In 2017, a new definition of dry eye disease was introduced:
‘Dry eye is a multifactorial disease of the ocular surface characterised by a loss of homeostasis of the tear film and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.’
(2017 International Dry Eye Workshop).
Dry eye is usually classified into two categories:
- Evaporative dry eye (EDE); this is caused by excessive water loss from the ocular surface in patients with normal lacrimal secretion and may be a result of intrinsic (meibomian oil deficiency) or extrinsic (vitamin A deficiency or related to contact lens wear) factors.1
- Aqueous-deficient dry eye (ADDE); a condition where tear production is reduced.2
Tear film instability and hyperosmolarity, ocular surface inflammation and damage as well as neurosensory abnormalities certainly play aetiological roles in this disease.3 Nevertheless, besides many ocular risk factors, there is much evidence to confirm that dry eye disease (DED) is associated with various systemic disorders.
This article offers a review of these associations.
Dry eye and heart disease
Cardiovascular disease (CVD) is a term used to describe a group of disorders of the heart and blood vessels that includes, among others, coronary heart disease (CHD), peripheral artery disease and hypertension. The main risk factors for CVD are increased blood pressure (BP), elevated blood glucose, raised blood lipids, along with weight increase and obesity. In the UK, there are around 7.6 million people with various forms of circulatory diseases.4
Dyslipidaemia, a risk factor for CVD, describes a group of metabolic abnormalities characterised by any or a combination of the following:
- Raised low-density lipoprotein cholesterol (LDL-C)
- Raised total cholesterol (TC)
- Raised triglycerides (TG) and low high-density lipoprotein cholesterol (HDL-C)
DED is associated with dyslipidaemia. Indeed, as lipid homeostasis is important for the stability of the tear film, the association between dyslipidaemia and DED is entirely justified. Moreover, disruption of cholesterol biosynthesis is also associated with meibomian gland (MG) dysfunctions (figure 1).5 Indeed, it has been previously proposed that the relationship between hypercholesterolemia and DED can be explained as increased levels of cholesterol in the meibomian lipid would increase its melting point, thus leading to increased viscosity and plugging of the meibomian orifice.6
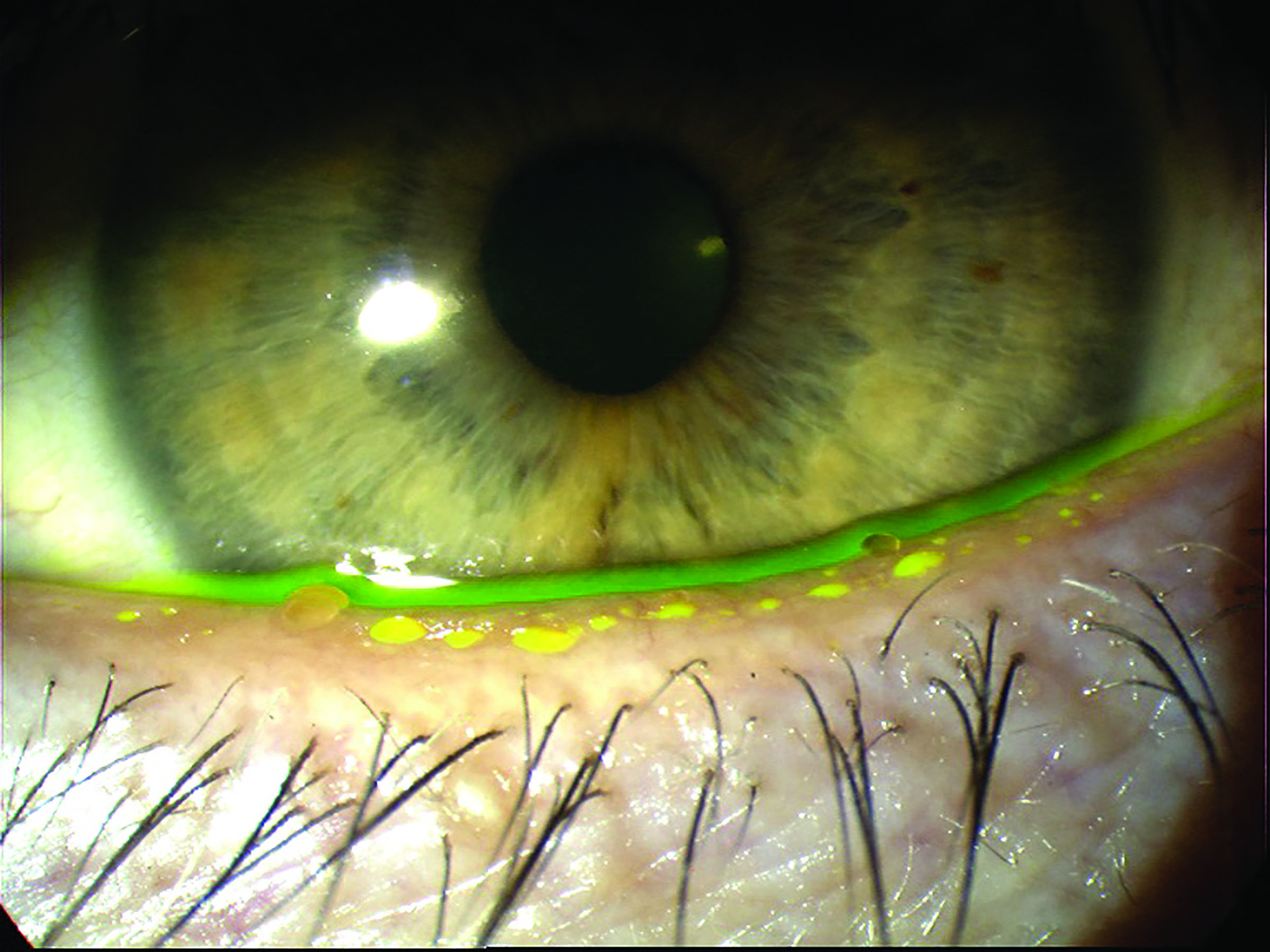 Figure 1: Meibomian gland dysfunction is associated with disruption of cholesterol biosynthesis
Figure 1: Meibomian gland dysfunction is associated with disruption of cholesterol biosynthesis
Dyslipidaemia also represents a significant risk factor for CVD. In particular, this is due to its contribution to the pathogenesis of atherosclerosis, not only in medium-sized and large arteries but also at the microvascular level. This has catastrophic effects on the balance between the physiological dilated and constricted vascular states which, in turn, will also affect other important circulatory functions and, most importantly, vascular protection against oxidation, inflammation and thrombosis.7 This imbalance characterises the so-called endothelial dysfunction (ED), an initial reversible step in the development of atherogenesis. Nevertheless, it is also one of the most important stages in the development of CVD. Therefore, its identification as early as possible represents a key factor in CVD prevention.7
Besides having common risk factors, other relationships between DED and CVD are not yet well understood. Recently, the author and co-researchers have found that individuals with a positive diagnosis of DED exhibit abnormal retinal microvascular function and a possible higher risk for CVD (figure 2).8
 Figure 2: A possible link between dry eye disease and cardiovascular disease risk has been established
Figure 2: A possible link between dry eye disease and cardiovascular disease risk has been established
