In the first part of this series, we established a link between nutrition and health and underlined why this is relevant to the eye care practitioner. In this article, I will take a closer look at the role of individual nutrients in maintaining eye health and focus in particular upon the healthy macula.
Dietary Differences
Food components impact the richness and diversity of the gut microbiota. A high intake of animal proteins, saturated fat, refined sugar and salt appears to promote the growth of pathogenic bacteria to the detriment of beneficial bacteria, thereby leading to potential alterations of the intestinal barrier. The consumption of complex polysaccharides and plant protein appears to promote beneficial bacteria, so stimulating short-chain fatty acid (SCFA) production. Moreover, omega-3, polyphenols and micronutrients appear to have the potential to confer health benefits via modulation of the gut microbiota.1
Dietary habits can strongly influence gut microbiota composition.1 The Western diet is associated with a higher intake of red and processed meat, high-fat dairy products, fried potatoes, refined grains and eggs may reduce gut microbial diversity in terms of phyla and genus leading to dysbiosis, alteration of barrier function and permeability, and abnormal activation of immune cells. This ultimately leads to high incidences of chronic diseases, including increased odds for both early and advanced AMD.2,3
The Mediterranean diet (figure 1) is typically characterised by a high consumption of fruits, vegetables, legumes, whole grains and nuts; moderate consumption of fish, poultry and dairy; and a limited consumption of red meat. Olive oil is used instead of butter and low to moderate amounts of red wine may be consumed.3 Adherence to the Mediterranean diet optimally modulates microbiota diversity and stability as well as the regular permeability and activity of immune functions. This contributes to improved metabolic health4 as well as providing a protective mechanism in the progression of AMD.2,5,6
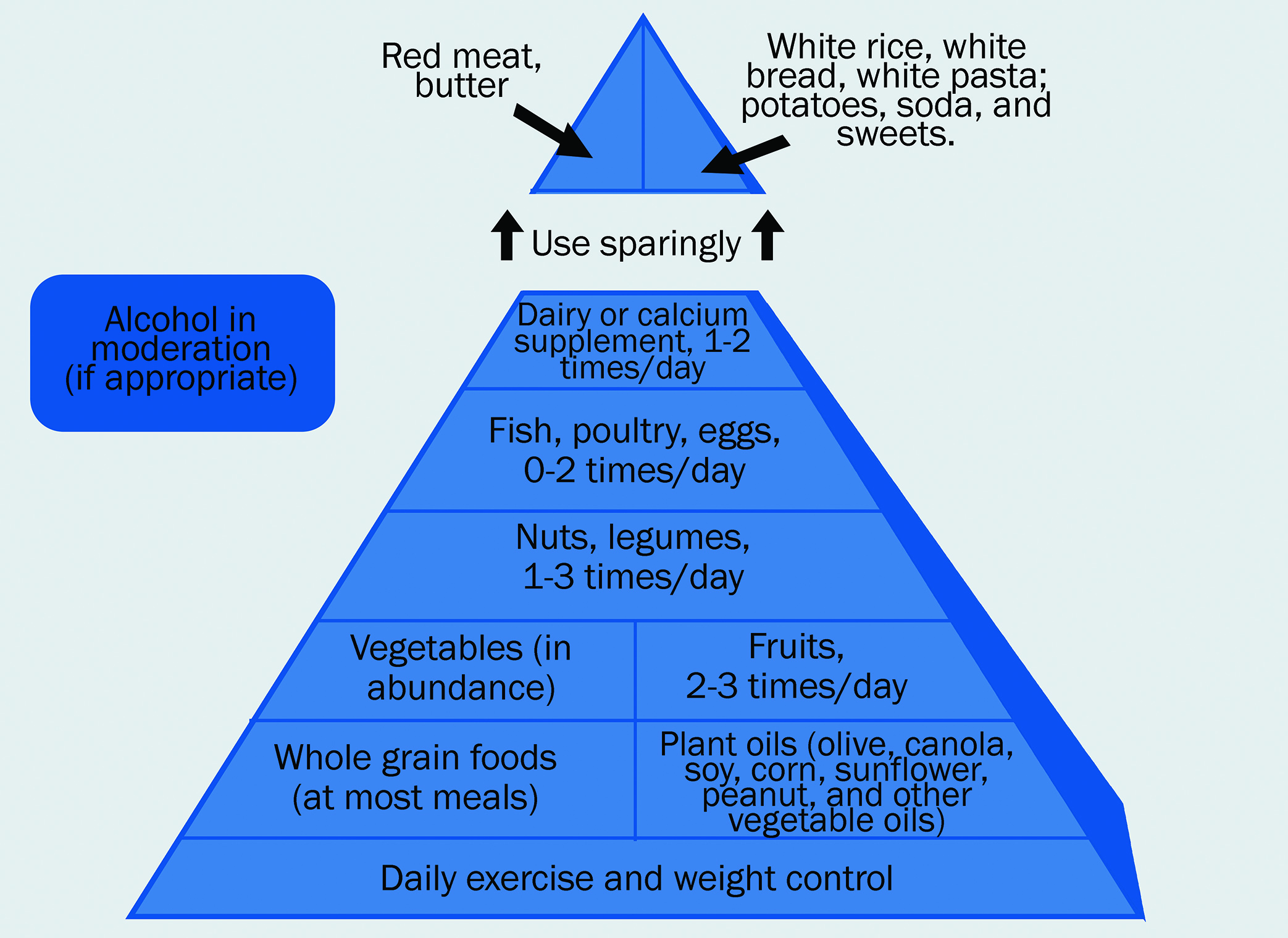 Figure 1: The Mediterranean diet
Figure 1: The Mediterranean diet
The Oriental diet pattern is similar to the Mediterranean diet, defined as having a higher intake of tofu, fruit, vegetables, legumes, whole grains, tomatoes and seafood.3 Other modified versions of the Mediterranean diet include the Prudent diet.7 Results for diets such as these have suggested a protective role in both early6 and advanced AMD.2
The role of dietary patterns offers a more holistic approach when considering the impact on the composition and function of the gut microbiota and general well-being.8 However, there is value in looking at nutrient components that may be of interest to the clinician.3,9 Nutrients can be grouped into macronutrients and micronutrients. We will now focus on these in a bit more detail.
