In keeping with guidance from the College of Optometrists (2015), routine optometric assessments typically include the measurement of visual acuity, refractive and binocular vision status, visual fields and ocular health. However, it is less common for optometrists to assess contrast sensitivity (CS). This raises the question as to whether measuring contrast sensitivity is relevant to optometric practice. Do CS measurements provide additional information? Are there valid reasons for including contrast sensitivity in a routine eye examination? Should this test be part of a standard low vision assessment?
After a brief refresher of what contrast sensitivity is, this article will explore CS from a medical perspective with a focus on assessing visual function to aid diagnosis and treatment of ocular and general medical conditions. It will then consider the social perspective of contrast sensitivity and its influences, with a particular focus on functional vision, task performance and participation.
Brief revision of contrast sensitivity
The ability to resolve targets varies significantly with the contrast of the target. The visual world a patient inhabits is one of varying target size and contrast and, furthermore, diseases affecting vision may affect the ability to resolve these targets in a selective manner.1 Therefore, the use of high contrast targets for acuity testing has been criticised as non-representative of the visual world and less than sensitive at reflecting visual reduction due to disease. If a patient is shown a sine-wave grating of a constant
spatial frequency, as shown in figure 1, their ability to resolve the grating reduces as the contrast is reduced until a point is reached when it can no longer be resolved. This point, the contrast threshold (the reciprocal of which is called the contrast sensitivity), is different for different spatial frequencies and the plot of the threshold values against spatial frequency is described as the contrast sensitivity function (CSF) as shown in figure 2.
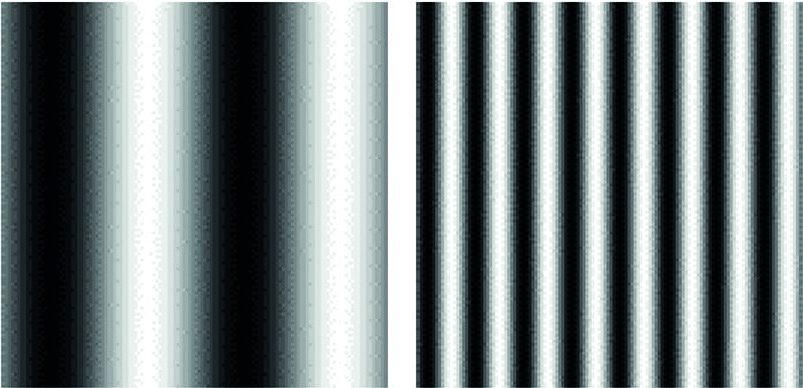 Figure 1: A sine wave grating with a low spatial frequency (left) and a higher spatial frequency (right). As contrast is reduced, the point at which the viewer can no longer discern the lines is called the contrast threshold, the inverse of which is the contrast sensitivity
Figure 1: A sine wave grating with a low spatial frequency (left) and a higher spatial frequency (right). As contrast is reduced, the point at which the viewer can no longer discern the lines is called the contrast threshold, the inverse of which is the contrast sensitivity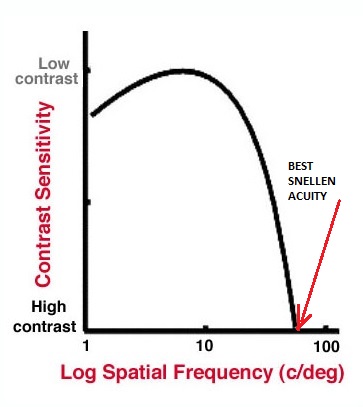 Figure 2: The contrast sensitivity function or curve. Note that the CS differs for different spatial frequencies
Figure 2: The contrast sensitivity function or curve. Note that the CS differs for different spatial frequencies
Snellen acuity relates to the resolution of a high contrast target of maximum spatial frequency and is therefore represented as the cut-off point on the horizontal axis on this curve. This is sensitive to conditions affecting mainly high spatial frequencies, such as refractive error, but less sensitive if lower spatial frequencies are affected, as with cataract, corneal disturbance and contact lens wear.
Increasingly clinicians are using targets of different contrast to assess the influence on acuity. LogMAR charts are available in different contrasts so assessing the patient’s ability to resolve increasing spatial frequencies at a given contrast value. Computerised acuity charts, such as the Thomson Test Chart 2020 shown in figure 3, allow any contrast value to be pre-set.

 Figure 3: Electronic test charts (such as the Thomson Test Chart 2020 here) can display a range of different charts at different percentage contrast settings. For accuracy, the screen needs to be calibrated correctly
Figure 3: Electronic test charts (such as the Thomson Test Chart 2020 here) can display a range of different charts at different percentage contrast settings. For accuracy, the screen needs to be calibrated correctly
Other charts, such as the Pelli-Robson (figure 4), use a constant letter size (approximating to one cycle per degree if viewed at 1m) and gradually reducing contrast as the chart is read by the patient. In theory, varying the working distance would allow the whole contrast sensitivity function to be assessed with a Pelli-Robson chart, but in practice this is rarely needed as high and low
contrast acuity scores combined with contrast sensitivity at 1m with a Pelli-Robson is usually sufficient to suggest any visual compromise. The Mars Letter Contrast Sensitivity Test (initially known as the Lighthouse Letter Contrast Sensitivity Test) is similar in design to the Pelli-Robson Test and has been found to have somewhat improved repeatability.
 Figure 4: The Pelli-Robson chart is standard for measuring CS in many low vision clinics. The lowest contrast triplet discerned is recorded as a numerical value
Figure 4: The Pelli-Robson chart is standard for measuring CS in many low vision clinics. The lowest contrast triplet discerned is recorded as a numerical value
Other contrast charts available for clinical practice include the Vistech (figure 5). This is a poster with five rows of circles upon which is printed a grating. Each row represents a constant spatial frequency but, as the patient looks along the row from left to right, the contrast of the grating decreases until it is no longer possible to state in which direction the grating is orientated (to the left or to the right for example). This way, a score representing the limit of contrast resolution for each pre-defined spatial frequency may be noted and plotted as a CSF curve. The test is limited, however, by the necessarily limited number of targets viewed.