It is interesting to note that patients with DED have previously been shown to exhibit abnormal microvascular response and reduced blood flow at the level of the conjunctival vessels after trigeminal stimulation. This finding suggests that these patients suffer an imbalance in the autonomic nervous system (ANS).9 Indeed, conjunctival vessels have a dual autonomic innervation (figure 3). Moreover, DED has been proposed to be associated with various ANS dysfunctions, as autonomic nerves are abundant in MG tissue and play an important role in regulating the secretory activities of MG in animals.10
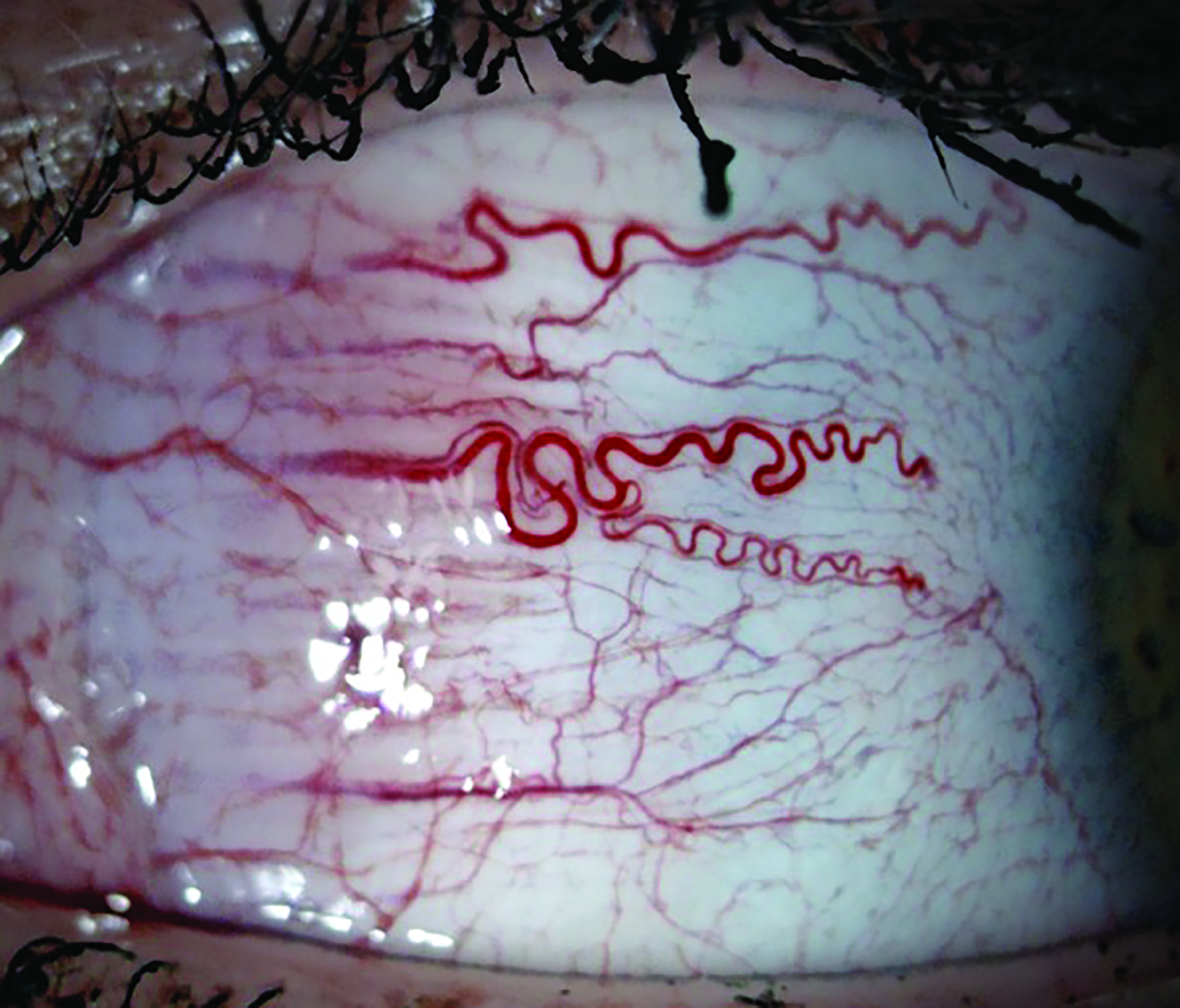 Figure 3: A possible link between dry eye disease and cardiovascular disease risk has been established
Figure 3: A possible link between dry eye disease and cardiovascular disease risk has been established
Endothelial dysfunction (ED) and ANS imbalance often coexist in the development of various CVD processes. At the retinal microvascular level, in the absence of autonomic innervation, metabolic and myogenic stimuli are more involved in retinal autoregulation of the microvascular calibre. Although the normal functioning of retinal micro-vessels is not under the influence of ANS, the results of our above-mentioned study could suggest that both the ANS and endothelial dysregulation co-exist in individuals with DED and that the results of this imbalance are evident and may be measurable at different vascular levels. Moreover, we found a positive correlation between the retinal microvascular function parameters and the levels of circulating HDL-C in DED individuals. As this circulatory parameter is a strong indicator of risk for CVD, this observation is very important and, in addition to the above-mentioned microcirculatory abnormalities, points to the fact that DED individuals could exhibit higher risk for CVD than age- and sex-matched normal individuals. Functional retinal assessments could therefore be useful for early vascular screening, possibly contributing to a reduced risk for CVD morbidity in these individuals (figure 4).
 Figure 4: Retinal assessment may have a use in screening for cardiovascular disease
Figure 4: Retinal assessment may have a use in screening for cardiovascular disease
Dry eye and obesity
In 2019, it was estimated that 28% of adults in England are obese and a further 36.2% are overweight. The impact of obesity on health and its deleterious effects on the body are well known.11 Excess weight and obesity are established risk factors for the development of CVD and metabolic diseases, such as type 2 diabetes. Obesity is also an important risk factor for other non-communicable diseases, such as stroke, asthma and cancer.
In addition to its systemic complications, some studies have also established associations between obesity and various ocular pathologies (figure 5). These include:
- Glaucoma
- Cataract
- Diabetic retinopathy
- Floppy eyelid syndrome
 Figure 5: There is an established link between obesity and a range of ocular disease. A link with dry eye disease is less certain
Figure 5: There is an established link between obesity and a range of ocular disease. A link with dry eye disease is less certain
Nevertheless, the influence of obesity upon the health of the ocular surface is less clear, with many conflicting reports having been published. Indeed, although obesity is associated with abnormal lipid metabolism and dyslipidaemia, which are associated with dry eye disease,12 to date there is no research strongly linking obesity to DED. In addition, although high levels of body fat have been associated with dry eye symptoms in a general population, a high body mass index (BMI), which represents the main parameter considered when a person is classified as obese (table 1), was not found to be linked to changes in the ocular surface.
 Table 1: Interpreting body/mass indices
Table 1: Interpreting body/mass indices
Moreover, although tear hyperosmolarity, along with reduced tear stability and volume, was reported in patients suffering from metabolic syndrome (a combination of DM, high blood pressure and obesity13), a systematic review of this disease concluded that all its components were risk factors for dry eye disease with the exception of obesity.14 So, the link between obesity and changes to the ocular surface are controversial, perhaps due to the many confounding environmental and comorbidity factors in the cross-sectional cohorts studied.