Fatty acids
Macronutrients supply the body with energy and include carbohydrates, proteins and fats. Omega-3 fatty acids (DHA and EPA) are essential polyunsaturated fatty acids that are required for metabolism and must be obtained from the diet (figure 2). The three major types of omega-3 fatty acids are as follows:
- Alpha-linolenic acid (ALA)
- Docosahexaenoic acid (DHA)
- Eicosapentaenoic acid (EPA)
 Figure 2: Vegetable sources of omega-3 are rich in alpha-linolenic acid
Figure 2: Vegetable sources of omega-3 are rich in alpha-linolenic acid
All three are considered to have anti-inflammatory activity.10 ALA is found in seeds, nuts and vegetable oils. DHA and EPA are found in high concentration in fish oils and dark-meat fish, such as salmon, anchovy, mackerel, tuna, salmon, sardines and swordfish. DHA is present in retinal photoreceptors and synaptic membranes and is required for retinal function. A diet rich in omega-3 fatty acids is beneficial to reducing the development of intermediate and late AMD.11,12
Omega-6 in the diet comes mainly from vegetable oils and animal fats and is generally considered to be pro-inflammatory.13 Although some debate surrounds the ideal omega-3 to omega-6 ratio in a diet (1:5 or less is an accepted ratio3), the concept of simply decreasing the intake of omega-6 fatty acids to maintain the omega-3 to omega-6 ratio may be more meaningful than merely increasing the intake of omega-3 to counteract a high intake of Omega-6.3,14
The greatest benefit in terms of reducing the impact of AMD that can be linked with omega-3 intake appears to come from when it is taken as part of a combined intake of all three omega-3 fatty acids (ALA + DHA + EPA).15,16 The consumption of trans-fats, partially hydrogenated vegetable oils, should be kept to a minimum.15
Carbohydrates
Carbohydrates are groups of organic compounds present in food and living tissue as sugars, starch and fibre/cellulose. The digestive system breaks down carbohydrates into glucose, which can then be used by the body as energy.
The glycaemic index (GI) ranks carbohydrate-containing foods based on their effect on blood sugar levels over a period of two hours. Pure glucose is given the reference GI of 100. Examples of high-GI (>70) foods include white bread, potatoes, white rice, cereals, honey and refined sugar. Low-GI (<55) foods include whole fruit and vegetables, whole wheat bread, pasta, oats, bran, legumes, milk and yoghurt (see figure 3). A low GI diet is considered preferable.3
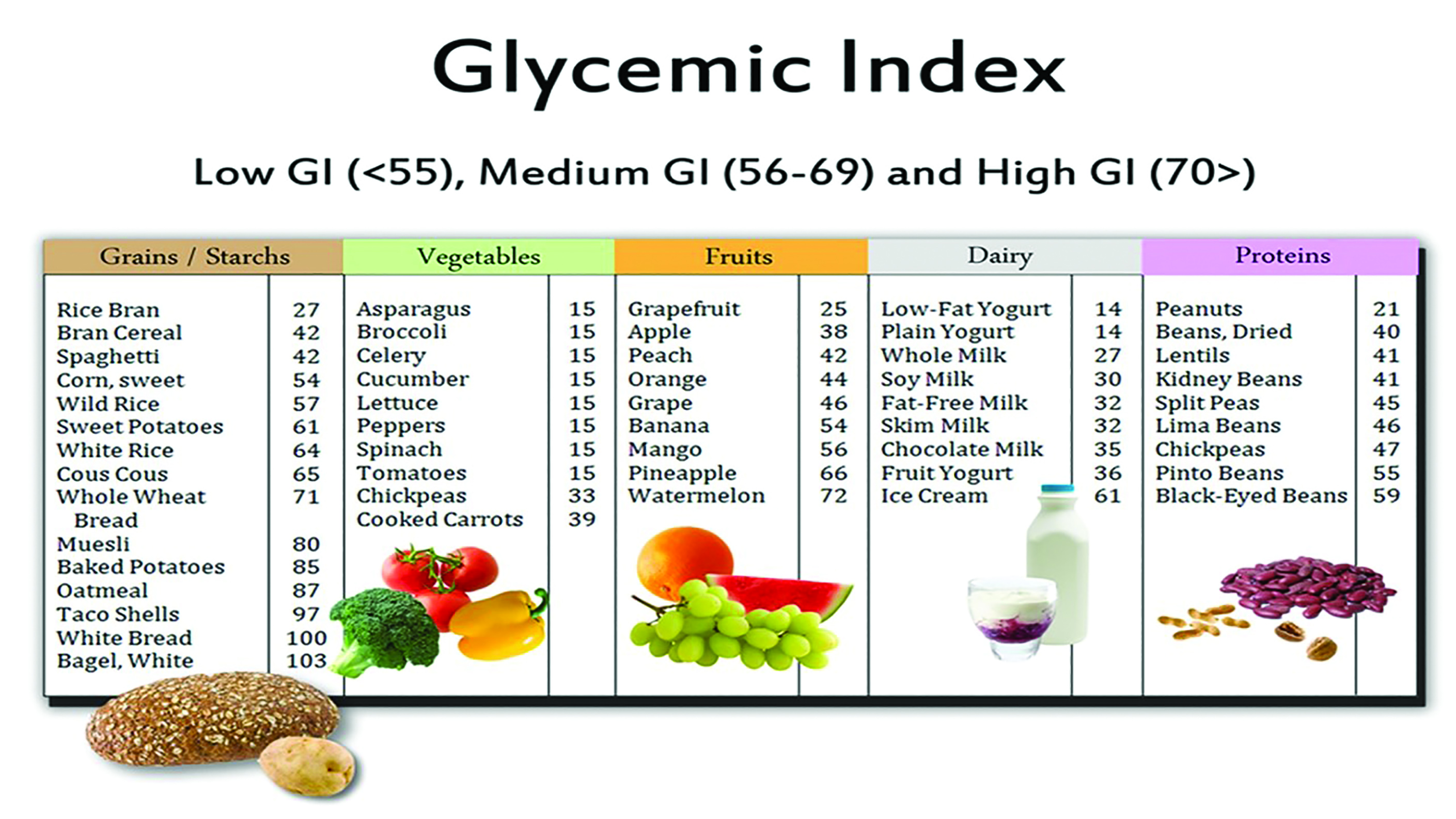 Figure 3: The glycaemic index and some common foodstuffs
Figure 3: The glycaemic index and some common foodstuffs
A low-GI diet reduces inflammatory processes (Pahwa 2019) when compared with a higher GI diet17 and has been recommended for minimising the impact of both early and late AMD.2,18
Micronutrients and AREDS
Micronutrients are required in small amounts by the body and include vitamins and minerals and have a profound impact on our general well-being. Their role in macular degeneration has been covered extensively19 and forms the foundation for the AREDS and AREDS2 formulation of supplements.20,21
AREDS was a large (4,757 patients) complex randomised clinical trial designed to evaluate the effect of higher than NRV (nutrient reference value, formerly known as recommended daily allowance, or RDA) dose supplements as follows:
- Vitamin C (500mg)
- Vitamin E (400 IU)
- Beta- carotene (15mg)
- With or without zinc (80mg)
- Copper (2mg)
The aim was to gauge their impact on the progression of AMD.23 Most of the patients were taking nutritional supplements at the time of the study enrolment and, to standardise this occurrence, a daily dose of a (Centrum) multivitamin and minerals tablet was provided. The patients were followed up for a mean period of 6.3 years.24
The results showed that treatment with zinc alone or in combination with antioxidants reduced the risk of progression to advanced AMD in patients of Categories 3 and 4 (meaning those patients with intermediate AMD showing extensive intermediate drusen in one or both eyes, one or more large drusen in at least one eye, or non-subfoveal geographic atrophy in one eye, or advanced AMD revealed as subfoveal geographic atrophy or a choroidal neovascular membrane in one eye). The AREDS results provide evidence that risk of progression in moderate, high risk AMD to the severe form of AMD can be reduced by up to 25% when taking a combination of high-dose antioxidants and zinc. The treatment effect persisted following five additional years of follow-up after the end of the clinical trial.24
The AREDS formula showed no beneficial effect for any other stage of AMD.24 Observational data from AREDS and other studies suggested that the increased dietary intake of lutein and zeaxanthin, and omega-3 long-chain polyunsaturated fatty acids (LCPUFAs) found in fish reduced the risk of late AMD.25-32
AREDS2 was designed to investigate whether other nutrients supplements, specifically lutein/zeaxanthin and omega-3 fatty acids in addition to the AREDS supplements, might further reduce the risk of progression to advanced AMD.33,34
The AREDS2 was a multicentre, phase three randomised, double-masked, placebo-controlled trial conducted between 2006 and 2012, which enrolled participants with either bilateral intermediate AMD, or advanced AMD in one eye. The primary randomisation included the daily AREDS supplement plus one of the following:
- Lutein (10mg)
- Zeaxanthin (2g)
- DHA/EPA (350/650mg)
- A combination of the above
- A placebo.