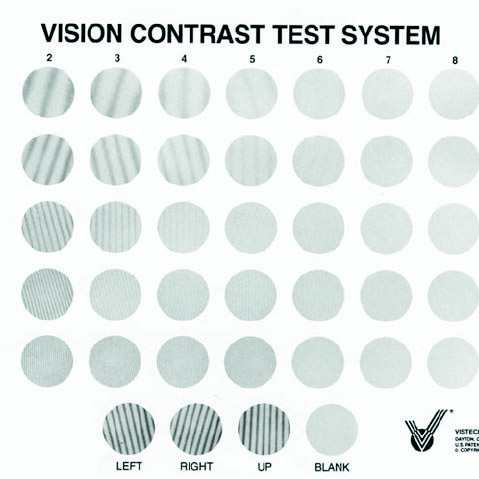 Figure 5: The Vistech CS chart
Figure 5: The Vistech CS chart
Medical perspective
The primary purpose of a medical assessment is to diagnose and treat conditions with a view to preserve and restore health. In the optometric practice this concerns ocular health in the first place, but it also includes general health. For example, a retinal examination may reveal ocular disease but may also reveal signs of general health conditions, such as cardiovascular disease. Some conditions, however, show reduced contrast sensitivity before the onset of ocular manifestations. In this case, contrast sensitivity could act as a biomarker.
Contrast sensitivity and systemic disease
Optometrists play a key role in the early detection of diabetes.2 Early diagnosis and intervention leads to a better health outcome for diabetic patients. Traditionally, early detection of diabetes in the optometric practice is based on retinal signs (figure 6) and changes in visual acuity and refractive error. However, there is evidence that CS is often reduced in early diabetic retinopathy and in pre-diabetes before changes in visual acuity.3-7 This suggests that neural dysfunction can occur before other structural changes develop.
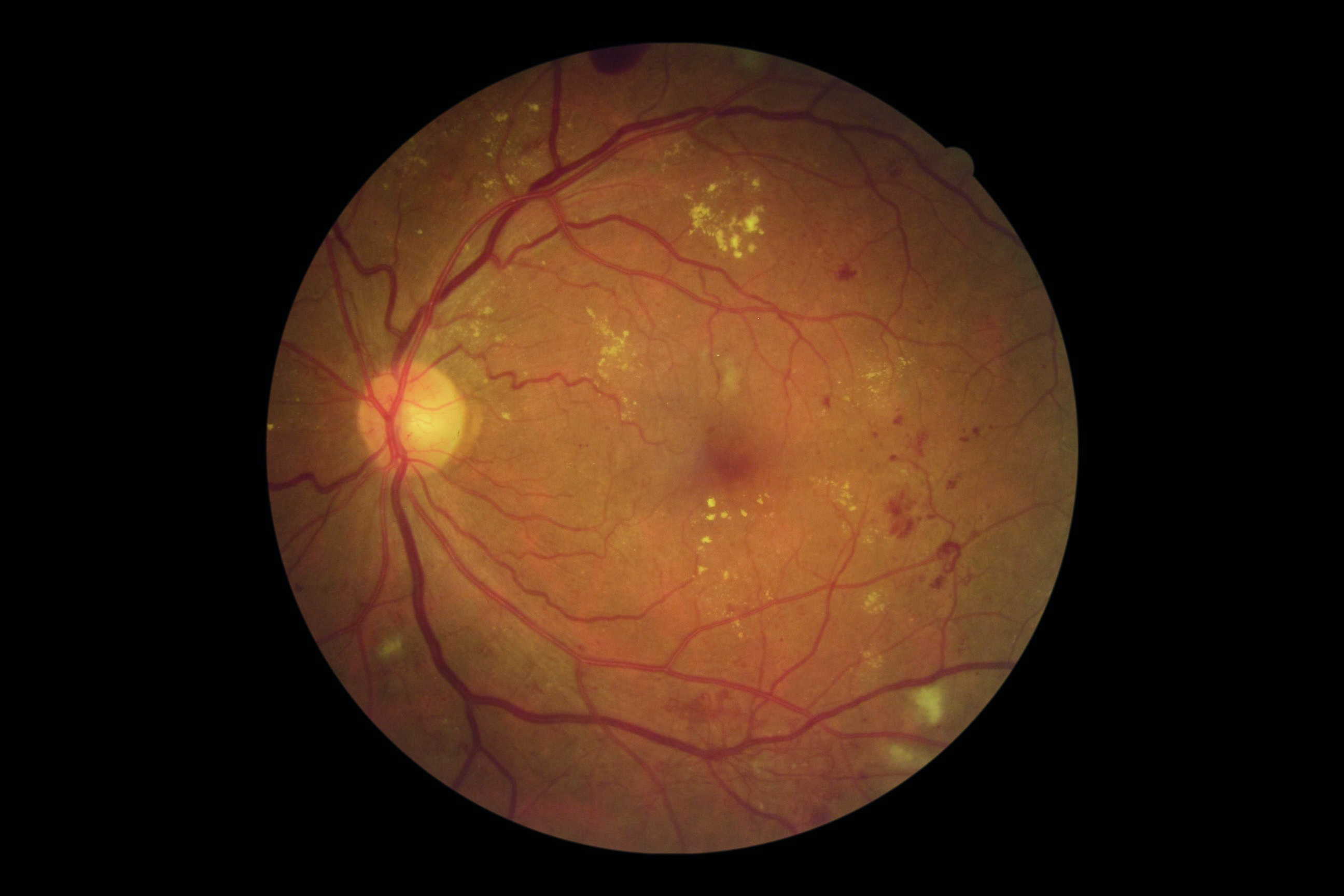 Figure 6: Diabetic retinopathy. Reduction in contrast sensitivity may be a useful early biomarker
Figure 6: Diabetic retinopathy. Reduction in contrast sensitivity may be a useful early biomarker
Reduced CS and colour vision can also be indicators of neurodegenerative and neurological conditions.8 These biomarkers can aid in early diagnosis in conditions such as Huntington’s disease,9,10 Parkinson’s disease,11 Alzheimer’s disease,12 multiple sclerosis13 and optic chiasm compression due to pituitary adenomas.14
Contrast sensitivity and ocular disease
Although CS loss could be an early sign of the aforementioned conditions, CS loss is also associated with a number of common ocular conditions.15
Tear film instability and ocular surface irregularity
Patients with tear film or ocular surface abnormalities often complain of reduced vision despite normal visual acuity. It is worth assessing CS in these cases to explain their symptoms. Meibomian gland dysfunction, epiphora, dry eye-related superficial punctate keratopathy and reduced tear-film stability are associated with reduced CS.16-19 Also, an irregular corneal shape due to keratoconus is associated with reduced CS rather than reduced acuity (figure 7).20
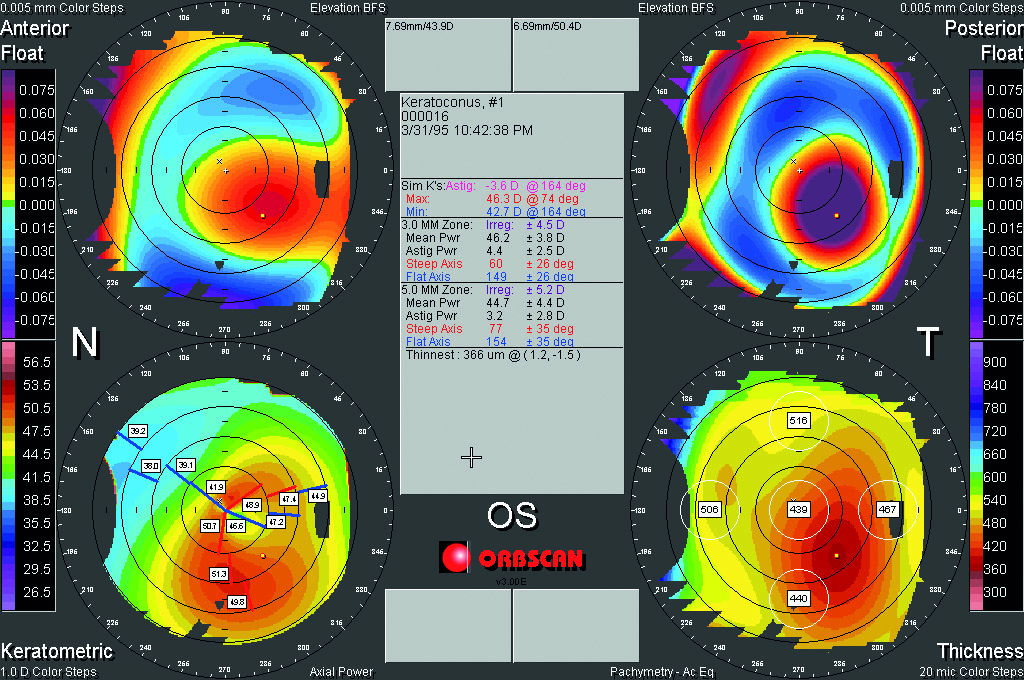 Figure 7: Irregular cornea due to keratoconus may impact CS before any major acuity change
Figure 7: Irregular cornea due to keratoconus may impact CS before any major acuity change
Cataract
Cataract is known to have an impact on CS, although in many optometric practices and cataract assessment clinics, this function is not measured routinely. Cataract extraction and intraocular lens implantation can improve visual outcomes in terms of visual acuity, near vision and CS.21-24 In patients with concurring macular degeneration, CS is likely to improve after cataract surgery.25
Diabetic eye disease
As mentioned in an earlier section, diabetic retinopathy is associated with reduced CS. This tends to be more severe as the disease progresses.26,27 This can lead to problems in functional vision. For example, the risk of falls is associated with DRP severity28 and with low CS in Type 2 diabetes.29
