The overall aim of this series is to consider the role of preventative medicine in the maintenance of eye health and to suggest that eye care practitioners are well placed to undertake this role.
“The best way to treat eye disease is to avoid it in the first place”
The Concept of Lifestyle Medicine
Lifestyle goes a long way in determining our state of health; a poor lifestyle leads to poor health, and a good lifestyle generally leads to good health.1
The Western lifestyle has been associated with three major conditions endemic to the Western World: inflammation, obesity, and type 2 diabetes. They are intricately interrelated and promote further chronic illnesses including cardiovascular disease and cancer. These conditions are largely the result of poor lifestyle choices, usually fuelled by poor nutrition and physical inactivity. We, as clinicians, are gaining an appreciation that these conditions are associated with an increased risk of ocular pathology, in particular age-related macular degeneration (AMD).2-4
This leads us to the concept of ‘lifestyle medicine’; a focus on prevention rather than the treatment of chronic diseases. It has been estimated that almost 80% of chronic conditions could be avoided through the adoption of healthy lifestyle recommendations.5 The appropriate interventions through recommendation and advice regarding lifestyle medicine generally concern the following:
- Healthy eating6
- Physical activity and health
- Maintenance of a healthy weight
- Promotion of emotional resilience1
Emotional resilience is defined as one’s ability to respond to an adverse situation. Factors impairing or affecting emotional resilience include depression, anxiety, stress, insomnia and the presence of comorbidities, namely, additional chronic conditions. Depression and anxiety are the most common issues that negatively affect emotional resilience in the Western population.1
These four interventions have been described by Bodai1 as the lifestyle medicine quadrants as represented in figure 1.
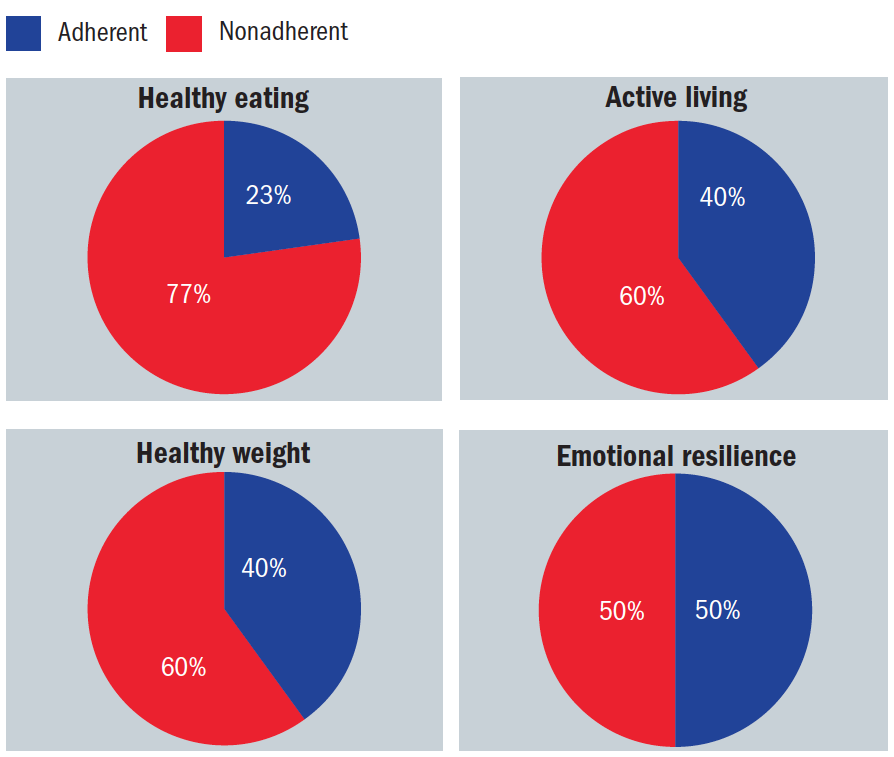 Figure 1: The quadrants of total health; the red zones relate to the percentage of the Western population that fails to adhere to such recommendations1
Figure 1: The quadrants of total health; the red zones relate to the percentage of the Western population that fails to adhere to such recommendations1
Many attributes of our Western lifestyle promote an inflammatory response, which acts as a causative factor in nearly all chronic diseases.7-9 Obesity is an escalating problem, with almost two thirds of the western population either overweight or obese.1 Obesity is nowadays considered to be an inflammatory disease,10-12 where Inflammatory protein levels are elevated, triggering an inflammatory response in the adipose tissue.13,14 This inflammation has been linked with insulin resistance, and the development of type 2 diabetes12,14 as well as ocular complications.15
Obesity and AMD
A varieties of risk factors for AMD exist and have been covered in detail elsewhere.15,16 Of particular interest in the context of this series is the impact obesity has on AMD.
