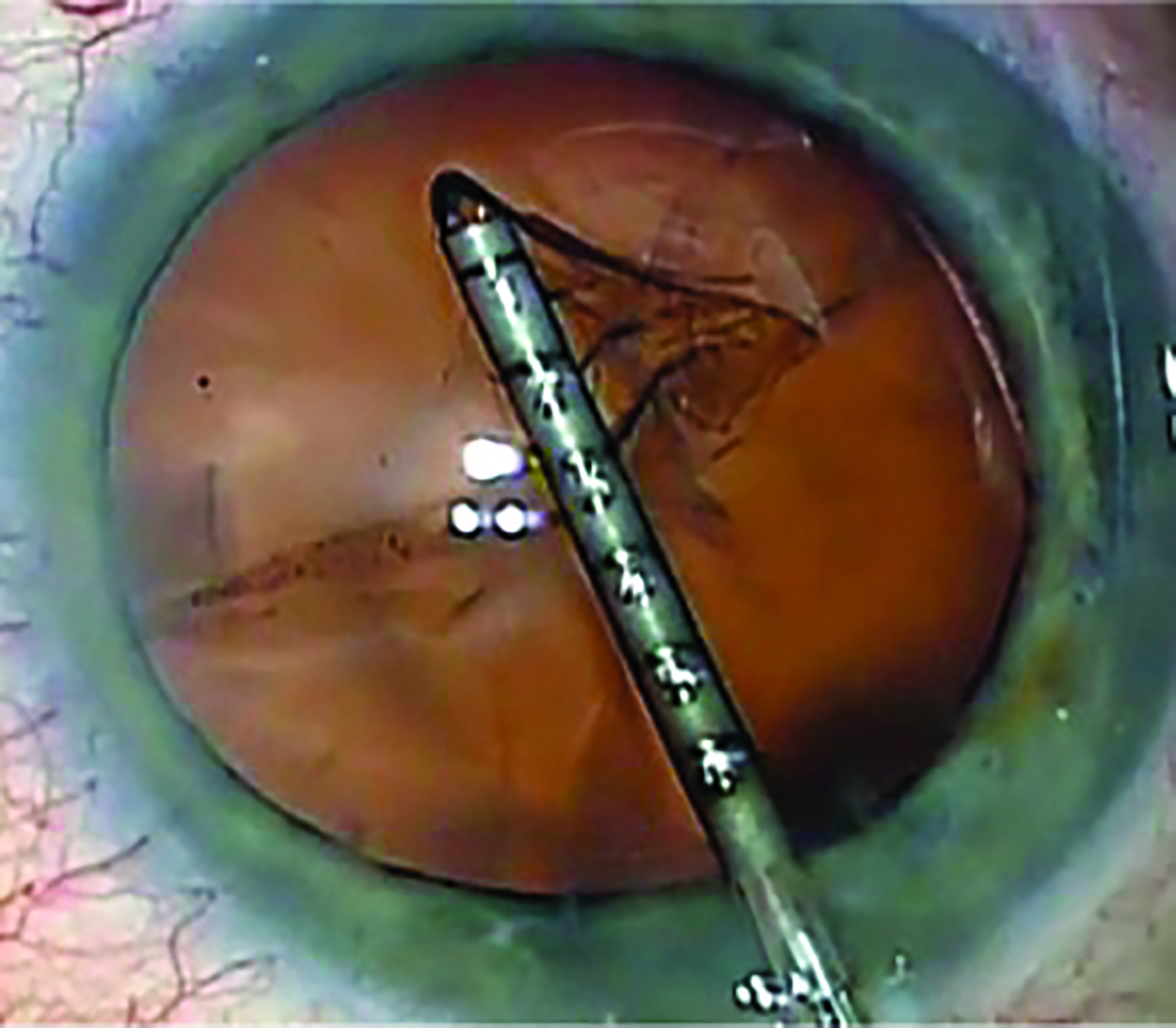 Figure 1: Capsulorhexis procedure
Figure 1: Capsulorhexis procedure
Despite great progress both in research and clinical development, a consensus for the optimal approach to DED diagnosis has been not reached. The latest TFOS DEWS 2 report recommends a series of clinical steps based on scientific evidence to reduce test variability in DED practice which will boost DED diagnosis and treatment. These clinical steps take into account subjective and objective metrics considered from the outcomes of many robust randomised clinical trials.10 Therefore, in our research we decided to apply a series of minimally to non-invasive diagnostic DED tests recommended by the latest TFOS DEWS 2 report to help to improve the understanding of the impact of dry eye on the refractive and visual outcomes in ophthalmic surgery and on the impact of ophthalmic surgery on the ocular surface.