Retinal vascular disease
Retinal vascular disease can impact upon CS as well as visual acuity. The impact of treatment on CS is an important outcome measure for patients with conditions such as central or branch retinal vein occlusion (figure 8). It has been demonstrated that intravitreal ranibizumab injections can improve CS, even in the absence of improved visual acuity.30 Systemic cardiovascular conditions can also impact on CS. For example, variability in orthostatic blood pressure is associated with reduced CS.31
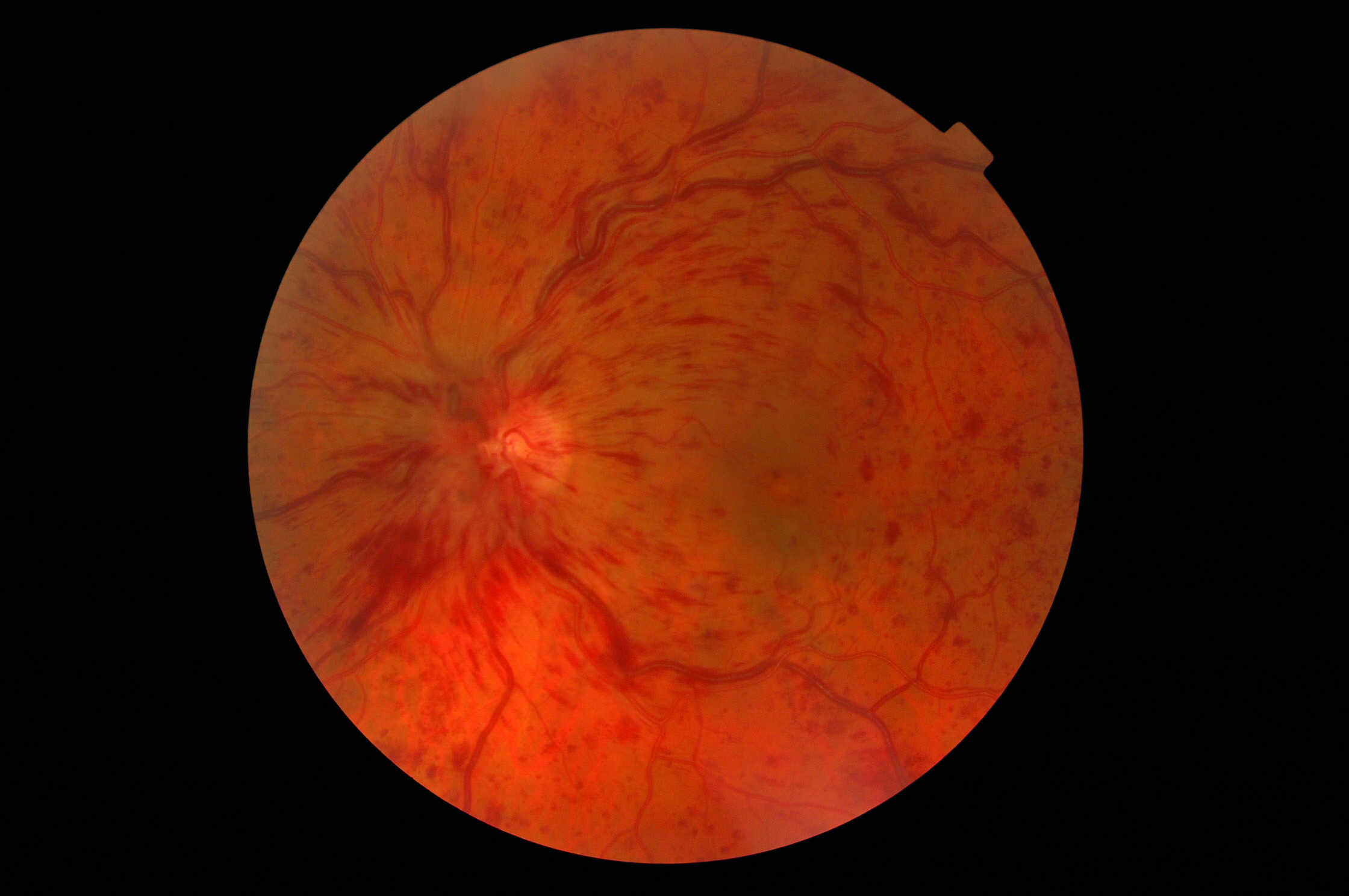 Figure 8: Contrast sensitivity is an important indicator of the effectiveness of management of retinal vein occlusion
Figure 8: Contrast sensitivity is an important indicator of the effectiveness of management of retinal vein occlusion
Macular degeneration
CS typically reduces as AMD progresses.32 This is important as CS impacts upon health-related and vision-related quality of life in the many people with AMD. Reduced CS, as well as reduced visual acuity and low luminance visual acuity, have an negative effect on face recognition and reading speed.33-38
Glaucoma
In terms of monitoring disease progression, visual acuity and visual fields are the mainstream visual function tests for glaucoma. Visual field impairments are associated with an increase of falls and self-reported difficulties with mobility in glaucoma.39,40 However, binocular visual acuity and CS appear to be the best predictors for the ability to perform daily activities in patients with glaucoma.41 Reduced CS is a common feature of glaucoma42-44 and it increases the risk of impaired face recognition45 and Charles Bonnet syndrome.39 Appropriate advice about contrast, lighting and glare management is indicated. This will also be helpful in view of the common issue of reduced dark adaptation and glare symptoms in glaucoma.46
Retinitis pigmentosa (RP)
It is common for patients with inherited retinal degenerations, such as retinitis pigmentosa (RP, figure 9) to experience poor
visual function due to a loss of CS when visual acuity is relatively unaffected.47 Other symptoms that are associated with RP are visual field restriction, impaired dark adaptation and reduced visual acuity. Each of these parameters have an impact on reading, mobility and scanning.48
 Figure 9: It is common for patients with retinitis pigmentosa to have a loss of contrast sensitivity even when visual acuity is relatively unaffected
Figure 9: It is common for patients with retinitis pigmentosa to have a loss of contrast sensitivity even when visual acuity is relatively unaffected
Cerebral visual impairment
Cerebral visual impairment (CVI) is a complex condition which affects basic processing functions as well as higher processing functions. Clinical presentation is variable. Reduced visual acuity is found in the majority of patients. Reduced CS is common among children with CVI.49-51 Other functional impairments include abnormal optokinetic nystagmus, strabismus, ocular motility disorders and field impairments. The majority of patients with CVI have visual-perceptual disorders.49 Children and families benefit from a thorough assessment of visual functions, including CS, to characterise the visual impairments. This informs the practitioner about the visual world as perceived by the child and aids in optimising the child’s functioning and learning. When one understands what is not seen, one can offer learning material in a format that can be perceived and accessed by the child.