Obesity represents a threat as a risk factor for various systemic diseases and is also a known risk factor for AMD2,15 where it is known to be associated with elevated inflammatory factors.16 The use of the body mass index (BMI) is considered to be a less reliable indicator of abdominal obesity than the waist-hip ratio (WHR).17 It has been demonstrated that a raised WHR is associated with a greater risk of AMD progression.18,19 Adipose tissue is a storage site for carotenoids, and the implication follows that, the greater the bodyweight, the fewer carotenoids will be available to supply the macula.20,21 This has implications for the argument promoting eye care practitioners becoming involved in advising obese patients. To further strengthen the argument, there is also evidence that a reduction in the WHR is associated with decreased odds of AMD developing.19
Diet and Diabetic Retinopathy
The conventional approach to the management of type II diabetes mellitus focuses on maintaining tight control of blood glucose levels, the aim being to reduce microvascular complications which include diabetic retinopathy (figure 2). This approach has recently been under question.22 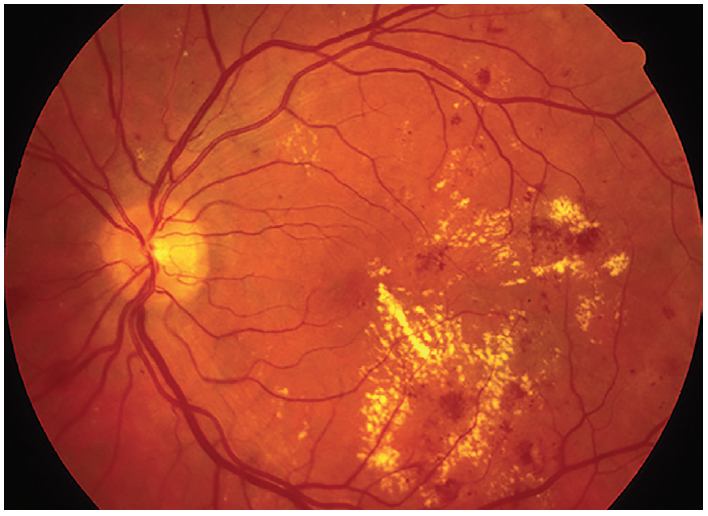 Figure 2: Diabetic retinopathy; a common cause of sight loss. But is pharmacological glycaemic control the best way to avoid this?
Figure 2: Diabetic retinopathy; a common cause of sight loss. But is pharmacological glycaemic control the best way to avoid this?
The use of medication to maintain glycaemic control may itself also cause a number of adverse side effects.23 Changes in lifestyle, in fact, may be as effective as medication but without the side effects.1 Improved glycaemic control through diets formulated specifically for diabetics are well known, as is the finding that plant-based diets (with abundant fruit, vegetables, whole grains, beans, nuts and seeds) have a superior effect in reducing the HbA1c measurement (a longer-term indication based on glucose bound to haemoglobin).24,25 Adequate physical activity, adopted as a lifestyle change, along with the normalisation of body weight, decreases the chronic inflammatory reaction of the body26,27 and improves HbA1C levels comparable to those achieved through medication.22,23
A Brief Review of the Gut Microbiota
The common link between inflammation and obesity, as well as other chronic diseases, may well reside in the gastrointestinal (gut) microbiome,28,29 where the microbiota diversity is known to be affected. Although covered in detail in a previous article (Optician 25.9.20), a brief review of the gut microbiome may be helpful.