The effect of significant weight loss, either through non-surgical or surgical methods, upon the quality of the ocular surface is also unclear. While an improvement in the circulatory levels of lipids associated with significant weight loss could lead, in theory, to beneficial effects on the quality of the ocular surface, to date there is no research to clearly reflect this. Moreover, although bariatric surgery is associated with significant beneficial effects overall, including higher remission of chronic diseases such as diabetes, it can also result in severe nutritional deficits due to restricted diets and changes in the anatomy and physiology of the digestive tract. These deficiencies could have effects at multiple levels, including the ocular surface. Indeed, it has been shown that patients with a history of bariatric surgery demonstrate abnormal tear film and have higher risk of developing dry eye.15
Nevertheless, another study that performed pre- and post-operative measurements in different groups of patients did not find this effect. In a recent study (as yet unpublished data), the author and colleagues have found that, despite a significant reduction in weight and an improvement in systemic blood pressure and circulatory lipid levels, there were no significant changes after bariatric surgery on the ocular surface health, even in those with pre-existing ocular surface damage. While a clear link between obesity and various ocular surface damage is not clear, the role of adipokines, secreted by the adipose tissue, in causing systemic chronic inflammation is well established.16 Moreover, studies performed on animals demonstrated a link between adiponectin, a 30-kDa multimeric protein that is mainly secreted by white adipose tissue and tear secretion in ageing mice.17 Therefore, the presence of dry eye and/or various degrees of ocular surface inflammation in individuals with obesity is perfectly plausible. Moreover, in patients that undergo the so-called Roux-en Y gastric bypass (RYGB) procedure, a mixed restrictive and malabsorptive technique that improves circulatory cholesterol (HDL, LDL and total) and triglycerides, the procedure is also known to increase significantly the risk of various nutritional deficiencies. These include deficiencies of vitamin B12, folate and vitamin A, all very important for ocular surface health.18-20 In addition, deficiencies of vitamins B6 and B12 and folate also cause high levels of homocysteine, another systemic imbalance previously linked not only to an increased risk of CVD, but also to dry eye and other ocular conditions, such as glaucoma and retinal artery and vein occlusions (figure 6).
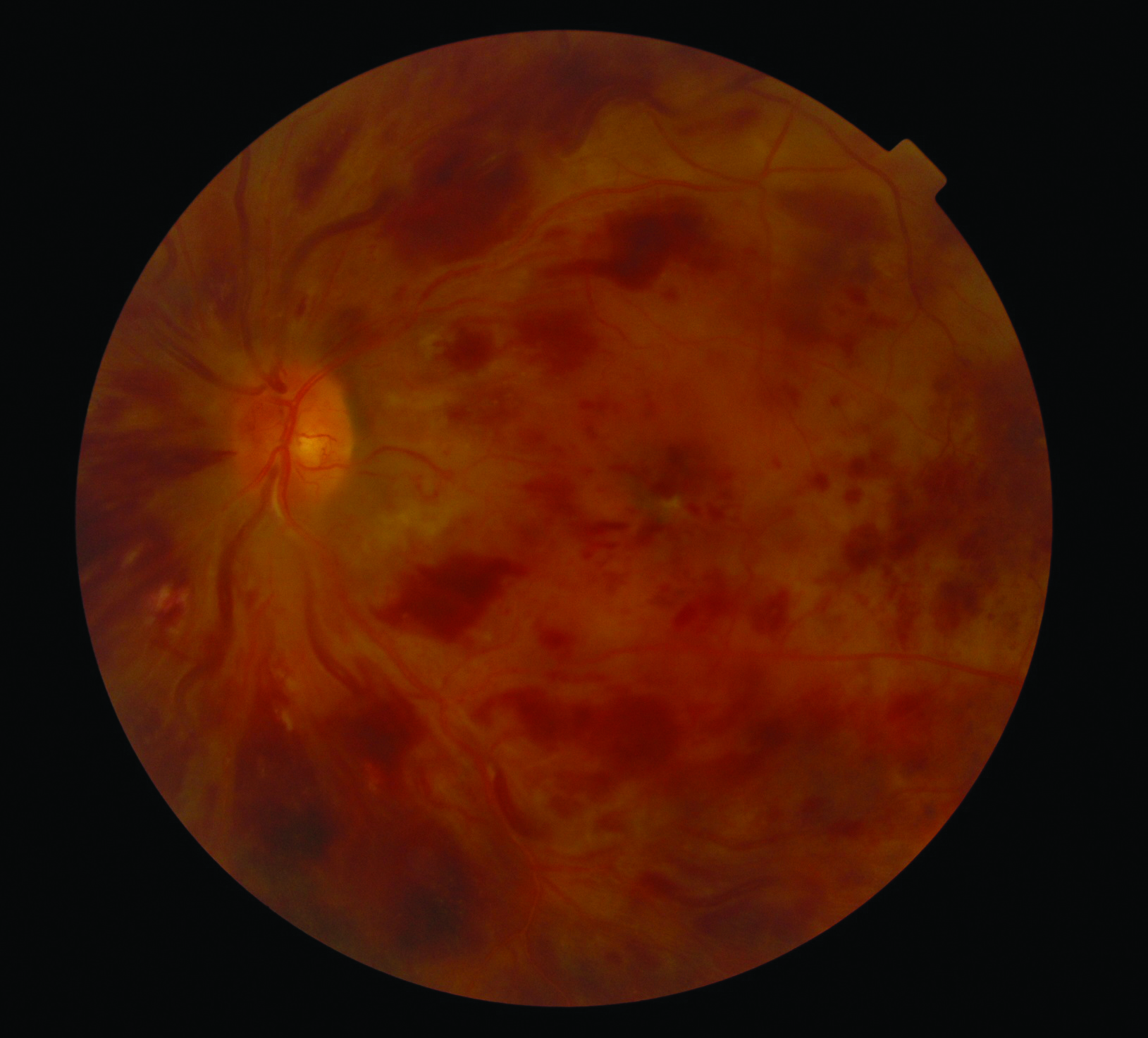 Figure 6: Some surgical treatments of obesity may result in increased levels of homocysteine which increases the risk of dry eye disease and vascular occlusions
Figure 6: Some surgical treatments of obesity may result in increased levels of homocysteine which increases the risk of dry eye disease and vascular occlusions
Dry eye and diabetes
In the UK, it is estimated that more than 4.8 million people have diabetes mellitus, 90% of whom have type 2 diabetes.21 Diabetes is one of the most important systemic causes of DED (figure 7). Indeed, between 20 and 50% of patients with diabetes also suffer from DED22 and the incidence is even higher in women. Moreover, there is also a positive correlation between DED and the duration of diabetes.