Stroke
Stroke patients often experience visual field impairments, such as hemianopia and lower field impairments. Primary pathway lesions tend to result in field impairment without reduced visual attention. However, field impairments from lesions in the higher processing pathway are often associated with myriad other features. Visual inattention and functional impairment in the ‘seeing field’ are common. CS can be severely reduced in the ‘seeing field’.52-54 This has consequences for daily functioning and rehabilitation strategies.
Other eye conditions
Reduced CS is associated with albinism.55 Prematurely born children, children with developmental delay, cerebral palsy or Down syndrome have a higher rate of reduced CS, and CS is often reduced in children with amblyopia, even in the non-amblyopic eye. Optic neuritis is also associated with impaired CS.56
In summary, CS is associated with a number of ocular and medical health conditions. Although it can be a helpful tool for early detection of these conditions, it can be difficult to discern to which condition the impaired contrast sensitivity is attributable or indeed if it is attributable to more than one condition. In any case, CS is a helpful tool for understanding the patient’s functional vision.
Social perspective
Task performance and quality of life
When a patient has impaired CS, the underlying condition needs to be addressed first. Any interventions to preserve or restore sight need to be considered. In some situations, impaired CS is permanent and one has to consider options to improve functional vision. In some cases, reduced CS can be a stronger predictor for experiencing poor vision compared to visual acuity and visual field impairment.37,47,57,58 Assessment of CS is therefore indicated if one wishes to understand how a condition changes the visual world for patients and how it impacts on daily functioning and quality of life.56
Mobility
Mobility performance depends on a number of factors, such as age, visual fields, central visual function, visual attention and CS.59 It is suggested that CS is a stronger predictive factor for mobility performance than visual acuity.60 In terms of visual field impairments, it appears that lower field defects tend to cause more problems with mobility,61,62 whereas peripheral field defects tend to cause more collisions with other pedestrians.63
A thorough assessment of all the different parameters of visual functions can form the basis for mobility training. Patients with visual field impairment could benefit from scanning techniques,64 while reduced CS necessitates the use of contrast to prevent falls in the home, school and work environment. Reduced CS renders it difficult to detect floor boundaries and to judge the depth of kerbs and steps. Tactile ground surface indicators in public spaces can aid mobility. The benefit is greater when these indicators have a high level of luminance contrast.66 The use of a cane or hiking pole can be used to feel the ground ahead, which helps with detecting uneven ground, kerbs and steps.