Table 1 summarises the AREDS2 formulation.
 Table 1: The AREDS2 formulation
Table 1: The AREDS2 formulation
The secondary randomisation consisted of the elimination of beta-carotene and/or lowering of zinc to 25mg instead of the original 80mg found in the AREDS formulation.24 The results, published in 2013, revealed that addition of lutein/ zeaxanthin, DHA/EPA or both to the AREDS1 formulation did not further reduce the risk of progression to advanced AMD in the primary analysis.24 As the design of AREDS2 was complex, any of the interventions need to perform 25% (or more) better than the AREDS1 formulation to reach statistical significance. This
represents a very high threshold, and it seems reasonable to question whether this is an achievable target for such a study. In addition, secondary analysis was performed regarding the effect of lutein/zeaxanthin, which showed effects in certain subgroups. Lutein/zeaxanthin was not related to increase in lung cancer incidence, unlike to beta-carotene that was associated with a rise in lung cancer in former smokers.
There are a number of limitations to the AREDS2 study that are important to note. Firstly, given that almost all patients included took the original (or modified) AREDS1 supplement, no real placebo group is available. Thus, the question as to whether supplementation with lutein/zeaxanthin (L/Z) or DHA/EPA alone is superior to placebo is still unanswered. In addition, it needs to be stated that, on average, the individuals participating the AREDS2 trial were mostly very well-nourished Caucasians. Whether this holds true for other populations or even is representative for the US population in general is uncertain. Furthermore, a large proportion of the participants took additional supplements such as Centrum, which makes the interpretation of the results even more difficult. In summary, the AREDS2 led to the recommendation that lutein/zeaxanthin may be considered as a replacement for beta-carotene. Due to the complex design and the fact that no real placebo group was included, several other questions, such as the efficacy of omega-3 fatty acids, remained unanswered.
Carotenoids
Of particular interest is the role of carotenoids in influencing AMD. Carotenoids can be considered as the antioxidant first line of defence35 and a biomarker for good health.36 Carotenoids are classified into carotenes and xanthophyls and both have positive effects on brain and ocular function.37,38
Lutein and zeaxanthin (L/Z) are xanthophyls (figure 4), found in the macular pigment located in the ganglion cells, cone axons and Mueller cells of the macula. Both L/Z have antioxidative properties and a protective effect by absorbing hazardous blue and ultraviolet light before it can reach the photoreceptors. L/Z are not synthesised in the body and must be obtained through diet. A third variation, meso-zeaxanthin, is synthesised in the retina from lutein.
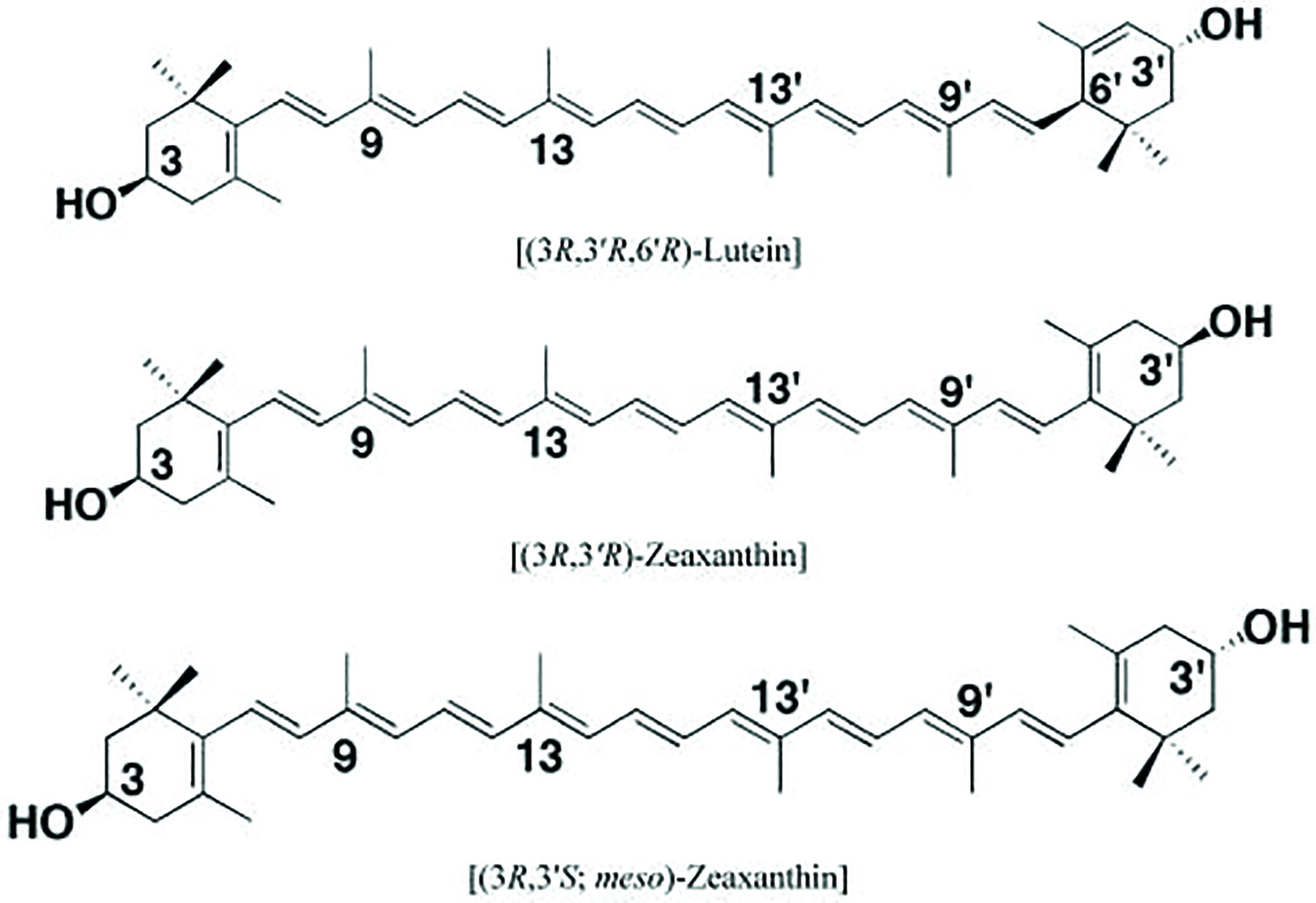 Figure 4: Molecular structure of retinal carotenoids
Figure 4: Molecular structure of retinal carotenoids
Foodstuffs rich in lutein and zeaxanthin are mainly dark-leafy vegetables, such as spinach and kale, and yellow/orange vegetables, such as carrots and bell peppers (figure 5). Table 2 shows some useful sources of L and Z. The intake of L/Z reduces the progression of late AMD.