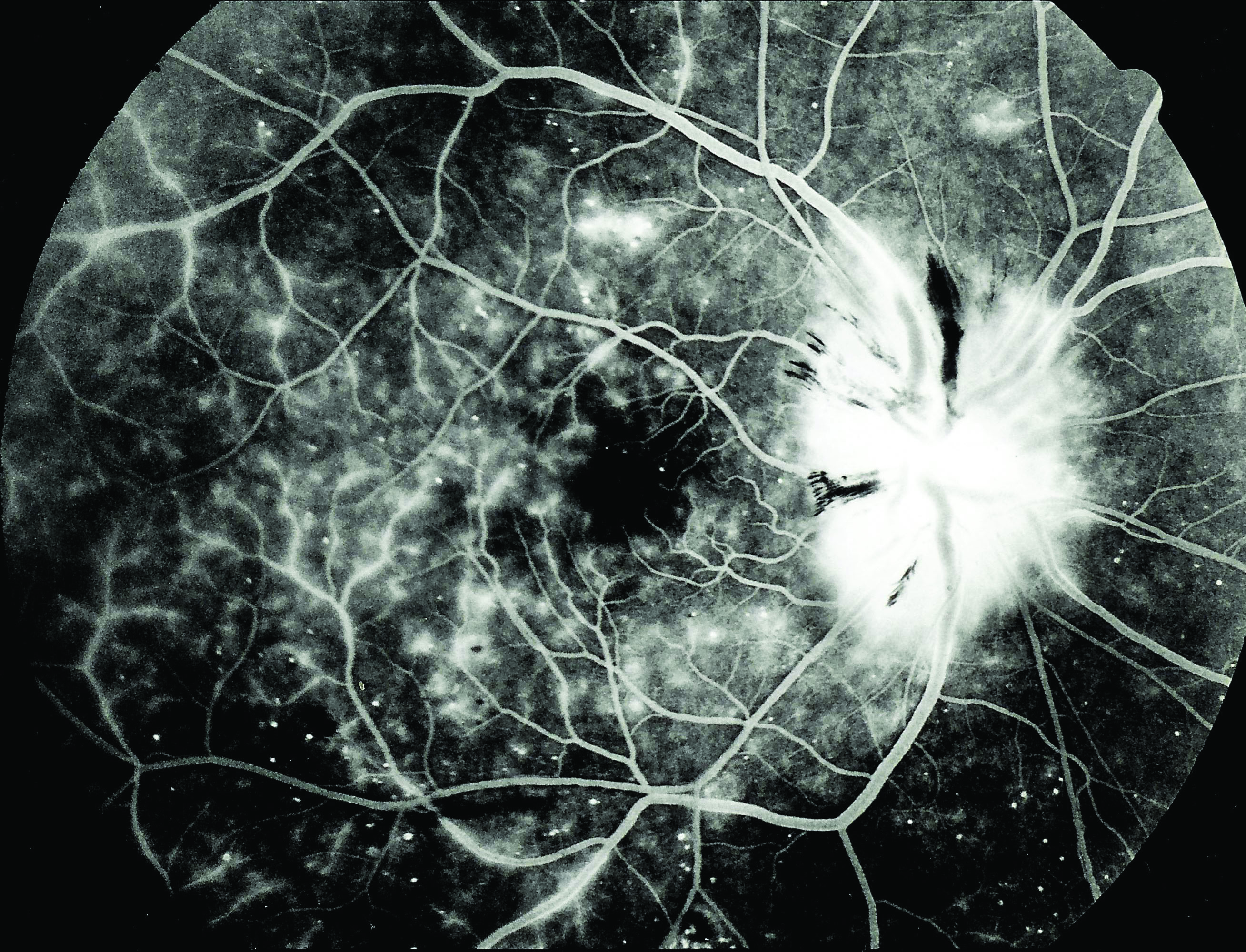 Figure 7: Diabetes is one of the most important systemic causes of dry eye disease
Figure 7: Diabetes is one of the most important systemic causes of dry eye disease
Chronic hyperglycaemia has a number of consequences, including diabetic neuropathy, microvasculopathies and osmotic disturbances. These, in turn, result in dysfunction of the lacrimal apparatus, abnormal tear dynamics and tear film dysfunctions. As a result, diabetic patients are prone to a number of challenges to ocular surface health.23 These include:
- Insufficient tear production
- Excess tear loss
- Abnormalities in blinking (figure 8)
- Changes in tear film composition
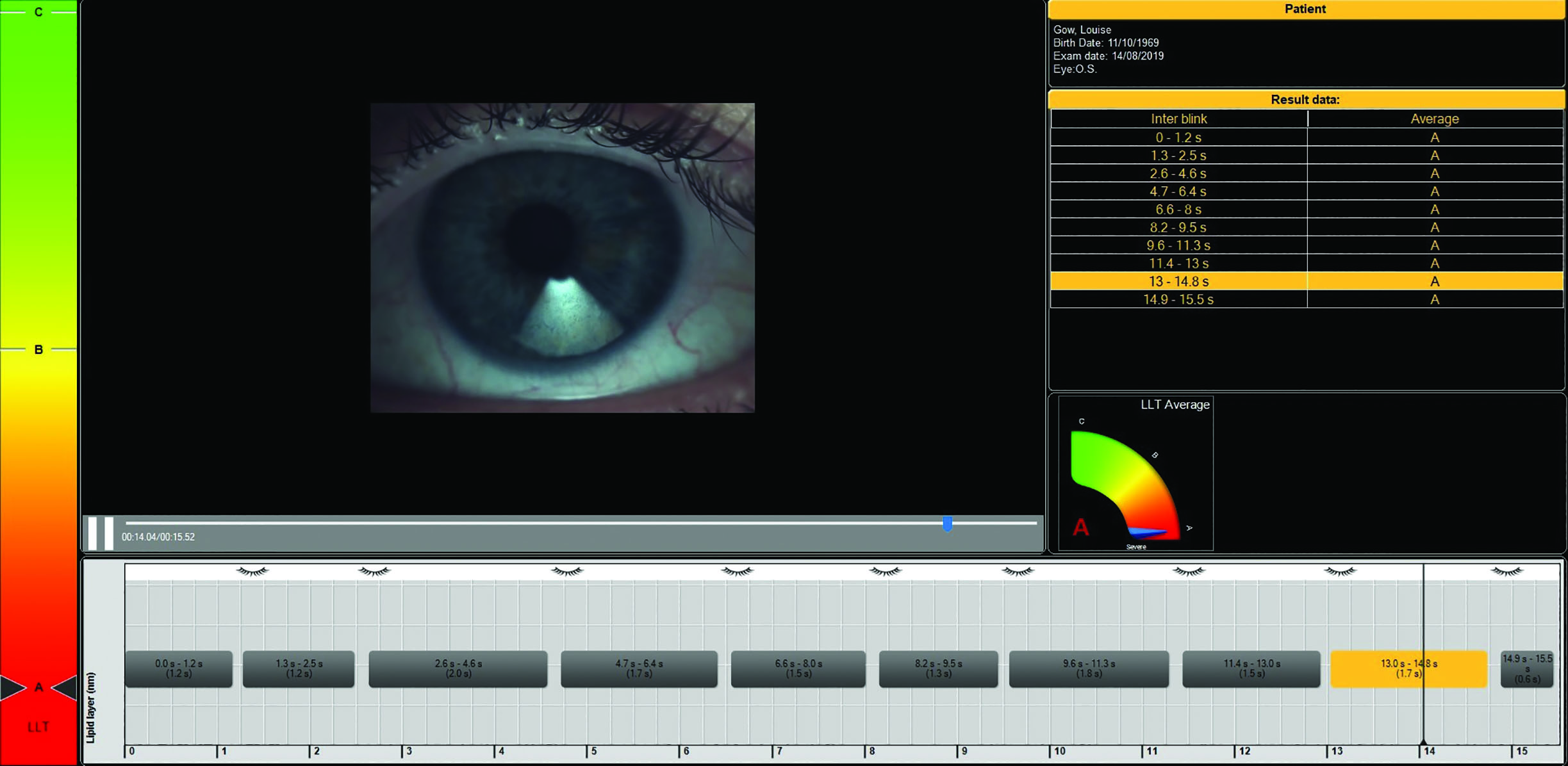 Figure 8: Blink pattern irregularities occur with diabetic hyperglycaemia
Figure 8: Blink pattern irregularities occur with diabetic hyperglycaemia
In addition, hyperglycaemia also triggers an inflammatory cascade that affects the lacrimal gland. DED is also associated with the existence of diabetic retinopathy (DR). Indeed, DR is linked with various tear-film abnormalities, such as decreased tear-break-up time (figure 9) and reduced Schirmer’s test measurements.24
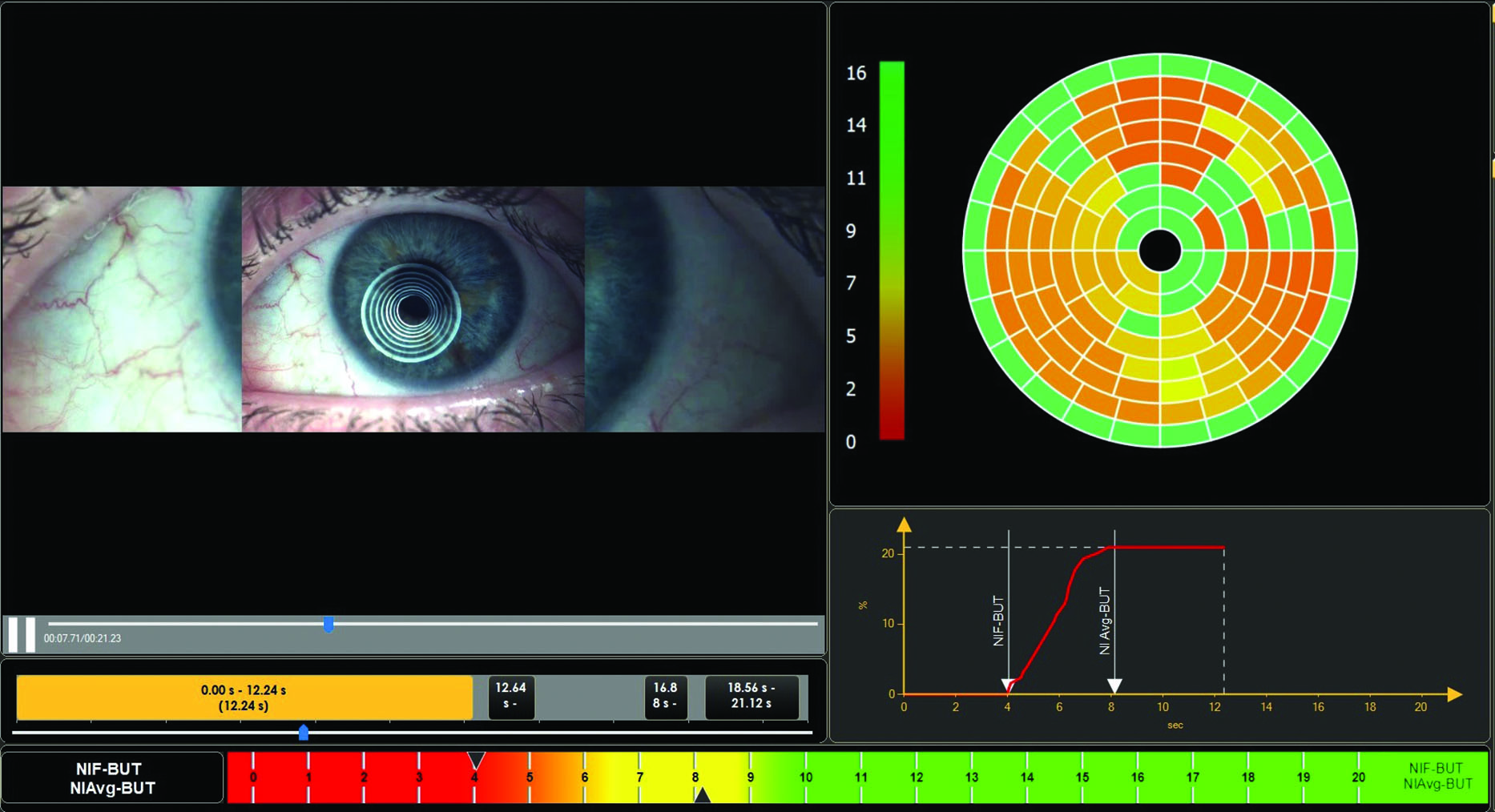 Figure 9: Reduced tear break-up time results from hyperglycaemia
Figure 9: Reduced tear break-up time results from hyperglycaemia
Due to the associated neuropathies and the high risk of infection in diabetic patients, DED can result in complications, such as conjunctivitis, keratopathies and recurrent corneal lesions (figure 10). Moreover, many diabetic patients lack or have diminished corneal sensation. A dangerous consequence of this situation