Reading
It is well known that visual acuity has an impact on reading speed. As a rule of thumb for fluent reading tasks, an acuity reserve of 2:1 or even 3:1 is desirable.66 When using magnifiers, one also has to bear in mind that a reduced field of view with stronger magnifiers affects reading speed as well.66 Another visual function that influences reading speed is CS.67,68 For fluent reading, the contrast of the print needs to be several times threshold,69,70 from 4x for spot reading up to 10x for fluent reading. Reduced reading speed and CS are associated with poorer vision related quality of life.38 Tables 1 and 2 provide approximate contrasts of everyday objects and reading material. Table 3 explains how contrast sensitivity values as a percentage relate to log contrast and severity of loss on different test charts.
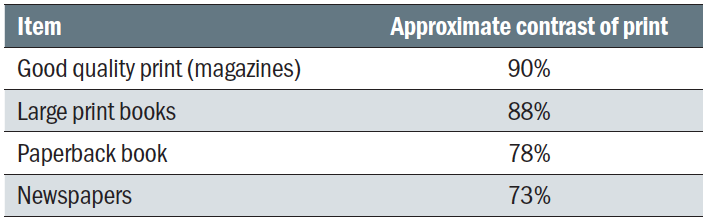 Table 1: Contrast of commonly encountered printed materials
Table 1: Contrast of commonly encountered printed materials
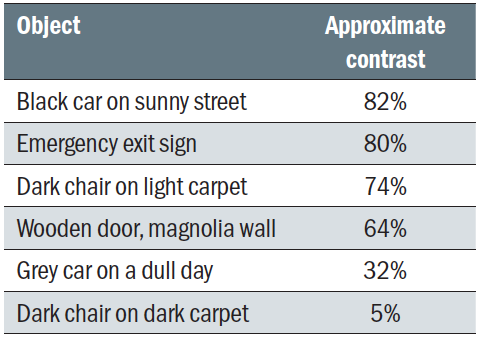 Table 2: Contrast of a range of everyday objects
Table 2: Contrast of a range of everyday objects
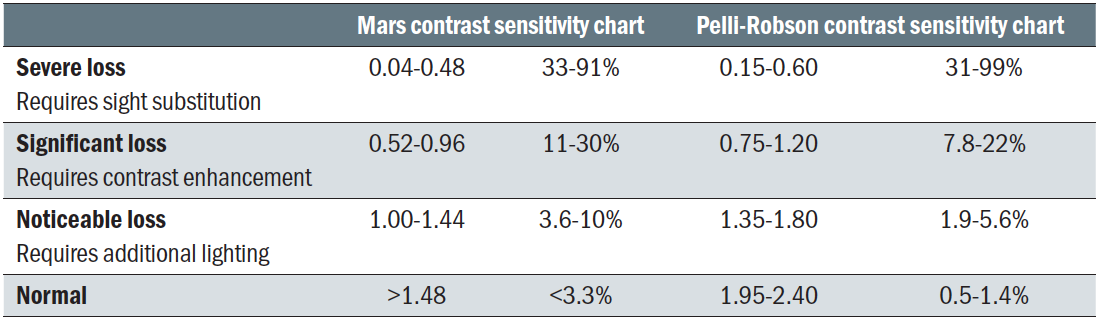 Table 3: Understanding contrast sensitivity measurements
Table 3: Understanding contrast sensitivity measurements
Driving
CS loss is common in elderly people, especially in the presence of eye conditions such as cataract and AMD. The impact of CS on driving safety is well documented.71,72 However, CS is not routinely tested in the context of fitness to drive. The College of Optometrists recommends regular sight tests for drivers, especially in people aged over 60 in order to ensure that peo ple meet driving standards. Currently, the Driver and Vehicle Licencing Agency (DVLA) vision standards state a minimum requirement of 6/12 (best corrected) Snellen vision.73
If patients attend for a sight test, the optometrist is responsible for giving the correct advice about driving standards. It is not safe to assume people with vision below the driving standards will have stopped driving of their own accord. Documentation of having advised patients of any risks of continued driving is particularly important in the case of driving due to the liability issues in the case of a road traffic accident. If a patient is not meeting driving standards, the optometrist needs to advise the patient that they have a legal duty to inform the DVLA about their condition.
The College of Optometrists’ (2020) guidance recommends that the practitioner should put any advice offered about driving in writing and give it to the patient, keep a copy of this letter in the records, and inform the patient’s GP (providing that the patient have given full consent for this). A report by The General Optical Council (in 2017) revealed that optometrists’ views and actions regarding vision and driving are not consistent. Patients who are unfit to drive do not always receive advice about their lack of fitness from their eye care professionals. In some instances, advice is given, but it is often not fully documented in the records.
Some practitioners find it challenging to initiate a conversation about fitness to drive, perhaps because this can be devastating news for the patient. Other practitioners are unsure about the criteria for driving, as a number plate test does not correlate well with the vision test in the consulting room. One has to bear in mind that optometrists can use their clinical judgement and advise the patient to contact the DVLA if in doubt. It is the DVLA’s responsibility to decide if a patient has to give up their licence. If the vision appears borderline for driving, it can be helpful to carry out a CS test to create a more informed clinical picture. This can also be helpful in terms of explaining to the patient why it is no longer safe to drive. Road signs are designed to be visible at a great distance, although poor contrast sensitivity does affect the ability to discriminate traffic signs (figure 10).74 If a driver only uses familiar routes, visual acuity may seem less relevant to their driving ability. However, the inability to see unexpected obstacles or people due to a loss of CS is a realistic concern. A patient is more likely to willingly give up driving when the evidence makes sense.