In summary, it is envisaged that the data from research studies detailed in this article will provide the readers with a useful overview of how using a series of advanced diagnostic techniques can improve our understanding of the role of DED in patients undergoing ophthalmic procedures for refractive and visual indications.
Modern corneal refractive procedures and dry eye
Several studies have reported on the incidence of DED after corneal refractive surgery.11-13 In a survey sent to more than 8,800 members of the American Society of Cataract and Refractive Surgery, 1,053 were returned highlighting that the most common complication after LASIK surgery was dry eye (95.2% of the total respondents).14 In 2015, Bower et al (2015) reported the results at 12 months, with DED incidence in 5% and 0.8% patients operated with photorefractive keratectomy (PRK) and laser-assisted in situ keratomileusis (LASIK) surgery, respectively. However, the advent of femtosecond laser technology has introduced several improvements in the past decade for refractive procedures.15 Femtosecond-LASIK and refractive lenticule extraction obtained FDA approval for correcting myopia in 2010 and 2016, respectively. Only subsequently, in 2018, the FDA approved the SMILE procedure for correcting compound myopic astigmatism. Since then, femtosecond laser surgery has increased the precision of corneal refractive surgery thanks to the advanced photodisruption process which target tissue diameters of approximately 0.001mm in one femtosecond (10-15s).
Two main refractive techniques are based on the femtosecond laser to create a slice of stromal tissue called a lenticule. These are:
- Femtosecond lenticule extraction (ReLEx FLEX)
- Small incision lenticule extraction (ReLEx SMILE)
In fact, by creating a stromal lenticule through a circular dissection using the femtosecond laser, the cornea can be reshaped to correct refractive error.
The main difference between the two techniques is the presence of the lifting flap in the FLEX procedure, SMILE being a flapless technique where a small incision, a side tunnel in a patient’s cornea, is created to extract the stromal lenticule. The results from initial studies on SMILE surgery16,17 have supported the flapless surgery to be less invasive compared to other corneal refractive procedures permitting better conservation of the biomechanical properties of the cornea while preserving the corneal nerves.18 However, with SMILE, during the initial learning curve, surgeons might experience different complications such as lenticule dissection and lenticule extraction.19
Therefore, in 2017 we decided to conduct a study to assess the clinical outcomes and tear film stability before and after the first cases of SMILE surgery undertaken by surgeons in their early learning curve at a private eye hospital in the UK.20 The refractive results obtained were in agreement with other research:21,22 in fact, 82% and 94% of our patients (n=37) were within ±0.50DS and ±1.00DS within three months after surgery, respectively. Post-operative visual acuity (VA) was 0.0 logMAR in 88% of the patients with no significant difference with the pre-operative corrected distance VA. Complications were observed in three eyes, which included a minor epithelial abrasion in two eyes and some difficulty removing the lenticule in one eye. None of the complications adversely affected the visual outcome.