 Figure 5: Carotenoid-rich foodstuffs
Figure 5: Carotenoid-rich foodstuffs
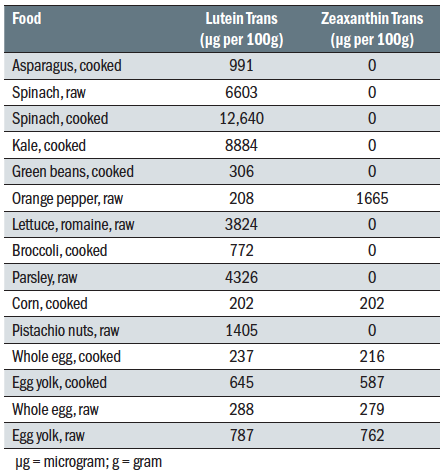 Table 2: Dietary sources of lutein and zeaxanthin
Table 2: Dietary sources of lutein and zeaxanthin
The benefits of lutein are not limited to the eye. Lutein has also been shown to be beneficial as a prebiotic, promoting the growth of ‘good bacteria’ and reducing other bacterial populations. Measuring ocular carotenoid levels would seem to be desirable and instruments exist to allow clinicians to make this assessment (figure 6). However, the subjective nature of many of these tests affects repeatability. There is, however, a correlation between skin, serum and macular carotenoid levels, opening up the possibility for the clinician to establish a baseline reading and future monitoring as needed.35,39,40
 Figure 6: The MPS II macular pigment screening device
Figure 6: The MPS II macular pigment screening device
The recommended intake of L/Z is usually stated as 10mg/day. However, most people, either with or without AMD, average a lot less in their diet, typically around 1.6mmg/day.41,42
The role of gastrointestinal (GI) health has an important role to play in the levels of L/Z absorbed. Generally, L/Z absorption occurs in the gut via a lipid substrate, after which they are transported to the liver. After this, L/Z are transported throughout the bloodstream as part of a lipid-protein complex, preferentially linked to high density lipoproteins (HDLs).42,43 The implication follows, therefore, that low HDL levels will limit the transport of L/Z to the retina, regardless of how much is consumed. The elevation of HDL is therefore desirable and can be achieved through medications, such as statins,44 and exercise.45,46
Adipose tissue competes with the retina for lutein,40,47 further depleting the amount of L/Z that reaches the retina. Obesity plays a role in L/Z absorption, and is an important topic to discuss with patients for whom L/Z would be beneficial.40
Age & Medication
The composition of the microbiota changes with chronological (simple passing of time) age, becoming more diverse.48 However, when we take into account biological aging, the overall richness decreases, while certain bacterial taxa associated with unhealthy ageing thrive. This sets up a compromise in gut homeostasis and a resultant gut dysbiosis (figure 7).49 There is no set age at which this occurs, and in most cases, it appears to occur gradually.48 These changes in GI microbiota appear to correlate more closely with biological age than chronological age.48,50
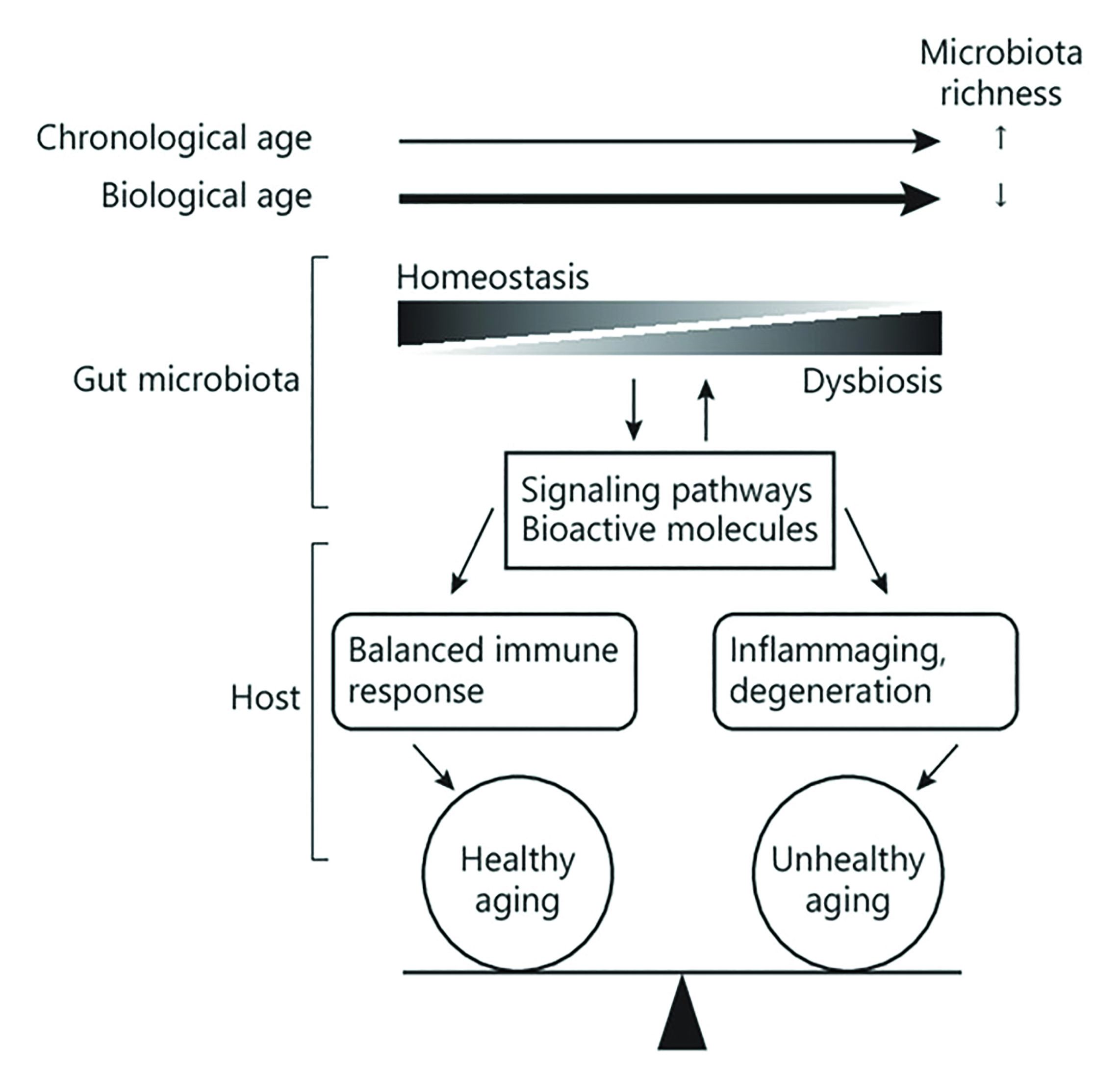 Figure 7: Influence of biological and chronological age on microbiota
Figure 7: Influence of biological and chronological age on microbiota
As one ages, a variety of processes, including reduced stomach hydrochloric acid secretion and loss of ‘good’ (Bifidobacteria) bacteria in the gut,1 occur and diminish the ability to breakdown and absorb nutrients. This has an important impact on the ability of the body to absorb specific nutrients59 and opens up the possibility of discussing the role of pre/probiotics with our patients. The choice of food consumed is important, as processed food is generally nutrient deficient due in part to reduced bioavailability.