 Figure 10: Road signs are designed to be visible at a great distance
Figure 10: Road signs are designed to be visible at a great distance
Face recognition
Visual acuity and CS are important factors for recognising faces and facial expressions.75 Difficulties with facial recognition and interpretation are a frequent complaint in a low vision clinic and can lead to social isolation, embarrassment and difficulties in communication. This problem is even more evident if a patient has a concurrent hearing impairment. Reducing back-light
scatter, by avoiding light sources behind the person being addressed, significantly improves the contrast of facial features.
Case scenario 1
An elderly housebound lady with disciform scars attends the low vision clinic. She has given up knitting due to her eyesight problems. Binocular distance acuity is 0.70 LogMAR (6/30 Snellen) and she reads N25 with her glasses, improved to N8 with a 3.5x illuminated hand magnifier. Her CS is significantly reduced (19% on MARS chart) and she cannot identify any plates on the Ishihara colour test.
In this example, magnification on its own is unlikely to solve the problem of not seeing the knitting stitches. However, if the colour and contrast impairment are taken into account, one can find a more suitable solution. This lady was advised to use thicker wool and needles in a contrasting colour (not too dark and contrasting against the colour of the knitting needles). She was also given some ideas for simple projects that do not require studying instructions from a knitting pattern. Furthermore, she was issued with a base for the 3.5x illuminated hand magnifier, which allowed her to see her knitting work without the need to hold the magnifier (figure 11). The next visit, she brought along some beautiful knitted scarfs, and more importantly, she was able to enjoy her hobby once more.
 Figure 11: Modern hand magnifiers may be supplied with a stand
Figure 11: Modern hand magnifiers may be supplied with a stand
Case scenario 2
A diabetic patient with background retinopathy, a binocular distance acuity of 0.32 LogMAR (6/12 Snellen) and no visual field impairment has lost confidence going down the stairs (figure 12). This has affected her independence. Her CS was reduced to 21% (significant loss). This patient was referred to the Eye Clinic Liaison Officer (ECLO) to arrange mobility training and a home assessment with a view to improve lighting around the staircase, to introduce contrasting banisters and floor markings indoors, and to improve visibility of the outdoor entrance to her home.

 Figure 12: Poor lighting around stairs represents a significant safety concern for those with reduced CS
Figure 12: Poor lighting around stairs represents a significant safety concern for those with reduced CS
Conclusion
In summary, the measurement of contrast sensitivity is an undervalued test in optometric practice. It is a valuable tool for the detection of general health and ocular conditions, monitoring change and appreciating treatment outcomes. Quality of life and daily functioning can be reduced due to impaired contrast sensitivity with a significant number of ocular conditions and health conditions. Understanding the impact of contrast sensitivity loss is a first step in offering appropriate advice and support.
References
- Harvey W, Doshi S. Eye Essentials; Investigative Techniques. Chapter 1, 2005, Butterworth Heinemann.
- Aldebasi Y, Reddy P, Nair V et al. 2015. Screening for diabetic retinopathy: the optometrist’s perspective. Available at: https://www.dovepress.com/screening-for-diabetic-retinopathy-the-optometristrsquos-perspective-peer-reviewed-fulltext-article-OPTO
- Chande PK, Raman R, John P et al. (2020). Contrast-sensitivity function and photo stress-recovery time in prediabetes. Clinical Optometry, 12, pp.151-155
- Eggers ED and Carreon TA. (2020). The effects of early diabetes on inner retinal neurons. Visual Neuroscience 37. Article Number E006
- Ferreira JT and Pinto L. (2017). New trends for early diabetic retinopathy diagnosis. Proceedings of the 5th International Conference on Photonics, optics and Laser Technology (Photoptics), Porto, pp.402-406
- Pramanik S, Chowdhury S, Ganguly U et al. (2020). Visual contrast sensitivity could be an early marker of diabetic retinopathy. Heliyon 6(10). Article Number e05336
- Rahman AA, Badarudin NE, Azemin MZC et al. (2018). Changes in contrast sensitivity in young adults with diabetes. Makara Journal of Health Research 22(1), pp.22-26
- Heinrich SP and Hoffmann MB. (2018). Visual acuity, contrast sensitivity, colour vision: thoughts on psychophysical exams in neuro-ophthalmology. Klinische Monatsblatter fur Augenheilkunde 235(11), pp.1212-1217
- Hamedani AG, Bardakjian T, Balcer LJ et al. (2020). Contrast acuity and the King-Devick Test in Huntington’s Disease. Neuro-Ophthalmology 44(4), pp.219-225
- Svetozarskiy SN. (2019). Contrast sensitivity and colour vision as biomarkers of the preclinical stage of neurodegeneration in Huntington’s disease. Sovremennye Technologii v Medicine 11(2), pp.77-84
- Ridder A, Mueller MLTM, Kotagal V et al. (2017). Impaired contrast sensitivity is associated with more severe cognitive impairment in Parkinson disease. Parkinsonism and Related Disorders 34, pp.15-19
- Risacher SL, WuDunn D, Tallman EF et al. (2020). Visual contrast sensitivity is associated with the presence of cerebral amyloid and tau deposition. Brain Communications 2(1). Article number 19
- Fisher JB, Jacobs DA, Markowitz CE et al. (2006). Relation of visual function to retinal nerve fibre layer thickness in multiple sclerosis. Ophthalmology 113(2), pp.324-332
- Sarnat-Kucharczyk M, Kos-Kudla B, Kajdaniuk D et al. (2019). The efficacy of microperimetry and contrast sensitivity test in the diagnosis of optic chiasm compression due to pituitary adenomas. Endokrynologia Polska 70(3), pp.241-247
- Xiong Y, Kwon M, Bittner AK et al. (2020). Relationship between acuity and contrast sensitivity: differences due to eye disease. Investigative Ophthalmology and Visual Science 61(6). Article Number 40