Nevertheless, a small reduction (~12%) of the fluorescein tear break-up time (FTBUT) was observed, but this is not likely to represent a clinically significant reduction. On the contrary, it should be seen as a preservation in the early post-operative FTBUT compared to more traditional surgery options (eg PRK and LASIK particularly with mechanical microkeratomes).7
Since the early ’90s, researchers showed results from denervated corneas that were more likely to suffer from corneal abnormalities, recurrent superficial erosion, impaired wound healing and infection.23,24 In fact, the corneal epithelium and the corneal nerves help each other in producing and secreting important growth factor to help corneal nerve trophism and neuromediators for epithelial cells regeneration.25,26 More importantly, an intact and functional corneal nerve loop maintains the homeostasis of the ocular surface by assuring the functionality of the connections with the lacrimal gland. Therefore, we can hypothesise that a less invasive refractive corneal surgery might help to preserve the ocular surface by maintaining adequate tear flow. Previous studies considered tear film metrics before and after femtosecond laser LASIK (FS-LASIK) which have reported a significant reduction in tear volume and reduced FTBUT when compared to SMILE.27,28 In vivo confocal microscopy (IVCM) has been intensively used in the last decade to investigate the structure and function of the corneal nerves. When applied to the ocular surface, IVCM can image epithelial cells, inflammatory cells, stromal cells and Meibomian glands.29 However, arguably one of the most important IVCM applications is to evaluate the overall structure of the corneal nerves, in particular, nerve fibre length and nerve fibre density.30 Vestergaard et al investigated the impact of new surgical procedures such as FLEX and SMILE in high myopes considering IVCM and dry eye metrics. Their findings suggested that SMILE surgery is less likely to induce dry eye than the laser flap surgery (FLEX) and highlighting that the corneal nerve density did not correlate with other DED metrics. Nevertheless, despite the increasing interest in research and clinical settings of using IVCM in DED, limitations in the way the scans from IVCM are analysed, has been an issue. In fact, if we consider the manual tracing of the corneal nerves imaged by IVCM, using programmes such as Matlab or Java algorithms have demonstrated several disadvantages, are time-consuming and are subject to bias which can impact on reproducibility and repeatability.31