The normal human gut microbiota consists predominantly of two main phyla; Bacteroides and Firmicutes. Bacteroides have a mainly positive influence when retained in the gut,30 while an overabundance of Firmicutes may have a less positive effect on health.31 Individuals classed as obese tend to have a higher Firmicutes/Bacteroides (F/B) ratio.32 Maintaining the right proportion of strains of Bacteroides and Firmicutes is therefore important, particularly when it comes to managing obesity and its associated metabolic conditions.33
The majority of the microbiota for the entire body resides in the gastrointestinal tract which is home for almost 70% of the human body’s immune system and they play an active role in modulating the immune response. Thus, it is not surprising that a disruption of the intestinal microbiota, known as intestinal dysbiosis, has been associated with various diseases known to have an inflammatory component.34
The intestine has a multi-layered mucous lining covering the surface. This allows the majority of gut bacteria to be kept at a safe distance from the epithelial cells of the intestinal wall. Thus, disruption of the mucus-bacteria interaction has the potential to promote diseases associated with gut inflammation or dysbiosis.35 This is typically associated with an increased F/B ratio and results in a thinning of the intestinal mucus layer and disruption of the gut/vascular barrier. This allows for the movement, or translocation, of gut commensal bacteria and bacterial products into the circulation where they travel to other tissues to induce pathological inflammation (figure 3).36
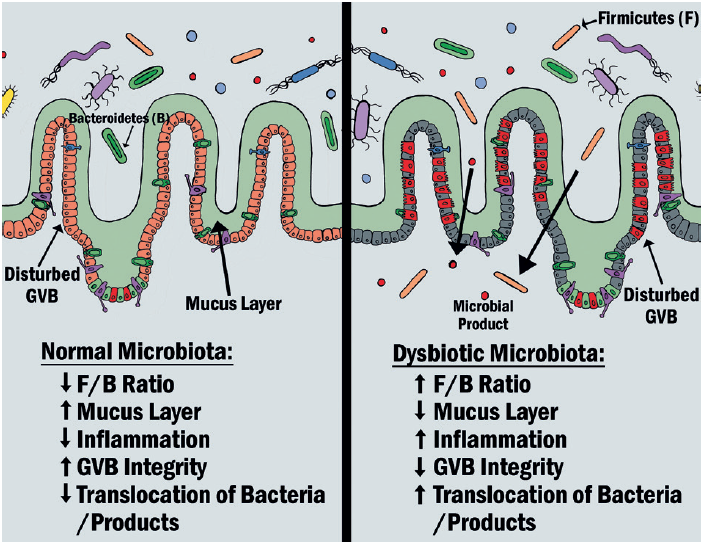 Figure 3: The effect of gut dysbiosis compared with a normal gut microbiota36
Figure 3: The effect of gut dysbiosis compared with a normal gut microbiota36
Antibiotics and the Gut
The role of antibiotics in impacting the microbiota is worth considering at this stage. Broad-spectrum antibiotics primarily reduce the number of pathogenic bacteria or eliminate them altogether. However, due to their broad spectrum of action, beneficial microbes can also be lost resulting in gut dysbiosis.37,38 During the period of antibiotic treatment, bacterial diversity and abundance decreases significantly, resulting in an altered F/B ratio. Depending on which bacteria are affected, the number of short chain fatty acids (SCFAs), produced by some microbiota and known to modulate inflammation, will be reduced, so creating a pro-inflammatory environment.38,39 Also associated with antibiotic treatment is an in surge of antibiotic-resistant bacterial strains, selective microbiota strains being eliminated and thereby giving antibiotic-resistant strains a growth advantage.38 Each class of antibiotics has different properties, resulting in different patterns of alteration to the microbiome composition.39 These effects can last from around six weeks40 to up to two years post-treatment.41 The use of prebiotics,42 probiotics and faecal microbiota transplantation (FMT) shows promise as strategies to combat antibiotic induced gut dysbiosis.38,43