- Koh S, Maeda N, Ikeda C et al. (2017). The effect of ocular surface regularity on contrast sensitivity and straylight in dry eye. Investigative Ophthalmology and Visual Science 58(5), pp.2647-2651
- Malhotra C, Singh S, Chakma P et al. (2015). Effect of oral omega-3 fatty acid supplementation on contrast sensitivity in patients with moderate meibomian gland dysfunction: a prospective placebo-controlled study. Cornea 34(6), pp.637-643
- Szczotka-Flynn LB, Maguire MG, Ying G et al. (2019). Impact of dry eye on visual acuity and contrast sensitivity: dry eye assessment and management study. Optometry and Vision Science 96(6), pp.387-396
- Tasaki K, Hoshi S, Hiraoka T, Oshika T (2020). Deterioration of contrast sensitivity in eyes with epiphora due to lacrimal passage obstruction. PLoS ONE 15(5): e0233295
- Liduma S, Luguzis A and Krumina G. (2020). The impact of irregular corneal shape parameters on visual acuity and contrast sensitivity. BMC Ophthalmology 20(1). Article Number 466
- Escaf LJ, Escaf LC, Polo S et al. Standard results and contrast sensitivity reestablishment after implantation of a trifocal intraocular lens. Current Eye Research 2020. http://doi/10.1080/02713683.2020.1828486
- Lian H, Ma W, Wei Q et al. (2020). A comparative study on early vision quality after implantation of refractive segmental and diffractive multifocal intraocular lens. Pakistan Journal of Medical Sciences 36(7), pp.1607-1612
- Sinha R, Sahay P, Saxena R et al. (2020). Visual outcomes of binocular implantation of a new extended depth of focus intraocular lens. Indian Journal of Ophthalmology 68(10), pp.2111-2116
- Tanabe, H., Tabuchi, H., Shojo, T., Yamauchi, T., & Takase, K. (2020). Comparison of visual performance between monofocal and multifocal intraocular lenses of the same material and basic design. Scientific reports, 10(1), 15490
- Armbrecht AM, Findlay C, Kaushal et al. (2000). Is cataract surgery justified in patients with age related macular degeneration? A visual function and quality of life assessment. British Journal of Ophthalmology 84(12), pp.1343-1348
- Gella L, Raman R, Pal SS et al. (2017). Contrast sensitivity and it determinants in people with diabetes: SN-DREAMS-II, Report no 6. Eye 31(3), pp.460-466
- Khan A, Petropoulos IN, Ponirakis G et al. (2017). Visual complications in diabetes mellitus: beyond retinopathy. Diabetic Medicine 34(4), pp.478-484
- Gupta P, Aravindhan A, Gan ATL et al. (2017). Association between the severity of diabetic retinopathy and falls in an Asian population with diabetes- The Singapore Epidemiology of Eye Disease Study. JAMA Ophthalmology 135(12), pp.1410-1416
- Deshpande N, Hewston P and Aldred A. (2017). Sensory functions, balance and mobility in older adults with type 2 diabetes without overt diabetic peripheral neuropathy: a brief report. Journal of Applied Gerontology 36(8), pp.1032-1044
- Sugiura Y, Okamoto F, Murakami T, Morikawa S, Hiraoka T, Oshika T. Time course of changes in contrast sensitivity following intravitreal ranibizumab injection for branch retinal vein occlusion. Japanese Journal of Ophthalmology. 2020 Sep;64(5):497-505
- Bhuachalla BN, McGarrigle CA, O’Leary N et al. (2019). Orthostatic blood pressure variability is associated with lower visual contrast sensitivity function: findings from The Irish Longitudinal Study on Aging. Experimental Gerontology 119, pp.12-14
- Schneck ME, Lott LA, Haegerstrom-Portnoy G, Hewlett S, Gauer BM, Zaidi A. Visual Function in Eyes with Intermediate AMD with and without Retinal Pigment Abnormalities. Optometry and Vision Science. 2021;98(1):64-72
- Bansback N, Czoski-Murray C, Carlton J et al. (2007). Determinants of health related quality of life and health state utility in patients with age related macular degeneration: the association of contrast sensitivity and visual acuity. Quality of Life Research 16(3), pp.533-543
- Ghoshal R, Shananjeet-Kaur S, Fadzil NM et al. (2020). Correlation between visual functions and retinal morphology in eyes with early and intermediate age-related macular degeneration. International Journal of Environmental Research and Public Health 17(17). Article Number 6379
- Logan AJ, Gordon GE and Loffler G. (2020). The effect of age-related macular degeneration on components of face perception. Investigative Ophthalmology and Visual Science 61(6). Article Number 38
- Pondorfer SG, Terheyden JH, Heinemann M et al. (2019). Association of vision-related quality of life with visual function in age-related macular degeneration. Scientific Reports 9. Article Number 15326
- Roh M, Selivanova A, Shin HJ et al. (2018). Visual acuity and contrast sensitivity are two important factors affecting vision-related quality of life in advanced age-related macular degeneration. Plos One 13(5). Article Number e196-481
- Rossouw P, Guichard MM and Hatz K. (2020). Contrast sensitivity and binocular reading speed best correlating with near distance vision-related quality of life in bilateral AMD. Ophthalmic and Physiological Optics 40(6), pp.760-769
- Subhi, Y., Schmidt, D. C., Bach-Holm, D., Kolko, M., & Singh, A. (2020). Prevalence of Charles Bonnet syndrome in patients with glaucoma: a systematic review with meta-analyses. Acta Ophthalmologica. https://doi.org/10.1111/aos.14567
- Wang Y, Alnwisi S and Ke M (2017). The impact of mild, moderate and severe visual field loss in glaucoma on patients’ quality of life measured via the Glaucoma Quality of Life-15 Questionnaire: a meta-analysis. Medicine 96(48). Article Number e8019
- Richman J, Lorenzana LL, Lankaranian D et al. 2010. Importance of visual acuity and contrast sensitivity in patients with glaucoma. Archives of Ophthalmology 128(12), pp.1576-1582
- Bambo MP, Ferrandez B, Guerri N et al. (2016). Evaluation of contrast sensitivity, chromatic vision and reading ability in patients with primary open angle glaucoma. Journal of Ophthalmology 2016. Article Number 7074016