Assessing Post-operative DED
To address the gaps in the current knowledge, in order to compare the early clinical outcomes in patients undergoing FS-LASIK (n=16 eyes) and SMILE (n= 13 eyes) surgery, we have considered a recommended protocol for DED assessment taken from the latest TFOS DEWS 2 report and we have applied a fully automated quantification of the corneal nerve fibre metrics with a validated software before and one month after the procedures (ACCMetrics, University of Manchester Research Group, UK). The refractive results obtained from LASIK and SMILE (up to 87% and 77% were within 0.50 D with FS-LASIK and SMILE, respectively) were in agreement with previous studies, where no complications were reported, showing good safety and efficacy.16,32 Post-operative VA was 0.0 logMAR in 100% and 92% with FS-LASIK and SMILE, respectively.
The DED protocol has included both OSDI and DEQ-5 questionnaires which have shown a significant drop on the subjective comfort only in the FS-LASIK patients. No significant changes were observed from a single osmolarity reading using a TearLab system in both procedures where the non-invasive tear meniscus height (TMH) readings obtained by using an Oculus Keratograph K5M (Oculus Optikgeräte GmbH, Wetzlar, Germany) clearly showed a significant reduction with the laser flap technique (figure 2). A shortening of up to 40% of the non-invasive Keratograph tear break-up time (NITBUT, figure 3) against only 3% was observed in the FS-LASIK cohort versus the SMILE group with no difference in the ocular staining and in the meibomian gland loss (% of area) before and after surgery. Using the ACCMetrics analysis, a significant reduction in the FS-LASIK group in terms of subbasal corneal nerve density (up to 75% reduction) after surgery was observed compared to the SMILE group where the impact was less (up to 23%). Therefore, FS-LASIK and SMILE safely corrected the refractive error providing a favourable visual outcome in both study cohorts. However, our results showed that FS-LASIK surgery had more impact on DED symptomatology, TMH and NIKBUT comparing the pre-operative values within the post-operative values compared to SMILE. In conclusion, SMILE, due to its ‘flap-less’ feature, can provide better preservation of the ocular surface metrics and subbasal corneal nerves structure compared to more traditional laser vision correction procedures.33