Ageing can result in patients becoming malnourished and maybe linked to ‘breaking point’ events that disrupt lifestyle and eating habits. Having an awareness of social breaking points (such as retirement, loss of partner) as well as medical breaking points (new diagnosis of dementia, progression of existing disease, medications) may result in newly adopted and inappropriate eating habits and mark the beginning of an unbalanced diet. The elderly population with visual impairment appear to have greater challenges achieving a healthy diet and suffer more obesity and undernourishment, so exacerbating the situation.63
The issue of appropriate nutrition is compounded when one considers that medications have the potential to interfere with nutrient absorption (figure 8) and be an example of a medical ‘breaking point’ event that clinicians should be aware of.
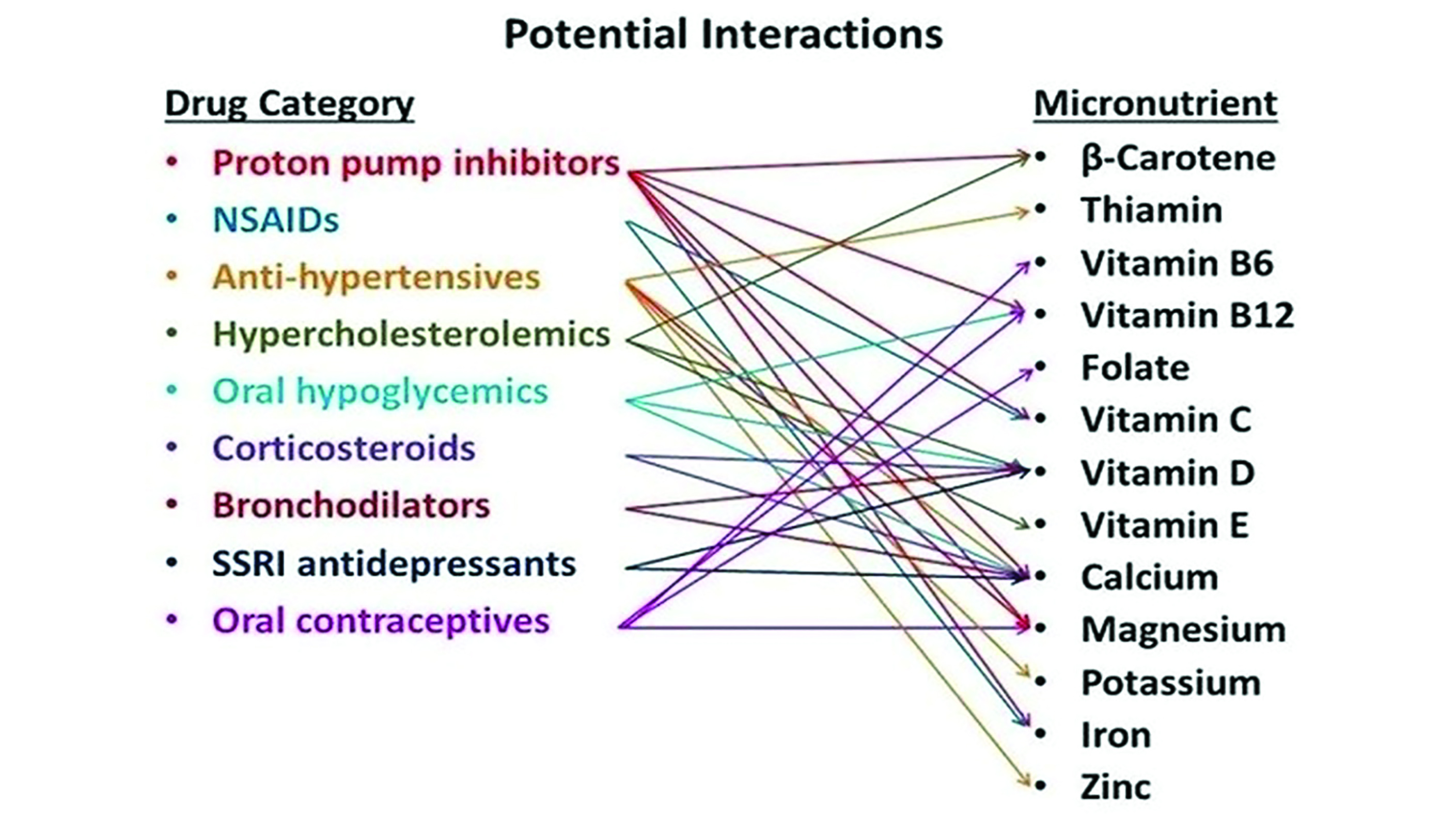 Figure 8: Some potential drug and nutrient interactions64
Figure 8: Some potential drug and nutrient interactions64
Zinc is an essential trace element and plays a key role in many cellular processes.16 In developed countries, up to 30% of elderly people are zinc deficient and it has recognised that zinc may have an important role in degenerative disorders of ageing,51 as a regulator of the antioxidant pathway and as an anti-inflammatory agent and has been implicated in AMD.52,53
The Use of Genetic Testing
As to whether genetic testing for AMD is a research or a clinical tool, the debate continues. In addition, the debate rages on about whether genotyping should be standard care for AMD patients taking antioxidants and zinc.54 Research suggests that zinc in the AREDS formula actually increases progression risk in some individuals, primarily those with specific genetic variants.55 However, others feel that the results of such studies are mixed, instead suggesting that patients should be offered antioxidants with zinc without consideration of their genetic profile, provided they meet the criteria for supplementation to help prevent AMD.57 ‘Genetic testing is important for research, but not for management at this point,’ says Dr Emily Chew, who ran both the AREDS1 and AREDS2 studies. ‘I would love to be able to use genetic testing to personalise treatment, but we just don’t have the treatment or the genetic data to suggest that the patients respond differently because of genetic interactions.’54
Resveratrol
Resveratrol is a polyphenol mainly found in grape seed and skin and some other fruit berries.57 It is known to possess a variety of positive influences, including antioxidant, anti-angiogenic, anti-cancer and anti-inflammatory effects.58-60 Resveratrol has been shown to exert beneficial effects in AMD.61,62
Meat
The consumption of different types of meat appears to have an impact on AMD risk. A diet high in red meat, particularly when processed in a form such as salami or continental sausage, increases the risks of developing late AMD. On the other hand, chicken reduces the risk. The exact mechanism is not clear. The consumption of red and processed meat has been linked with increased risk of cancers,66,67 and this has led the International Agency for Research on Cancer to conclude that processed meat and red meat are ‘carcinogenic, or probably carcinogenic (respectively) to humans,’68 where the act of preparation causes damage to protein DNA.