Influence of Different Diets on Disease Risk
Diet is a particularly important factor in determining the microbiota composition of the gut. A variety of processed food contains detergent-like emulsifiers that negatively impact the mucous layer. Likewise, certain food and diet patterns exert a similar effect (see figure of 4). Thus, vegans, vegetarians, and omnivores have distinct microbiomes.42
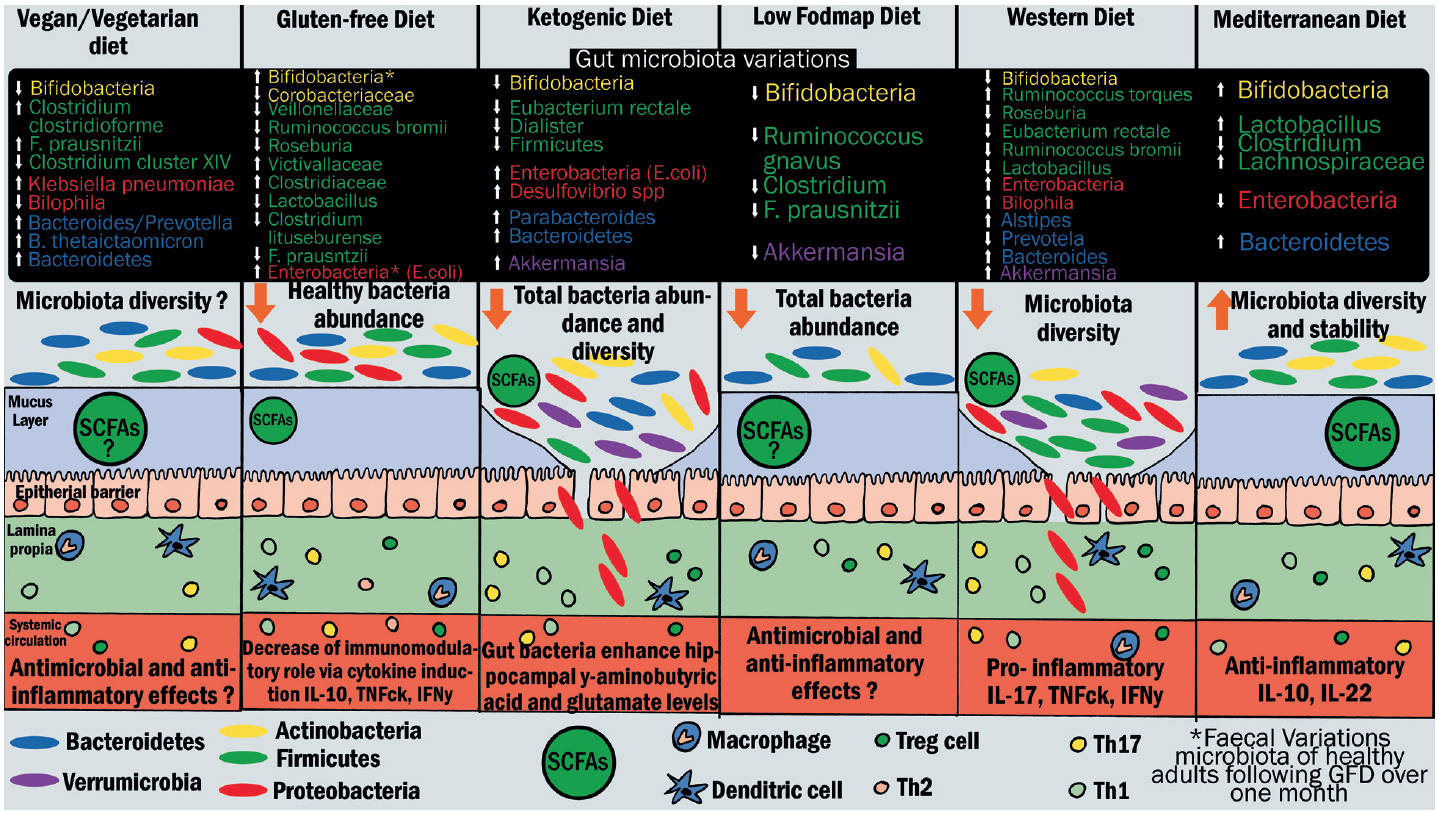 Figure 4: Effects of different types of diet on gut microbiota, mucus layer, and immune cells. Bacteria species variations are indicated in rectangular frames. The arrows pointing up or down respectively indicate an increase or decrease of bacteria abundance. Each colour of the rectangular frames represents one phylum: yellow for Actinobacteria, green for Firmicutes, red for Proteobacteria, blue for Bacteroides, and purple for Verrumicrobia. In the illustration of the intestinal epithelium, oval shapes represent microbiota. Each colour represents one phylum. Abbreviations: FODMAP: fermentable oligo-, di-, mono-saccharides, and polyols; GFD: gluten-free diet; SCFAs: short-chain fatty acids (adapted from Rinanella44)
Figure 4: Effects of different types of diet on gut microbiota, mucus layer, and immune cells. Bacteria species variations are indicated in rectangular frames. The arrows pointing up or down respectively indicate an increase or decrease of bacteria abundance. Each colour of the rectangular frames represents one phylum: yellow for Actinobacteria, green for Firmicutes, red for Proteobacteria, blue for Bacteroides, and purple for Verrumicrobia. In the illustration of the intestinal epithelium, oval shapes represent microbiota. Each colour represents one phylum. Abbreviations: FODMAP: fermentable oligo-, di-, mono-saccharides, and polyols; GFD: gluten-free diet; SCFAs: short-chain fatty acids (adapted from Rinanella44)
Even a short-term change of diet can alter the microbial community structure.42 A gluten-free diet (GFD) is the most commonly adopted specialised diet worldwide and it has been shown that just 13 weeks of a GFD has a significant effect on the microbiome diversity.45
Recent attention to diet quality as it pertains to diabetes prevention has focused on the reduction of refined sugar consumption. One serving per day of sugar-sweetened beverages (typically, soft drinks) has been associated with a 25% increased risk of diabetes.1 Alongside a reduction in refined sugar consumption, eating less meat has also been shown as beneficial in reducing the prevalence of diabetes mellitus (DM). Also, processed meat has been associated with increased risk of developing type II diabetes.46 Substituting as little as 5% of the calories from meat with those from plant protein can reduce the risk of DM by 23%.47 It has been proposed that a reduction in processed meats as well as other harmful dietary factors, such as partially hydrogenated vegetable oils, high-sodium foods, and refined grains, starches, and sugars, is likely to produce the greatest net benefits for both individual and population health.46