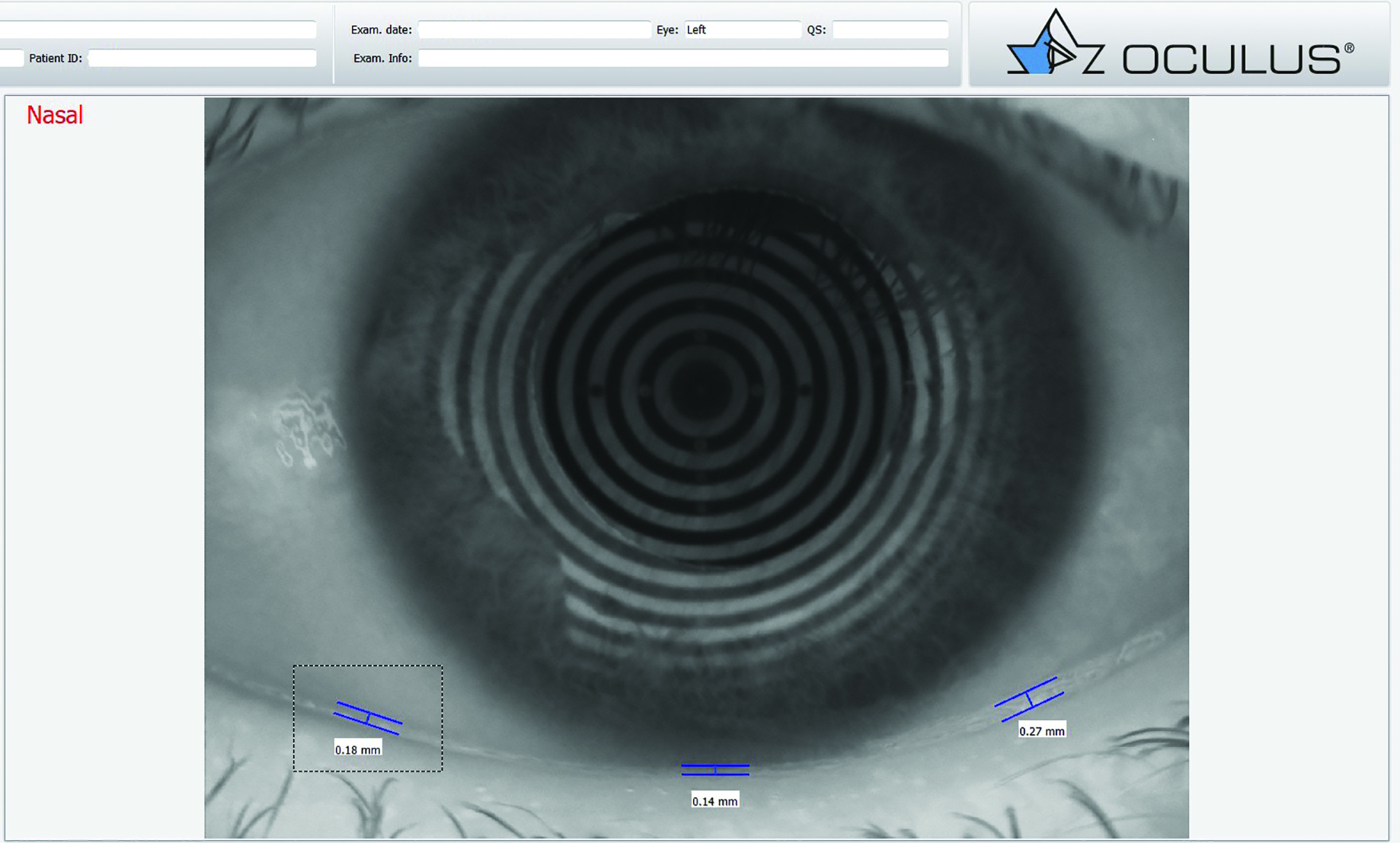 Figure 2: Tear prism height measurement on the Oculus Keratograph
Figure 2: Tear prism height measurement on the Oculus Keratograph
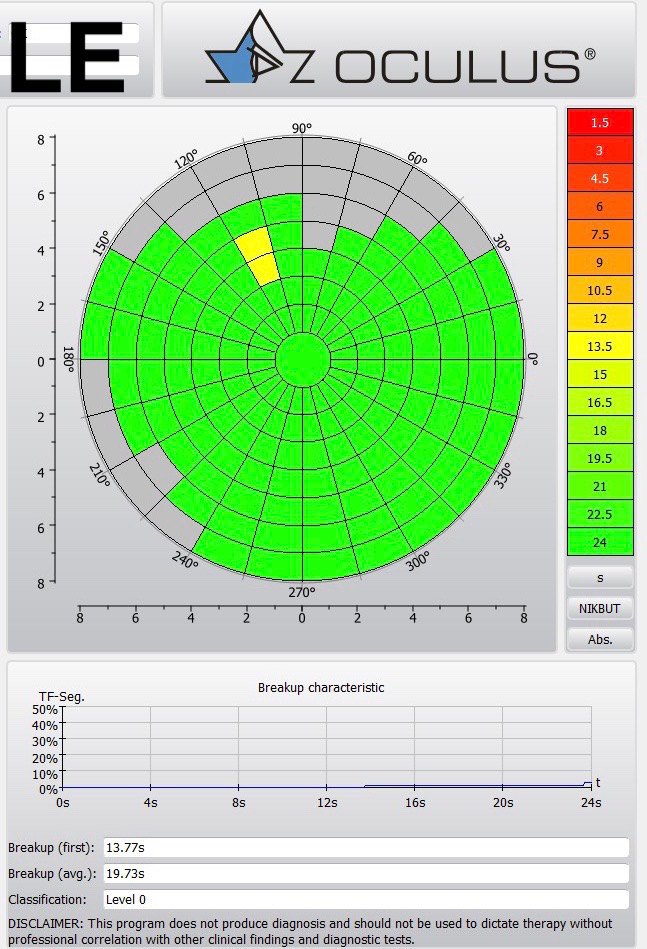
Figure 3: Non-invasive tear break data from the Oculus Keratograph
Lens surgery and dry eye
There are two main points to consider when analysing dry eye: before and after IOL surgery. Pre-operatively, as cataract is more prevalent in people older than 50 years,34 DED can be present as it is more prevalent after that age with a peak prevalence greater than 50% of the global population.2 Research has been undertaken in relating ageing and DED but not many studies have considered populations undergoing lens surgery with the potential presence of DED. For example, Epitropoulos35 reported variability of up to 1 D in measured corneal astigmatism (keratometry readings) in patients with ocular surface disturbance which might account up to 0.50 D of variation in the final refractive outcomes post-surgery.
Findings from the ‘Prospective Health Assessment of Cataract Patients’ Ocular Surface’ (PHACO) study with patients interested in having cataract surgery showed that 50% of the total (n= 136) suffered from reduced tear film stability while 45% presented with ocular surface staining.36 Later, Cochener37 presented data with 52% of patients undergoing cataract surgery with MG dysfunction (MGD) in the absence of lipid layer abnormalities. No correlations were found between MGD and symptoms and the authors enforced the message to consider subjective and objective metrics in patients attending for cataract surgery.
The second point to consider that relates lens surgery and DED is the impact of the surgical procedure and the consequent temporary post-operative DED. Kasetsuwan38 summarised the most common causes of post-operative DED due to surgery: transection of corneal nerves, corneal epithelial cells loss, topical anaesthesia, microscope light, etc.
Additionally, the post-surgical drug regime might be a potential risk factor for DED development, in particular, if preservative eyedrops are used (antibiotics and steroids).