is that, due to lack of symptoms, these patients are likely to ignore their condition and present very late with advanced, infected corneal lesions, visual impairments and scarring. Therefore, all patients with diabetes should be screened for the existence of DED or ocular surface disease and treatment should be prescribed appropriately.
 Figure 10: Recurrent corneal erosions are associated with diabetes
Figure 10: Recurrent corneal erosions are associated with diabetes
Dry eye and thyroid disease
The most common types of thyroid disease are hypothyroidism and hyperthyroidism. Grave’s disease is an autoimmune disorder associated with hyperthyroidism. It is caused by a combination of genetic, epigenetic, and environmental factors. When there is ocular involvement, this is described as Graves’ ophthalmopathy (GO), also sometimes called thyroid-associated orbitopathy, thyroid eye disease, or Graves’ orbitopathy. GO is characterised by the following:
- Upper eyelid retraction
- Lid oedema
- Erythema (reddening) of the periorbital tissues and conjunctivae
- Proptosis
DED is very common in GO. However, the mechanism behind this relationship is still unclear. Corneal exposure could be one reason, though aqueous tear deficiency is also common in these patients.25 The so-called ‘evaporative dry eye’ encountered in GO is due to the mechanical impairment of the lids that results from a combination of factors including hypertrophy of the extraocular muscles, fibrosis of the levator muscle complex, and an increase in the amount of orbital fat and connective tissue.26 In addition, incomplete blinking leads to an inadequate tear distribution over the ocular surface.