A Note on Processed Foods
Processed, nutrient-deficient, food consists primarily of four constituents: refined sugar, refined white wheat flour, polyunsaturated vegetable oils, and artificially produced trans-fats. These have formed the bulk (63%) of the American diet for decades, a diet that is also largely lacking in vitamins and minerals.48 Processed food has a higher association with AMD.49,50 Prevention of macular degeneration, as well as treatment for existing AMD, should include eliminating from the diet the four food groups currently expected to be causative: refined white flour, refined sugars, polyunsaturated fatty acids containing vegetable oils, and artificially created trans fats.48
The average Western diet contains a high level of processed food, a high level of animal-based foodstuffs (25%) and low levels of plant-based food sources (12%).51
This combination promotes inflammatory processes in our bodies which are known to be drivers of chronic diseases including those found within the eye. This presents an opportunity for diet modification and the use of bioactive ingredients52 in an effort to reduce the progression of such diseases, including AMD.50,53
The microbiota in patients with AMD differs from that of those who do not.54 A large percentage of AMD patients do not have any identified genetic predisposition for the disease, implying the significant influence of environmental factors. They do also show increased complement activity, despite no obvious genetic variation or predisposition.55 It has been proposed that the profile of the bacteria in the gut could promote over-activation of the complement cascade,56 and indeed an increase in the F/B ratio is observed in the microbiota of AMD patients resulting in a pro-inflammatory state.54
The consumption of a high-glycaemia (HG) diet has been attributed to many AMD features, whereas consumption of the lower-glycaemia (LG) diet has not. Switching from an HG to a LG diet late in life has been found to arrest AMD.54 There appears to be a functional interaction between dietary carbohydrates and gut microbiota. The HG diet promotes a greater amount of the Clostridiales order and has been associated with AMD features, whereas a low GI diet which promotes Bacteroides gut bacteria are associated with protection from end stage AMD. This suggests dietary intervention that may be useful in patients to arrest AMD.56
The Mediterranean Diet
What exactly constitutes a Mediterranean diet will vary, as the geographical region consists of 17 countries. However, it is predominantly based upon dietary habits from Southern Italy and Greece57 and broadly consists of nine components,57,58 coupled with a general higher preference for monounsaturated fats,59 as follows:
- A high intake of;
- Whole grains
- Fruit
- Vegetables
- Legumes
- Nuts
- Moderate amounts of;
- Fish
- Alcohol
- Low consumption of;
- Red and processed meat
- Dairy products
This is often represented as the Mediterranean diet food pyramid (figure 5).