Despite the increasing awareness of post-op dry eye issues, pre-operative DED screening is not always included in the pre-operative clinical pathway and therefore many patients undergoing lens surgery could potentially risk unwanted refractive outcomes due to the ocular dryness.
Hence, we conducted a study to determine the post-operative refractive predictability and visual and patient-reported outcomes in normal (n= 16 with 299 mOsm/L) and hyperosmolar (n= 11 with 314 mOsm/L) populations presenting for lens surgery considering a tear film hyperosmolarity cut-off ≥ 308 mOsml/L. Our findings suggested that the presence of pre-operative DED symptoms was found in about 58% of the total (n=27) where only 19% of those have previously identified as DED patients by their GP or eye practitioner (ophthalmologist or optometrist). However, the hyperosmolar group was found to have a deviation from the predicted post-operative refraction (PPOR) of more than 0.50 D which led to a slightly decreased unaided distance VA compared to the patients with normal tear film osmolarity: -0.1 logMAR (~6/5 vision) was achieved by 9% of the hyperosmolar patients vs 31% of the normal osmolarity patients. Again, the hyperosmolar group after surgery was found to achieve a residual error within ± 0.50 D in only 54% of the total against 81% of the normal patients. Nonetheless, both groups had ocular staining and reduced NIKBUT, although not significantly different. Finally, a series of correlation analyses were conducted to address the correspondence of the tear film osmolarity with the other DED metrics considered in the study although again statistically significant differences were not found of the two groups. In closing, hyperosmolarity of the tear film might lead to suboptimal refractive outcomes (up to 0.60 D of deviation from the PPOR) which may impact on visual outcomes after the procedure.
Conclusion
A review of current research studies has illustrated the role of DED in patients undergoing ophthalmic procedures for refractive and visual indications. Despite the safety and efficacy of corneal and lens treatments to restore optimal levels of vision, a significant part of the population undergoing those surgeries might develop surgically-induced DED which could burden their quality of life.
The development of less-invasive surgical procedures to correct the refractive errors such as myopia, hyperopia, astigmatism and presbyopia (if multifocal IOLs are considered) should begin with an assessment of the ocular surface. While the research in the field of DED has rapidly evolved in the past years, the inclusion of those techniques in routine clinical practice have not progressed quite as rapidly. Therefore, we suggest that an evidence-based approach could help clinicians to mitigate the effects of DED development before and after surgery.
Dr Alberto Recchioni is a research optometrist based at the Institute of Inflammation and Ageing, University of Birmingham.
- This article is based on his PhD which was one of the first in the UK to be supported by Optegra.
References
- Schiffman, R M, Walt, J G, Jacobsen, G, Doyle, J J, Lebovics, G & Sumner, W. 2003. Utility assessment among patients with dry eye disease. Ophthalmology, 110, 1412-9.
- Stapleton, F, Alves, M, Bunya, V Y, Jalbert, I, Lekhanont, K, Malet, F, NA, K S, Schaumberg, D, Uchino, M, Vehof, J, Viso, E, Vitale, S & Jones, L. 2017. TFOS DEWS II Epidemiology Report. Ocular Surface, 15, 334-365.
- Zhang, X, Wang, L, Zheng, Y, Deng, L & Huang, X. 2020. Prevalence of dry eye disease in the elderly: A protocol of systematic review and meta-analysis. Medicine, 99, e22234.
- Alio, J, Plaza-Puche, A, Fernanez-Buenaga, R, Pikkel, J & Maldonado, M. 2017. Multifocal intraocular lenses: An overview. Survey of Ophthalmology, 62, 611-634.
- Toda, I. 2018. Dry Eye After LASIK. Investigative Ophthalmology and Vision Science, 59, 109-115.
- Szakats, I, Sebestyen, M, Toth, E & Purebl, G 2017. Dry Eye Symptoms, Patient-Reported Visual Functioning, and Health Anxiety Influencing Patient Satisfaction After Cataract Surgery. Current Eye Research, 42, 832-836.