Alcohol
It has been suggested that, to reduce the chance of early AMD, alcohol consumption should be limited to less than two standard drinks per day.3,69
Concluding Thoughts
With the ever-growing evidence of specific dietary influences upon macular health, it is somewhat bewildering for an eye care professional to feel able to offer complete and accurate advice. That said, a useful approach in the form of a flow chart has been developed by researchers at Aston University and is represented in figure 9.
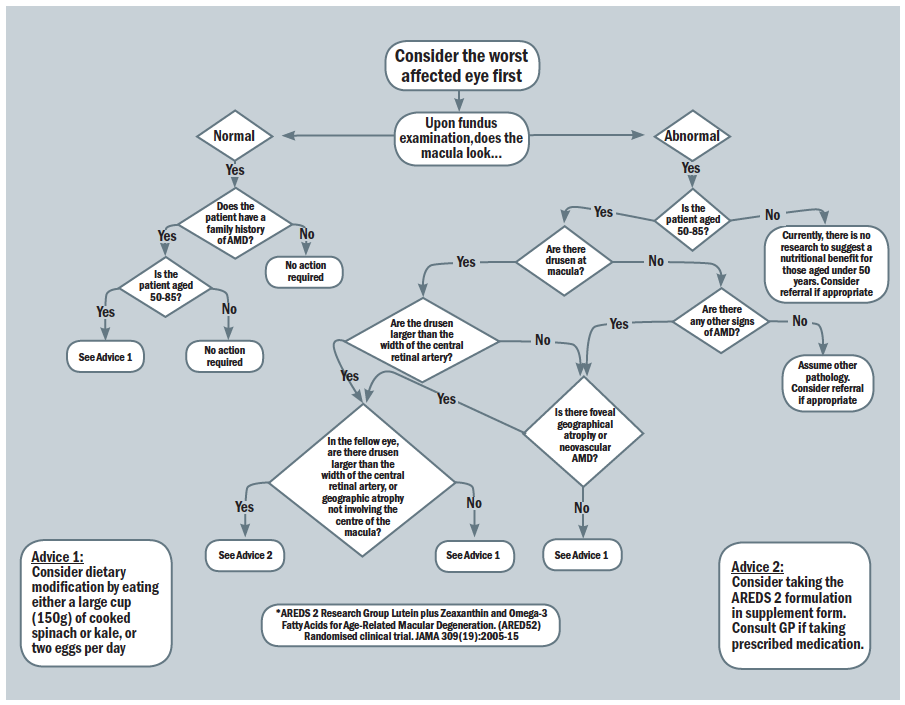 Figure 9: Nutrition advice for people with, or at risk of, AMD70,71
Figure 9: Nutrition advice for people with, or at risk of, AMD70,71
Dr Rohit Narayan is a therapeutic optometrist and visiting clinician at Aston University.
- In the next in this series, our attention will turn to the use of dark adaptation as a means of assessing macular function.