Patients with GO have an abnormally high tear film osmolarity,27 which can increase ocular surface inflammation (figure 11). It has also been shown that patients with GO have an abnormal tear film protein profile, which implies dysfunction of the lacrimal gland.28
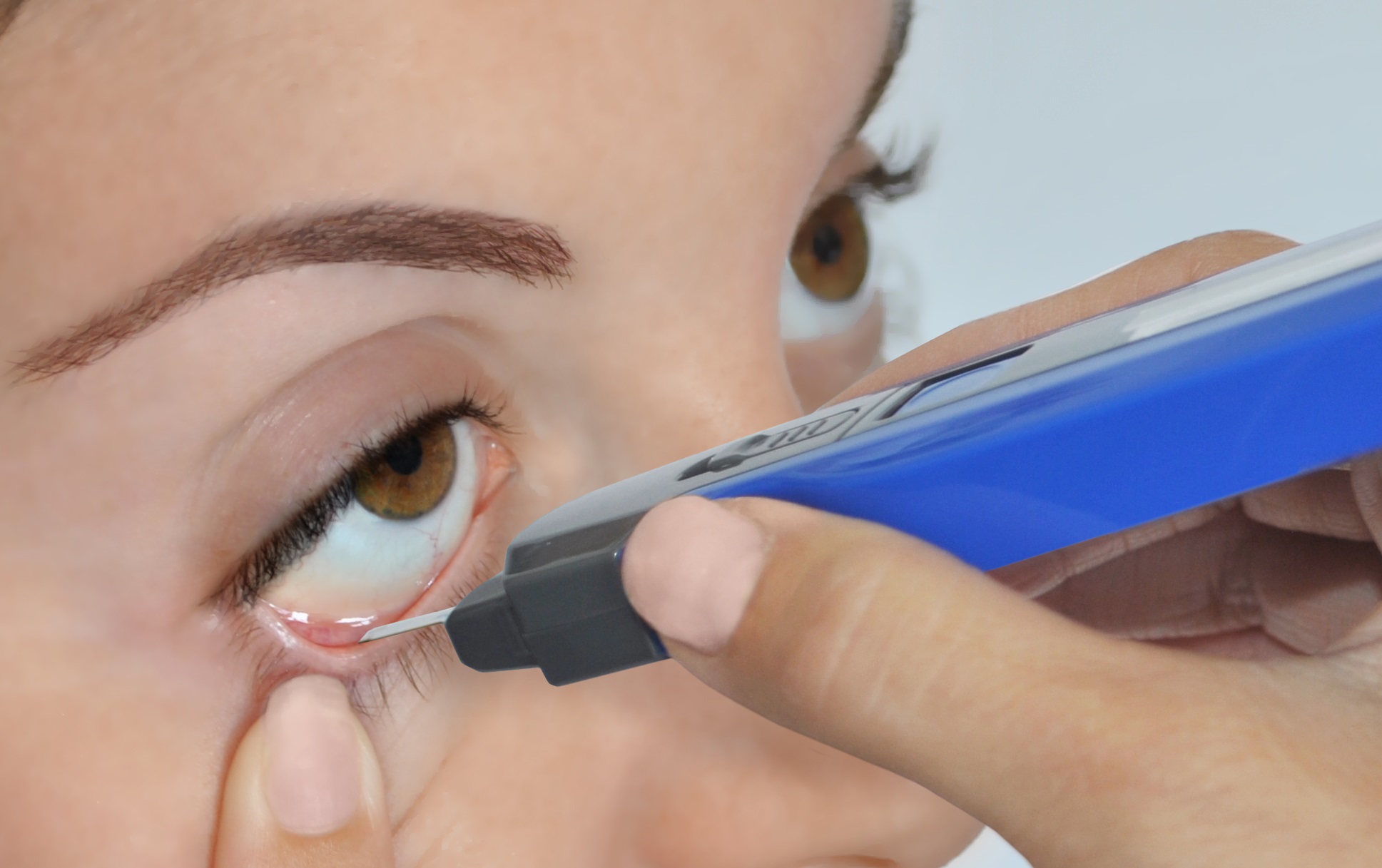 Figure 11: Hyperosmolarity of the tears is found in Grave’s ophthalmic disease
Figure 11: Hyperosmolarity of the tears is found in Grave’s ophthalmic disease
Hypothyroidism, due to underactivity of the thyroid gland, can be caused by diseases such as Hashimoto’s thyroiditis. Although ocular complications are less frequent than in hyperthyroidism, it is possible for patients with Hashimoto’s disease to also suffer from dry eye.29 Therefore, ECPs should work closely with endocrinologists to actively detect and treat DED found in patients with various types of thyroid diseases.
Dry eye and Sjögren’s syndrome
Sjögren’s syndrome (SS) is an autoimmune disease caused by the infiltration of exocrine glands by lymphocytes resulting in varied glandular dysfunction.30 It usually appears in middle- to older-aged adults, with onset typically occurring in the fourth or fifth decades of life. Salivary and lacrimal glands are the most commonly affected and, therefore, the main associations with SS are dry mouth and dry eyes.
SS may be primary, occurring in the absence of other autoimmune diseases, or secondary, presenting alongside other autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus. The symptoms are very commonly attributed to other conditions and it is possible that SS might be misdiagnosed in more than 50% of cases.31
Primary SS is characterised by the presence of keratoconjunctivitis sicca (dry eyes) and xerostomia (dry mouth), collectively called the sicca syndrome.32 The occurrence of SS is attributed to a mixture of factors,33 including:
- Genetic
- Environmental
- Infections; with viruses such as hepatitis C, Epstein-Barr or human T-cell leukaemia
- Hormonal; oestrogen deficiency
A positive diagnosis of SS requires salivary gland biopsy, lacrimal gland biopsy, salivary function tests, as well as serological tests for autoantibody biomarkers.
SS is associated with a specific type of DED, namely aqueous-deficient dry eye (ADDE), which results from a failure of lacrimal secretion. Therefore, when we have a patient with DED, a differential diagnosis between ADDE and evaporative dry eye (EDE) is important. Where ADDE is found, there is a strong possibility that the patient could have SS.
Meibomian gland dysfunction (MGD) is also present in patients with SS. It has been shown that meibomian gland dropout in SS is more severe than in healthy individuals or non-SS dry eye patients (figure 12).34

Figure 12: Meibomian gland dropout is more severe in Sjögren’s syndrome
It seems that destruction of the meibomian glands in SS involves both the upper and lower eyelids.35 Corneal nerve destruction and corneal staining is also present in SS patients. Indeed, the most recently classification criteria for SS by the American College of Rheumatology/European League Against Rheumatism includes the following two indicators:36
- Ocular staining score equal to or greater than five
- Schirmer’s type 1 test of less than 5mm in five minutes
Patients with SS-related dry eye will typically complain of ocular burning and itching, grittiness, sore eyes and a foreign body sensation. However, due to the destruction of the corneal nerves, symptoms might be less severe than might be expected from the signs. Patients may also complain of tired eyes, photosensitivity, fluctuating visual acuity, discharge and intolerance to contact lenses. These patients are also likely to have signs and symptoms of dry mouth, such as a lack of taste, difficulty swallowing and speaking, sore mouth, sore and cracked tongue and a dry cough. In women, SS is also associated with vaginal dryness.
In addition to ascertaining symptoms, ECPs can also ask about other systemic pathologies, such as rheumatoid arthritis or systemic lupus erythematosus, or confirm a history of skin rashes and joint pain.37 In the US, optometrists can use an in-office finger prick or blood collection (the Sjö from Bausch & Lomb) that can give an early diagnosis of SS.
In the UK, once identifying patients with serious ADDE, practitioners should consider referring them for possible SS.
Dry eye and dermatological conditions
Atopic disorders are genetically mediated allergic diseases that include rhinitis, asthma and atopic dermatitis. They are associated with inflammatory responses to various allergens.38 They start during childhood and have seasonal variability, being worse in spring, autumn and winter.