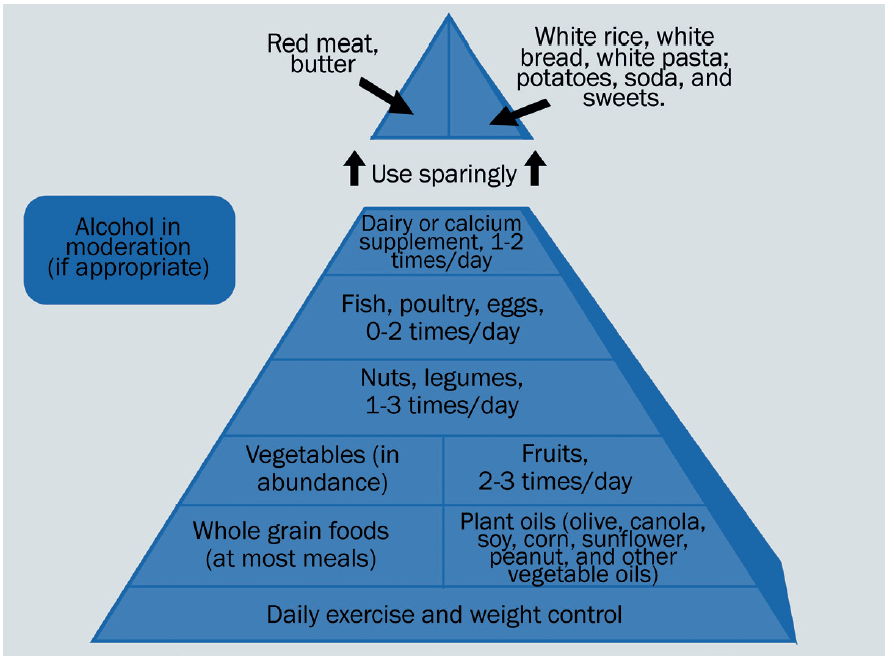 Figure 5: The Mediterranean diet food pyramid
Figure 5: The Mediterranean diet food pyramid
Adopting the Mediterranean diet has been shown to reduce the risk of cardiovascular events and chronic diseases, and so indirectly reduce the likelihood of ocular disease.60,61 Indeed, adopting a Mediterranean diet may be of benefit to all levels of AMD severity,58,62 having been linked to lower rates of early AMD, reduced drusen size63 and progression to late AMD.63,64 This would suggest it is never too early or too late to adopt a Mediterranean diet.58
Story So Far
I hope this first in our series has offered an overview of lifestyle factors for which there is good evidence as to their influence on both systemic and general health, with a particular focus on dietary influences. This reflects a good proportion of the influences that have been summarised by Richer65 and can be seen in figure 6. For an overall summary of healthy diet options, see table 1.
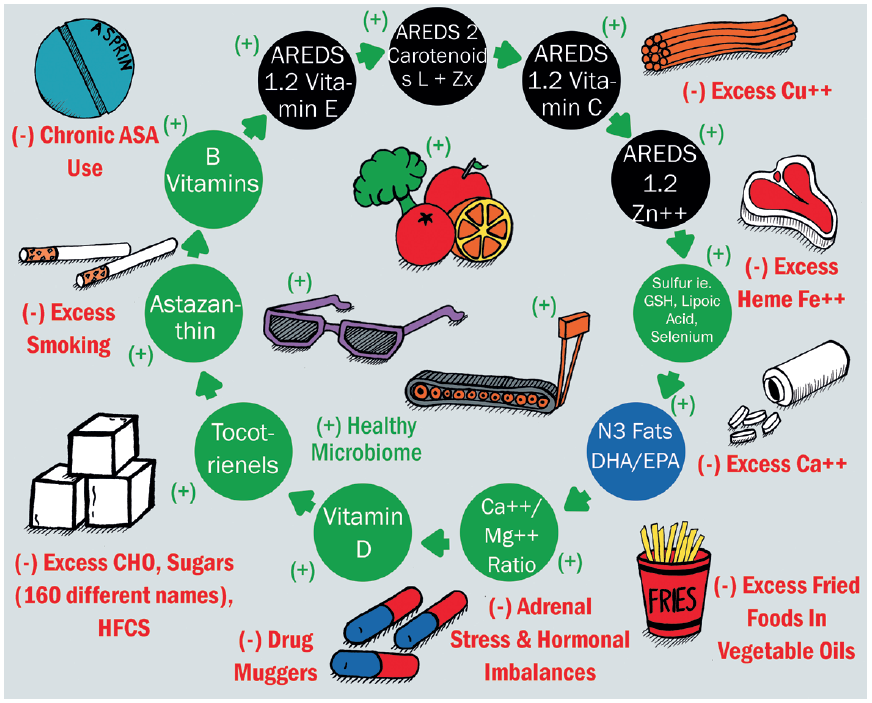 Figure 6: Positive and negative health habits and nutritional factors for lifetime ocular health65
Figure 6: Positive and negative health habits and nutritional factors for lifetime ocular health65
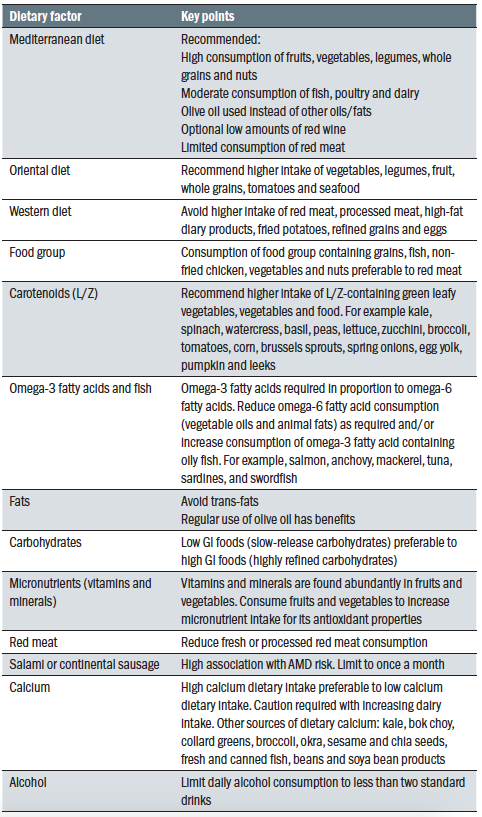 Table 1: Some key points about different diets and dietary components
Table 1: Some key points about different diets and dietary components
Preventing degenerative diseases through a blend of the frequent and regular monitoring of eye and vision health, coupled with educating patients that disease processes occur not just as a function of age, but through unhealthy lifestyle choices. These can be addressed through wellness and nutritional strategies.66
In the next in this series we will take a closer look at some specific nutrient influences and focus on macular degeneration.
Dr Rohit Narayan is a therapeutic optometrist and Visiting Clinician at Aston University.
References
- Bodai, BI, Nakata, TE, Wong, WT, Clark, DR, Lawenda, S, Tsou, C, Liu, R, Shiue, L, Cooper, N, Rehbein, . and Ha, BP, 2018. Lifestyle medicine: A brief review of its dramatic impact on health and survival. The Permanente Journal, 22.
- Adams, MK, Simpson, JA, Aung, KZ, Makeyeva, GA, Giles, GG, English, DR, Hopper, J, Guymer, RH, Baird, PN and Robman, LD, 2011. Abdominal obesity and age-related macular degeneration. American journal of epidemiology, 173(11), pp.1246-1255
- Kauppinen A, Paterno JJ, Blasiak J, Salminen A, Kaarniranta K. Inflammation and its role in age-related macular degeneration. Cell Mol Life Sci. 2016;73(9):1765-1786