References
- Rinninella, E, Cintoni, M, Raoul, P, Lopetuso, LR, Scaldaferri, F, Pulcini, G, Miggiano, GAD, Gasbarrini, A and Mele, MC, 2019. Food components and dietary habits: Keys for a healthy gut microbiota composition. Nutrients, 11(10), p.2393
- Chiu, CJ, Chang, ML, Zhang, FF, Li, T, Gensler, G, Schleicher, M and Taylor, A, 2014. The relationship of major American dietary patterns to age-related macular degeneration. American Journal of Ophthalmology, 158(1), pp.118-127
- Chapman, NA, Jacobs, RJ and Braakhuis, AJ, 2019. Role of diet and food intake in age-related macular degeneration: a systematic review. Clinical & Experimental Ophthalmology, 47(1), pp.106-127
- Bailey, MA and Holscher, HD, 2018. Microbiome-mediated effects of the Mediterranean diet on inflammation. Advances in Nutrition, 9(3), pp.193-206
- Islam, FMA, Chong, EW, Hodge, AM, Guymer, RH, Aung, KZ, Makeyeva, GA, Baird, PN, Hopper, JL, English, DR, Giles, GG and Robman, LD, 2014. Dietary patterns and their associations with age-related macular degeneration: the Melbourne collaborative cohort study. Ophthalmology, 121(7), pp.1428-1434
- Merle, BM, Silver, RE, Rosner, B and Seddon, JM, 2015. Adherence to a Mediterranean diet, genetic susceptibility, and progression to advanced macular degeneration: a prospective cohort study. The American journal of clinical nutrition, 102(5), pp.1196-1206
- Hu, FB and Willett, WC, 2002. Optimal diets for prevention of coronary heart disease. Jama, 288(20), pp.2569-2578
- Sonia Ramos, María Ángeles Martín. Impact of diet on gut microbiota. Current Opinion in Food Science, Volume 37, 2021, pp 83-90
- Hart, KM, Abbott, C, Ly, A, Kalff, S, Lek, JJ, Milston, R, Page, G, Robertson, B and Ayton, L, 2020. Optometry Australia’s chairside reference for the diagnosis and management of age-related macular degeneration. Clinical and Experimental Optometry, 103(3), pp.254-264
- Calder, PC, 2006. n− 3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. The American Journal of Clinical Nutrition, 83(6), pp.1505S-1519S
- Chong, EW, Kreis, AJ, Wong, TY, Simpson, JA and Guymer, RH, 2008. Dietary ω-3 fatty acid and fish intake in the primary prevention of age-related macular degeneration: a systematic review and meta-analysis. Archives of Ophthalmology, 126(6), pp.826-833
- Chew, EY, 2020. Age-related Macular Degeneration: Nutrition, Genes and Deep Learning—The LXXVI Edward Jackson Memorial Lecture. American Journal of Ophthalmology, 217, pp.335-347
- Innes, JK and Calder, PC, 2018. Omega-6 fatty acids and inflammation. Prostaglandins, Leukotrienes and Essential Fatty Acids, 132, pp.41-48
- DiNicolantonio, JJ and O’Keefe, JH, 2018. Importance of maintaining a low omega-6 to omega-3 ratio for reducing inflammation. Open Heart, 5 (2)
- Chong, EWT, Simpson, JA, Robman, LD, Hodge, AM, Aung, KZ, English, DR, Giles, GG and Guymer, RH, 2009. Red meat and chicken consumption and its association with age-related macular degeneration. American Journal of Epidemiology, 169(7), pp.867-876
- Heesterbeek, TJ, Lorés-Motta, L, Hoyng, CB, Lechanteur, YT and den Hollander, AI, 2020. Risk factors for progression of age-related macular degeneration. Ophthalmic and Physiological Optics, 40(2), pp.140-170
- Pahwa R, Goyal A, Bansal P, Jialal I. Chronic Inflammation. In: StatPearls. StatPearls Publishing, Treasure Island; 2020
- Kaushik, S, Wang, JJ, Flood, V, Tan, JSL, Barclay, AW, Wong, TY, Brand-Miller, J and Mitchell, P., 2008. Dietary glycemic index and the risk of age-related macular degeneration. The American journal of clinical nutrition, 88(4), pp.1104-1110
- Narayan R. Beyond the tip of the iceberg – AMD and nutrition. Optician, 27 July 2018
- Aoki, A, Inoue, M, Nguyen, E, Obata, ., Kadonosono, K, Shinkai, S, Hashimoto, H, Sasaki, S and Yanagi, Y, 2016. Dietary n-3 fatty acid, α-tocopherol, zinc, vitamin D, vitamin C, and β-carotene are associated with age-related macular degeneration in Japan. Scientific Reports, 6, p.20723
- Gorusupudi, A, Nelson, K and Bernstein, PS, 2017. The age-related eye disease 2 study: micronutrients in the treatment of macular degeneration. Advances in Nutrition, 8(1), pp.40-53
- AREDS Age-Related Eye Disease Study Research Group, ‘A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age- related macular degeneration and vision loss: AREDS report no. 8.’ Archives of Ophthalmology, vol. 119, no. 10, pp. 1417–1436, 2001
- Ângela Carneiro and José Paulo Andrade, ‘Nutritional and Lifestyle Interventions for Age-Related Macular Degeneration: A Review,’ Oxidative Medicine and Cellular Longevity, vol. 2017, Article ID 6469138, 13 pages, 2017
- Chew EY, Clemons TE, Agron E et al. Long-term effects of vitamins C and E, beta-carotene and zinc on age-related macular degeneration. AREDS Report no. 35. Ophthalmology 2013;120:1604-1611
- JP SanGiovanni, EY Chew, TE Clemons et al. The relationship of dietary lipid intake and age-related macular degeneration in a case-control study: AREDS report no. 20. Archives of Ophthalmology, vol. 125, no. 5, pp. 671–679, 2007
- JP SanGiovanni, E Argon, TE Clemons and EY Chew. w-3 Long-chain polyunsaturated fatty acid intake inversely associated with 12-year progression to advanced age-related macular degeneration. Archives of Ophthalmology, vol. 127, no. 1, pp. 110–112, 2009
- Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC, Faber MD, Gragoudas ES, Haller J, Miller DT, et al: Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. JAMA 1994;272:1413–1420.
- Augood C, Chakravarthy U, Young I, Vioque J, de Jong PT, Bentham G, Rahu M, Seland J, Soubrane G, Tomazzoli L, Topouzis F, Vingerling JR, Fletcher AE: Oily fish consumption, dietary docosahexaenoic acid and eicosapentaenoic acid intakes, and associations with neovascular age-related macular degeneration. Am J Clin Nutr 2008;88:398– 406.
- Mares-Perlman JA, Fisher AI, Klein R, Palta M, Block G, Millen AE, Wright JD: Lutein and zeaxanthin in the diet and serum and their relation to age-related maculopathy in the third national health and nutrition examination survey. Am J Epidemiol 2001;153:424–432.
- Moeller SM, Parekh N, Tinker L, Ritenbaugh C, Blodi B, Wallace RB, Mares JA; CAREDS Research Study Group: Associations between intermediate age related macular degeneration and lutein and zeaxanthin in the Carotenoids in Age-Related Eye Disease Study (CAREDS): ancillary study of the Women’s Health Initiative. Arch Ophthalmol 2006;124: 1151–1162.
- Tan JS, Wang JJ, Flood V, Rochtchina E, Smith W, Mitchell P: Dietary antioxidants and the longer-term incidence of age-related macular degeneration: the Blue-Mountains Eye Study. Ophthalmol 2008;115:334–341.