DED, if present, can exacerbate allergic responses in these patients.39 In addition, many studies have linked asthma to DED and tear film instability.40 Antihistamines and anti-inflammatory drugs used to treat asthma and allergies may also exacerbate DED by causing tear film dysfunction.41
Rosacea represents a chronic inflammatory skin condition that affects the face (figure 13). Risk factors for rosacea and systemic associations include genetic background, smoking, alcohol consumption, obesity, and diseases as varied as metabolic syndrome, infections, inflammatory bowel disease, Crohn’s disease,
ulcerative colitis, some forms of cancer, rheumatoid arthritis and migraine.41
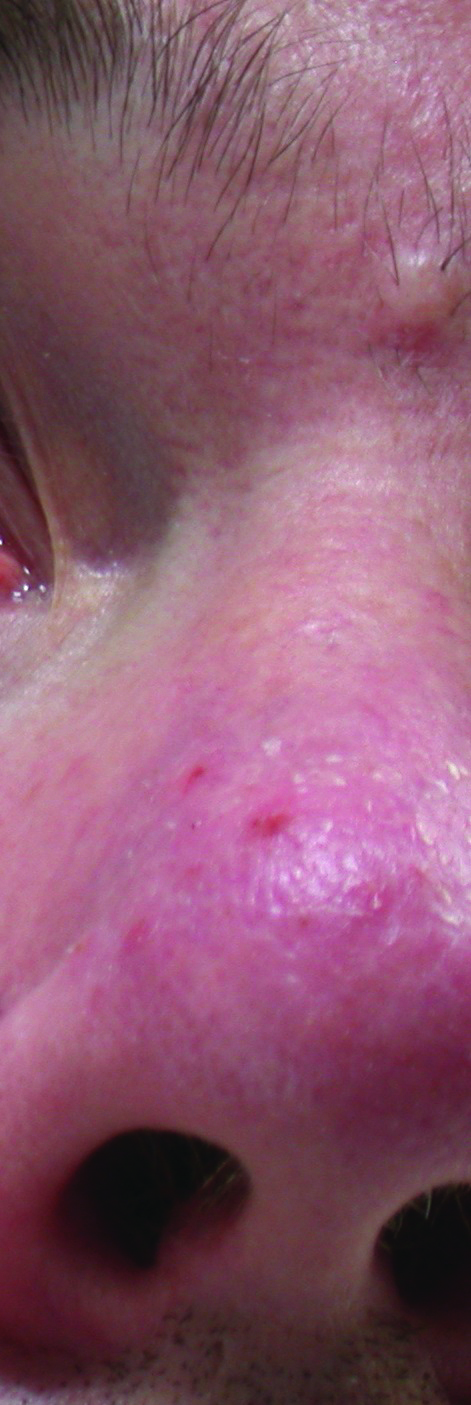 Figure 13: Rosacea manifesting on the nose and face
Figure 13: Rosacea manifesting on the nose and face
Rosacea is triggered by a number of factors, such as temperature fluctuation, sun exposure, spicy foods and drinking tea or coffee. Its clinical manifestations are many and can involve the scalp, the face and the neck but also, in some forms, the nose, ears, lids and chin. Ocular rosacea affects the MGs and leads to dry eye syndrome with reduced tear production.42
Other ocular complications of rosacea include blepharitis, keratopathy and chalazion (figure 14).
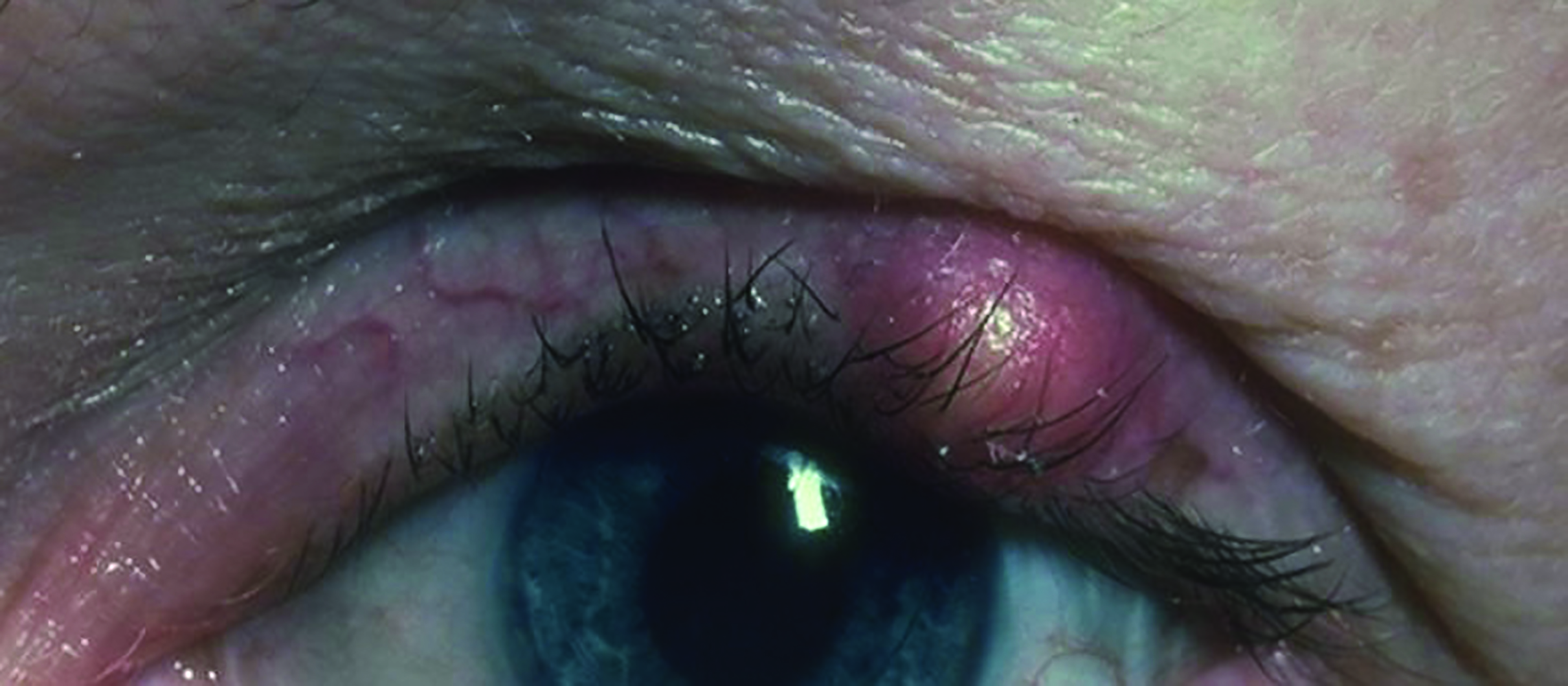 Figure 14: A chalazion might be associated with rosacea
Figure 14: A chalazion might be associated with rosacea
Dry eye and rheumatoid arthritis
Rheumatoid arthritis (RA) is the most common form of inflammatory arthritis and represents a chronic systemic autoimmune disease that is characterised by joint synovitis and multiple symmetric small and large joint lesions.43 It is usually first seen in middle-age adults and women are at higher risk than men to develop the disease.