- De Paiva, C, Chen, Z, Koch, D, Hamill, M, Manuel, F, Hassan, S, Wilhelmus, K. & Pflugfelder, S. 2006. The incidence and risk factors for developing dry eye after myopic LASIK. American Journal of Ophthalmology, 141, 438-445.
- Demirok, A, Ozgurhan, E B, AGCA, A, Kara, N, Bozkurt, E, Cankaya, KI & Yilmaz, OF. 2013. Corneal sensation after corneal refractive surgery with small incision lenticule extraction. Optometry and Vision Science, 90.
- Moreau, KL & King, JA. 2012. Protein misfolding and aggregation in cataract disease and prospects for prevention. Trends in molecular medicine, 18, 273-282.
- Wolffsohn, JS, Arita, R, Chalmers, R, Djalilian, A, Dogru, M, Dumbleton, K, Gupta, PK, Karpecki, P, Lazreg, S, Pult, H, Sullivan, BD, Tomlinson, A, Tong, L, Villani, E, Yoon, KC, Jones, L & Craig, JP. 2017. TFOS DEWS II Diagnostic Methodology report. Ocular Surface, 15, 539-574.
- Albietz, JM, Lenton, LM & Mclennan, SG. 2005. Dry eye after LASIK: comparison of outcomes for Asian and Caucasian eyes. Clinical & experimental optometry, 88, 89-96.
- Bower, K, Sia, R, Ryan, D, Mines, M & Dartt, D. 2015. Chronic dry eye in photorefractive keratectomy and laser in situ keratomileusis: Manifestations, incidence, and predictive factors. Journal of cataract and refractive surgery, 41, 2624-2634.
- Denoyer, A, Landman, E, Trinh, L, Faure, J, Auclin, F & Baudouin, C. 2015. Dry eye disease after refractive surgery: comparative outcomes of small incision lenticule extraction versus LASIK. Ophthalmology, 122, 669-676.
- Sandoval, HP, De Castro, LE, Vroman, DT & Solomon, KD. 2005. Refractive Surgery Survey 2004. Journal of Cataract and Refractive Surgery, 31, 221-33.
- Aristeidou, A, Taniguchi, EV, Tsatsos, M, Muller, R, Mcalinden, C, Pineda, R & Paschalis, EI. 2015. The evolution of corneal and refractive surgery with the femtosecond laser. Eye and Vision, 2, 12.
- Ganesh, S, Brar, S & Patel, U. 2017. Comparison of ReLEx SMILE and PRK in terms of visual and refractive outcomes for the correction of low myopia. International Ophthalmology, 1-8.
- Marino, GK, Santhiago, MR & Wilson, SE. 2017. Femtosecond Lasers and Corneal Surgical Procedures. Asia-Pacific Journal of Ophthalmology, 6, 456-464.
- Lee, JK, Chuck, RS & Park, CY. 2015. Femtosecond laser refractive surgery: small-incision lenticule extraction vs. femtosecond laser-assisted LASIK. Current Opinion in Ophthalmology, 26, 260-4.
- Titiyal, JS, Kaur, M, Rathi, A, Falera, R, Chaniyara, M & Sharma, N. 2017. Learning Curve of Small Incision Lenticule Extraction: Challenges and Complications. Cornea, 36, 1377-1382.
- Recchioni, A, Hartwig, A, Dermott, J, Vaswani, S, Bhatt, J, Morris, R & O’Donnell, C. 2017. Early clinical outcomes after small incision lenticule extraction surgery (SMILE). Contact Lens and Anterior Eye, 1-4.
- Vestergaard, A, Ivarsen, A, ASP, S & Hjortdal, J. 2012. Small-incision lenticule extraction for moderate to high myopia: Predictability, safety, and patient satisfaction. Journal of Cataract and Refractive Surgery, 38, 2003-10.