- He, MS, Chang, FL, Lin, HZ, Wu, JL, Hsieh, TC and Lee, YC, 2018. The association between diabetes and age-related macular degeneration among the elderly in Taiwan. Diabetes care, 41(10), pp.2202-2211
- Hyman, MA, Ornish, D and Roizen, M, 2009. Lifestyle medicine: treating the causes of disease. Alternative Therapies in Health & Medicine, 15(6), p.12
- Micha, R, Peñalvo, JL, Cudhea, F, Imamura, F, Rehm, C. and Mozaffarian, D., 2017. Association between dietary factors and mortality from heart disease, stroke, and type 2 diabetes in the United States. Jama, 317(9), pp.912-924
- Hunter, P, 2012. The inflammation theory of disease: The growing realization that chronic inflammation is crucial in many diseases opens new avenues for treatment. EMBO reports, 13(11), pp.968-970
- Kopp, W, 2019. How western diet and lifestyle drive the pandemic of obesity and civilization diseases. Diabetes, metabolic syndrome and obesity: targets and therapy, 12, p.2221
- Schwingshackl, L, Morze, J and Hoffmann, G, 2020. Mediterranean diet and health status: Active ingredients and pharmacological mechanisms. British Journal of Pharmacology, 177(6), pp.1241-1257
- Gregor, MF and Hotamisligil, GS, 2011. Inflammatory mechanisms in obesity. Annual Review of Immunology, 29, pp.415-445
- Solas, M, Milagro, FI, Ramírez, MJ and Martínez, JA, 2017. Inflammation and gut-brain axis link obesity to cognitive dysfunction: plausible pharmacological interventions. Current Opinion in Pharmacology, 37, pp.87-92
- Rogero, MM and Calder, PC, 2018. Obesity, inflammation, toll-like receptor 4 and fatty acids. Nutrients, 10 (4), p.432
- Carrillo, JLM, Campo, JOMD, Coronado, OG, Gutiérrez, PTV, Cordero, JFC and Juárez, ., 2018. Adipose tissue and inflammation. Adipose Tissue, p.93
- Zatterale, F, Longo, M, Naderi, J, Raciti, GA, Desiderio, A, Miele, C and Beguinot, F, 2020. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Frontiers in Physiology, 10, p.1607
- Singh, N, Srinivasan, S, Muralidharan, V, Roy, R, Jayprakash, V and Raman, R, 2017. Prevention of age-related macular degeneration. The Asia-Pacific Journal of Ophthalmology, 6(6), pp.520-526
- Heesterbeek, TJ, Lorés-Motta, L, Hoyng, CB, Lechanteur, YT and den Hollander, AI, 2020. Risk factors for progression of age-related macular degeneration. Ophthalmic and Physiological Optics, 40(2), pp.140-170
- Garn, SM, Leonard, WR, Hawthorne, VM. 1986. Three limitations of the body mass index, The American Journal of Clinical Nutrition, Volume 44, Issue 6, December 1986, Pages 996–997