- Seddon JM, Cote J, Rosner B: Progression of age-related macular degeneration: association with dietary fat, transunsaturated fat, nuts, and fish intake. Arch Ophthalmol 2003; 121:1728–1737
- The AREDS2 Research Group, Chew EY, Clemons T, SanGiovanni JP, Danis RP, Domalpally A, McBee W, Sperduto R, Ferris FL: The Age-Related Eye Disease Study 2 (AREDS2): study design and baseline characteristics (AREDS2 report number 1). Ophthalmology 2012;119:2282–2289
- Schmidl, D. , Garhöfer, G. and Schmetterer, L. (2015), Nutritional supplements in age-related macular degeneration. Acta Ophthalmol, 93: 105-121
- Sherman, J and Epstein, D., 2018. Treat your AMD patients as you would treat a family member with AMD. Optometry Today, (11), p.73
- Susan T Mayne. Oxidative Stress, Dietary Antioxidant Supplements, and Health: Is the Glass Half Full or Half Empty? Cancer Epidemiology Biomarkers Preview, December 1 2013 (22) (12) 2145-2147
- Johnson, E.J., 2014. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutrition Reviews, 72(9), pp.605-612
- Galasso, C., Orefice, I., Pellone, P., Cirino, P., Miele, R., Ianora, A., Brunet, C. and Sansone, C., 2018. On the neuroprotective role of astaxanthin: new perspectives? Marine drugs, 16(8), p.247
- Conrady, C.D., Bell, J.P., Besch, B.M., Gorusupudi, A., Farnsworth, K., Ermakov, I., Sharifzadeh, M., Ermakova, M., Gellermann, W. and Bernstein, P.S., 2017. Correlations between macular, skin, and serum carotenoids. Investigative Ophthalmology & Visual Science, 58(9), pp.3616-3627
- Obana, A., Gohto, Y., Nakazawa, R., Moriyama, T., Gellermann, W. and Bernstein, P.S., 2020. Effect of an antioxidant supplement containing high dose lutein and zeaxanthin on macular pigment and skin carotenoid levels. Scientific Reports, 10(1), pp.1-12
- Stevens, R., Bartlett, H. and Cooke, R., 2015. Dietary analysis and nutritional behaviour in people with and without age-related macular disease. Clinical nutrition ESPEN, 10(3), pp.e112-e117
- Renzi, L.M., Hammond, B.R., Dengler, M. and Roberts, R., 2012. The relation between serum lipids and lutein and zeaxanthin in the serum and retina: results from cross-sectional, case-control and case study designs. Lipids in Health and Disease, 11(1), p.33
- Thomas, S.E. and Harrison, E.H., 2016. Mechanisms of selective delivery of xanthophylls to retinal pigment epithelial cells by human lipoproteins. Journal of Lipid Research, 57(10), pp.1865-1878
- Barter, P.J., Brandrup-Wognsen, G., Palmer, M.K. and Nicholls, S.J., 2010. Effect of statins on HDL-C: a complex process unrelated to changes in LDL-C: analysis of the VOYAGER Database. Journal of lipid research, 51(6), pp.1546-1553
- Marques, L.R., Diniz, T.A., Antunes, B.M., Rossi, F.E., Caperuto, E.C., Lira, F.S. and Gonçalves, D.C., 2018. Reverse cholesterol transport: molecular mechanisms and the non-medical approach to enhance HDL cholesterol. Frontiers in Physiology, 9, p.526
- Nassef, Y., Lee, K.J., Nfor, O.N., Tantoh, D.M., Chou, M.C. and Liaw, Y.P., 2020. The impact of aerobic exercise and badminton on HDL cholesterol levels in Taiwanese adults. Nutrients, 12(5), p.1204
- Johnson, E.J., Hammond, B.R., Yeum, K.J., Qin, J., Wang, X.D., Castaneda, C., Snodderly, D.M. and Russell, R.M., 2000. Relation among serum and tissue concentrations of lutein and zeaxanthin and macular pigment density. The American Journal of Clinical Nutrition, 71(6), pp.1555-1562
- O’Toole, P.W. and Jeffery, I.B., 2015. Gut microbiota and aging. Science, 350(6265), pp.1214-1215
- Kim, S. and Jazwinski, S.M., 2018. The gut microbiota and healthy aging: a mini-review. Gerontology, 64(6), pp.513-520
- Maffei, V.J., Kim, S., Blanchard IV, E., Luo, M., Jazwinski, S.M., Taylor, C.M. and Welsh, D.A., 2017. Biological aging and the human gut microbiota. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences, 72(11), pp.1474-1482
- Gilbert, R., Peto, T., Lengyel, I. and Emri, E., 2019. Zinc Nutrition and Inflammation in the Aging Retina. Molecular Nutrition & Food Research, 63(15), p.1801049
- Newsome, David A., Michael V. Miceli, David J. Tate Jr, Nancy W. Alcock, and Peter D. Oliver. Zinc content of human retinal pigment epithelium decreases with age and macular degeneration, but superoxide dismutase activity increases. The Journal of Trace Elements in Experimental Medicine: The Official Publication of the International Society for Trace Element Research in Humans 8, no. 4 (1996): 193-199
- Lengyel, I. and Peto, T., 2008. Cure or cause: opposing roles for zinc in age-related macular degeneration. Expert Review of Ophthalmology, 3(1), pp.1-4
- Nalley C. 2020. Genetics in eyecare: DNA leads the way. Rev Of optometry, 15th October 2020. www.reviewofoptometry.com/article/genetics-in-eye-care-dna-leads-the-way#footnotes. Accessed November 10, 2020
- Kaufman, S.R., Yoganathan, P., Small, K.W., Rusia, D., Pachydaki, S.I., Conti, S.M., Wenz, R.E., Gersman, M.A., Shaya, F.S. and Kustra, R., 2020. Genetics and Age-Related Eye Disease Study Formulation Interaction in Neovascular Age-Related Macular Degeneration. Journal of VitreoRetinal Diseases, p.2474126420941713
- Assel, M.J., Li, F., Wang, Y., Allen, A.S., Baggerly, K.A. and Vickers, A.J., 2018. Genetic polymorphisms of CFH and ARMS2 do not predict response to antioxidants and zinc in patients with age-related macular degeneration: Independent statistical evaluations of data from the Age-Related Eye Disease Study. Ophthalmology, 125(3), pp.391-397
- Bungau, S., Abdel-Daim, M.M., Tit, D.M., Ghanem, E., Sato, S., Maruyama-Inoue, M., Yamane, S. and Kadonosono, K., 2019. Health benefits of polyphenols and carotenoids in age-related eye diseases. Oxidative Medicine and Cellular Longevity, 2019 vol. 2019, Article ID 9783429, p22
- Lançon, A., Frazzi, R. and Latruffe, N., 2016. Anti-oxidant, anti-inflammatory and anti-angiogenic properties of resveratrol in ocular diseases. Molecules, 21(3), p.304
- Richer, S., Ulanski, L., Popenko, N.A., Pratt, S.G., Hitchmoth, D., Chous, P., Patel, S., Sockanathan, S. and Sardi, B., 2016. Age-related Macular Degeneration Beyond the Age-related Eye Disease Study II. Advances in Ophthalmology and Optometry, 1(1), pp.335-369
- Salehi, B., Mishra, A.P., Nigam, M., Sener, B., Kilic, M., Sharifi-Rad, M., Fokou, P.V.T., Martins, N. and Sharifi-Rad, J., 2018. Resveratrol: A double-edged sword in health benefits. Biomedicines, 6(3), p.91
- Richer, S., Stiles, W., Ulanski, L., Carroll, D. and Podella, C., 2013. Observation of human retinal remodeling in octogenarians with a resveratrol based nutritional supplement. Nutrients, 5(6), pp.1989-2005.
- Richer, S., Patel, S., Sockanathan, S., Ulanski, L.J., Miller, L. and Podella, C., 2014. Resveratrol