- Lin, F, Xu, Yu & Yang, Y. 2014. Comparison of the visual results after SMILE and femtosecond laser-assisted LASIK for myopia. Journal of Refractive Surgery, 30, 248-254.
- Araki, K, KinoshitaI, S, Kuwayama, Y & Ohashi, Y 1993. Corneal epithelial wound healing in the denervated eye. Nippon Ganka Gakkai Zasshi, 97, 906-12.
- Araki, K, Ohashi, Y, Kinoshita, S, Hayashi, K, Kuwayama, Y & Tano, Y. 1994. Epithelial wound healing in the denervated cornea. Current Eye Research, 13, 203-11.
- Lambiase, A, Sacchetti, M, Mastropasqua, A & BONINI, S. 2013. Corneal changes in neurosurgically induced neurotrophic keratitis. JAMA Ophthalmology, 131, 1547-53.
- Mastropasqua, L, Massaro-Giordano, G, Nubile, M & Sacchetti, M. 2017. Understanding the Pathogenesis of Neurotrophic Keratitis: The Role of Corneal Nerves. Journal of Cellular Physiology, 232, 717-724.
- Xia, L, Zhang, J, Wu, J & Yu, K. 2016. Comparison of Corneal Biological Healing After Femtosecond LASIK and Small Incision Lenticule Extraction Procedure. Current Eye Research, 41, 1202-8.
- Gao, S, Li, S, Liu, L, Wang, Y, Ding, H, Li, L & Zhong, X. 2014. Early changes in ocular surface and tear inflammatory mediators after small-incision lenticule extraction and femtosecond laser-assisted laser in situ keratomileusis. Plos One, 9.
- Alhatem, A, Cavalcanti, B & Hamrah, P. 2012. In vivo confocal microscopy in dry eye disease and related conditions. Seminars in ophthalmology, 27, 138-148.
- Vestergaard, A, Grønbech, K, Grauslund, J, Ivarsen, A. & Hjortdal, J. 2013. Subbasal nerve morphology, corneal sensation, and tear film evaluation after refractive femtosecond laser lenticule extraction. Graefes Archive of Clinical Experimental Ophthalmology, 251, 2591-2600.
- Meijering, E. 2010. Neuron tracing in perspective. Cytometry, 77, 693-704.
- Yan, H, Gong, LY, Huang, W & Peng, YL. 2017. Clinical outcomes of small incision lenticule extraction versus femtosecond laser-assisted LASIK for myopia: a Meta-analysis. International Journal of Ophthalmology, 10, 1436-1445.
- Recchioni, A, Siso-Fuertes, I, Hartwig, A, Hamid, A, Shortt, AJ, Morris, R, Vaswani, S, Dermott, J, Cervino, A, Wolffsohn, JS & O’Donnell, C. 2020. Short-Term Impact of FS-LASIK and SMILE on Dry Eye Metrics and Corneal Nerve Morphology. Cornea.
- Thompson, J & Lakhani, N. 2015. Cataracts. Primary Care, 42, 409-23.
- Epitropoulos, A, Matossian, C, Berdy, G, Malhotra, R & Potvin, R. 2015. Effect of tear osmolarity on repeatability of keratometry for cataract surgery planning. Journal of Cataract and Refractive Surgery, 41, 1672-1677.
- Trattler, WB, Majmudar, PA, Donnenfeld, ED, Mcdonald, MB, Stonecipher, KG & Goldberg, DF. 2017. The Prospective Health Assessment of Cataract Patients’ Ocular Surface (PHACO) study: the effect of dry eye. Clin Ophthalmol, 11, 1423-1430.
- Cochener, B, Cassan, A & Omiel, L. 2018. Prevalence of meibomian gland dysfunction at the time of cataract surgery. Journal of Cataract and Refractive Surgery, 44, 144-148.
- Kasetsuwan, N, Satitpitakul, V, Changul, T & Jariyakosol, S. 2013. Incidence and pattern of dry eye after cataract surgery. Plos One, 8, 1-6.