- Seddon, JM, Cote, J, Davis, N and Rosner, B, 2003. Progression of age-related macular degeneration: association with body mass index, waist circumference, and waist-hip ratio. Archives of Ophthalmology, 121(6), pp.785-792
- Peeters, A, Magliano, DJ, Stevens, J, Duncan, BB, Klein, R and Wong, TY, 2008. Changes in abdominal obesity and age-related macular degeneration: the Atherosclerosis Risk in Communities Study. Archives of Ophthalmology, 126(11), pp.1554-1560
- Johnson, EJ, Neuringer, M, Russell, RM, Schalch, W and Snodderly, DM, 2005. Nutritional manipulation of primate retinas, III: effects of lutein or zeaxanthin supplementation on adipose tissue and retina of xanthophyll-free monkeys. Investigative Ophthalmology & Visual Science, 46(2), pp.692-702
- Obana, A, Gohto, Y, Nakazawa, R, Moriyama, T, Gellermann, W and Bernstein, PS, 2020. Effect of an antioxidant supplement containing high dose lutein and zeaxanthin on macular pigment and skin carotenoid levels. Scientific Reports, 10(1), pp.1-12
- Rodríguez-Gutiérrez, R and Montori, VM, 2016. Glycemic control for patients with type 2 diabetes mellitus: our evolving faith in the face of evidence. Circulation: Cardiovascular Quality and Outcomes, 9(5), pp.504-512
- American Diabetes Association, 2017. 8. Pharmacologic approaches to glycemic treatment. Diabetes Care, 40(Supplement 1), pp.S64-S74
- Barnard, ND, Cohen, J, Jenkins, DJ, Turner-McGrievy, G, Gloede, L, Jaster, B, Seidl, K, Green, AA and Talpers, S, 2006. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care, 29(8), pp.1777-1783
- Wolfram, T and Ismail-Beigi, F, 2011. Efficacy of high-fiber diets in the management of type 2 diabetes wmellitus. Endocrine Practice, 17(1), pp.132-142
- Knowler, WC et al. Diabetes Prevention Program Research Group, 2002. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New England journal of medicine, 346(6), pp.393-403
- Wu, H, Tremaroli, V, Schmidt, C, Lundqvist, A, Olsson, LM, Krämer, M, Gummesson, A, Perkins, R, Bergström, G and Bäckhed, F, 2020. The Gut Microbiota in Prediabetes and Diabetes: A Population-Based Cross-Sectional Study. Cell Metabolism, 32(3), pp.379-390
- Leigh, SJ and Morris, MJ, 2020. Diet, inflammation and the gut microbiome: Mechanisms for obesity-associated cognitive impairment. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, 1866(6), p.165767
- Bailey, MA and Holscher, HD, 2018. Microbiome-mediated effects of the Mediterranean diet on inflammation. Advances in Nutrition, 9(3), pp.193-206