- Ichhpujani P, Thakur S and Spaeth GL. (2020). Contrast sensitivity and glaucoma. Journal of Glaucoma 29(1), pp.71-75
- Jammal AA, Ferreira BG, Zangalli CS et al. (2020). Evaluation of contrast sensitivity in patients with advanced glaucoma: comparison of two tests. British Journal of Ophthalmology 104(10), pp.1418-1422
- Hirji SH, Hood DC, Liebmann JM et al. (2020). Association of patterns of glaucomatous macular damage with contrast sensitivity and facial recognition in patients with glaucoma. JAMA Ophthalmology. https://doi:10.1001/jamaophthalmol. 2020.4749
- Enoch J, Jones L, Taylor D et al. (2020). How do different lighting conditions affect the vision and quality of life of people with glaucoma? A systematic review. Eye 34(1), pp.138-154
- Alahmadi BO, Omari AA, Abalem MF et al. (2018). Contrast sensitivity deficits in patients with mutation-proven inherited retinal degenerations. BMC Ophthalmology 18. Article Number 313
- Szlyk, Janet, Seiple, William, Fishman, GA, Alexander, K, Grover, Sandeep, Mahler, C. (2001). Perceived and actual performance of daily tasks: relationship to visual function tests in individuals with retinitis pigmentosa. Ophthalmology. 108. 65-75
- Fazzi E, Signorini G, Bova SM et al. (2007). Spectrum of visual disorders in children with cerebral visual impairment. Journal of Child Neurology 22(3), pp.294-301
- Good WV, Hou C and Norcia AM. (2012). Spacial contrast sensitivity vision loss in children with cortical visual impairment. Investigative Ophthalmology and Visual Science 53912), pp.7730-7734
- Sakai S, Hirayama K, Iwasaki et al. (2002). Contrast sensitivity of patients with severe motor and intellectual disabilities and cerebral visual impairment. Journal of Child Neurology 17(10), pp.731-737
- Bola M, Gall C and sabel BA. (2013). ‘Sightblind’: perceptual deficits in the ‘intact’ visual field. Frontiers in Neurology 4. Article Number 80
- Clatworthy PL, Warburton EA, Tolhurst DJ et al. (2013). Visual contrast sensitivity deficits in ‘normal’ visual field of patients with homonymous visual field defects due to stroke: a pilot study. Cerebrovascular Diseases, 36(5-6), pp.329-335
- Dos Santos NA and Andrade SM (2012). Visual contrast sensitivity in patients with impairment of functional independence after stroke. BMC Neurology 12. Article Number 90
- Naipal S and Rampersand N (2020). Visual function and visual ability in adolescents with oculocutaneous albinism. British Journal of Visual Impairment. Article Number 0264619620973693
- Milling AF, O’Connor AR and Newsham D. (2014). The importance of contrast sensitivity testing in children. British and Irish Orthoptic Journal 11, pp.9-14
- Bennett CR, Bex PJ, Bauer CM et al. (2019). The assessment of visual function and functional vision. Seminars in Pediatric Neurology 31(SI), pp.30-40
- Havstam Johansson L, Skiljic D, Falk Erhag H et al. (2020). Vision-related quality of life and visual function in a 70-year-old Swedish population. Acta Ophthalmologica, 98(5), pp.521-529
- Leat SJ and Lovie-Kitchin JE. (2008). Visual function, visual attention, and mobility performance in low vision. Optometry and Vision Science 85(11), pp.1049-1056
- Duggan E, Donoghue O, Kenny RA et al. (2017). Time to refocus assessment of vision in older adults? Contrast sensitivity but not visual acuity is associated with gait in older adults. Journals of Gerontology Series A-Biological Sciences and Medical Sciences 72(12), pp.1663-1668
- Miyasike-da Silva V, Singer JC and McIlroy WE (2019). A role for the lower visual field information in stair climbing. Gait and Posture, 70, pp.162-167
- Subhi H, Latham K, Myint J et al. (2017). Functional visual fields: relationship of visual field areas to self-reported function. Ophthalmic and Physiological Optics 37(4), pp.399-408
- Peli E, Apfelbaum H, Berson EL et al. (2016). The risk of pedestrian collisions with peripheral field loss. Journal of Vision 16(5). Article Number 5
- De Haan GA, Melis-Donkers BJM, Brouwer WH et al. (2015). The effects of compensatory scanning training on mobility in patients with homonymous visual field defects: a randomized controlled trial. Plos One 10(8). Article Number e0134459
- Lukman AL, Bridge C, Dain SJ et al. (2020). Luminance contrast accessible tactile indicators for people with visual impairment. Ergonomics in Design 28(2), pp.4-15
- Lovie-Kitchin, J. E. a Whittaker, S. G. (1999). Interaction between acuity reserve and window size for scrolled reading rates in adults with normal and low vision. Investigative Ophthalmology & Visual Science 40(4), pp. S34-S34
- Brussee T, van den Berg TJTP, van Nispen RMA et al. (2018). Association between contrast sensitivity and reading with macular pathology. Optometry and Vision Science 95(3), pp.183-192
- Leat SJ and Woodhouse JM. (1993). Reading performance with low vision aids- relationship with contrast sensitivity. Ophthalmic and Physiological Optics, 13(1), pp.9-16
- Mohammed Z and Dickinson CM. (2000). The inter-relationship between magnification, field of view and contrast reserve: the effect on reading performance. Ophthalmic and Physiological Optics 20(6), pp.464-472
- Whittaker, S. G. and Lovie-Kitchin, J. 1993. Visual requirements for reading. Optometry and Vision Science 70(1), pp. 54-65
- Owsley C, Stalvey BT, Wells J et al. (2001). Visual risk factors for crash involvement in older drivers with cataract. Archives of Ophthalmology 119(6), pp.881-887
- Spreng L, Favrat B, Borrnat F et al. (2018). Cross-sectional study assessing the addition of contrast sensitivity to visual acuity when testing for fitness to drive. British Medical Journal Open 8(1). Article Number e018546
- UK Government. Driving eyesight rules. Available at: https://www.gov.uk/driving-eyesight-rules [Accessed 30 December 2020]
- Evans DW, Ginsbur AP. (1985). Contrast sensitivity predicts age-related differences in highway-sign discriminability. Human Factors 27(6), pp.637-642
- McCulloch DL, Loffler G, Colquhoun K et al. (2011). The effects of visual degradation on face discrimination. Ophthalmic and Physiological Optics 31(3), pp.240-248