Although it is mainly a disease that affects joints, RA can also present with extra-articular manifestations. Ocular manifestations include:
- Dry eye disease44
- Episcleritis (figure 15)
- Scleritis (figure 16)
- Peripheral ulcerative keratitis
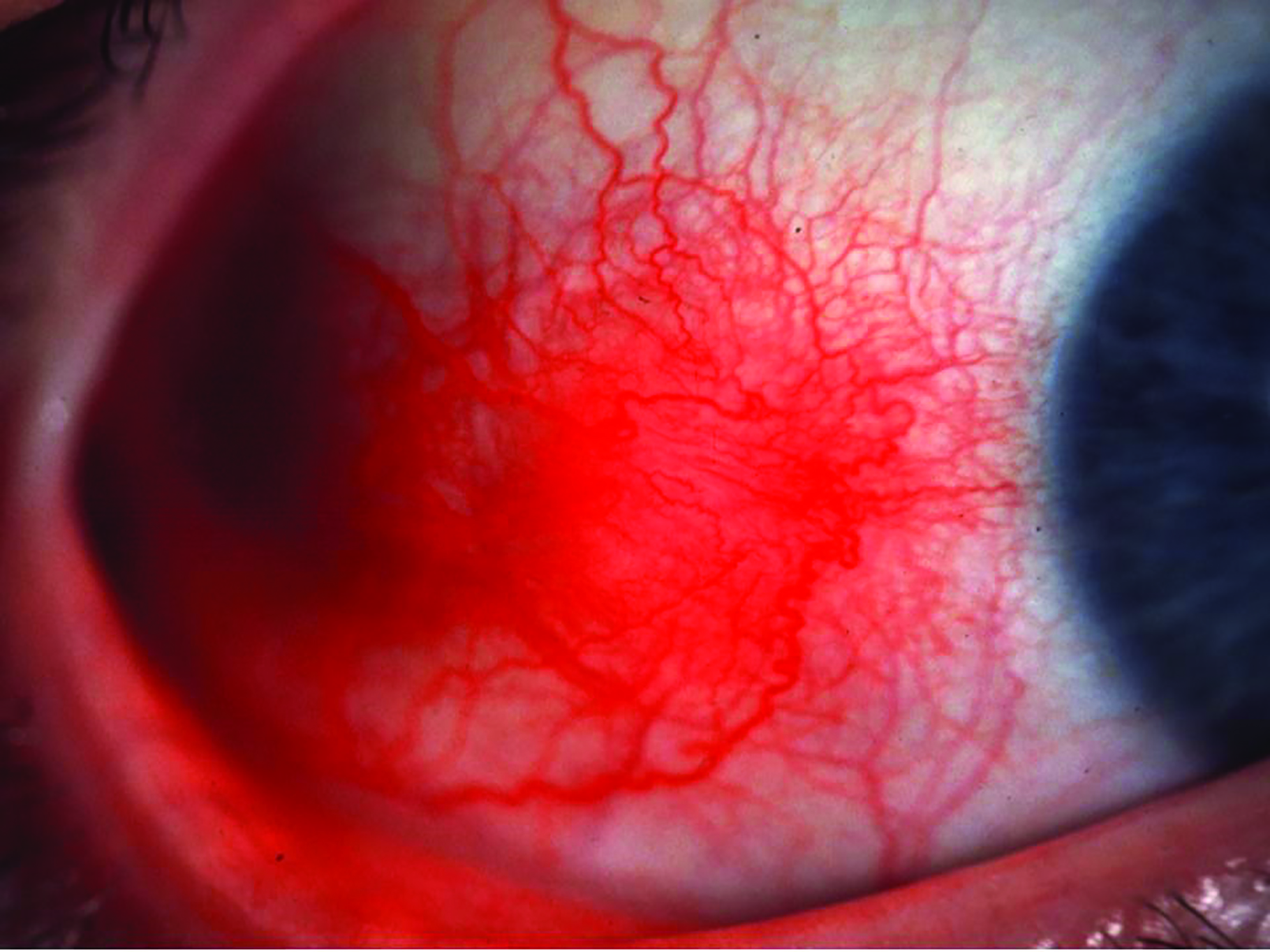 Figure 15: Episcleritis may be associated with rheumatoid arthritis
Figure 15: Episcleritis may be associated with rheumatoid arthritis
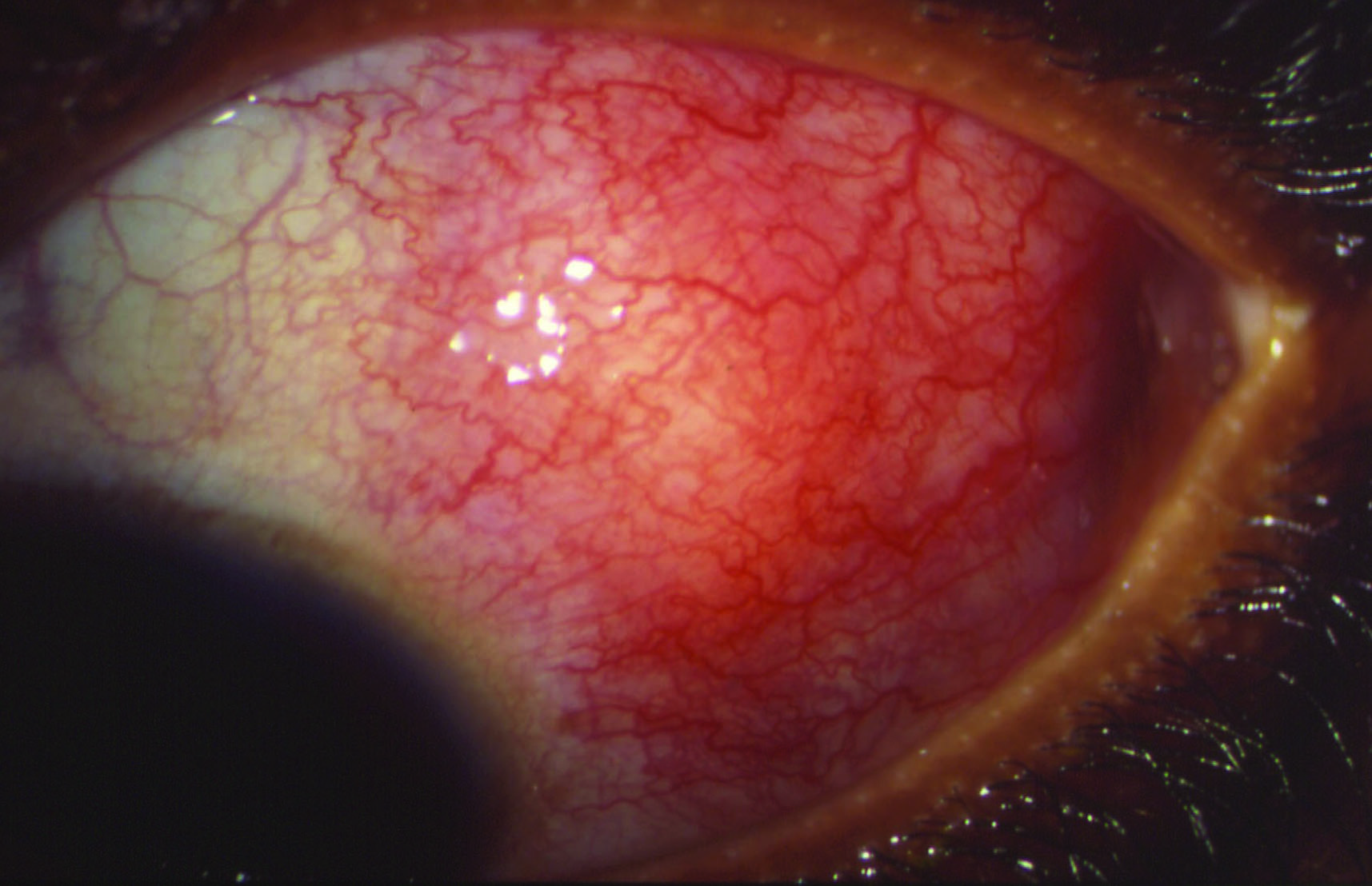 Figure 16: Scleritis may be associated with rheumatoid arthritis
Figure 16: Scleritis may be associated with rheumatoid arthritis
The association between dry eye and RA could signal secondary SS, but not in all cases. It seems that there is no direct relationship between the severity of RA and that of the dry eye.45 In addition, it has also been demonstrated that juvenile RA, a seronegative disease seen in children, is also associated with dry eye.46
Treatments for RA are also associated with ocular complications, including dry eye. Indeed, methotrexate, an antimetabolite and antifolate drug used in treating RA, is known to cause ocular irritation and dryness. Therefore, all patients with RA should be monitored for DED.
Dry eye and SLE
SLE is a chronic, autoimmune condition without known origin that results in inflammation of various organs including the skin, heart, joints, blood vessels, liver and kidneys. The diagnostic criteria for SLE were developed by the American College of Rheumatology (ACR) and is based on four of 11 criteria, either present or previously reported.47 These criteria are:
- Malar rash; a ‘butterfly rash’ on the face
- Discoid rash; circular or oval lesions on the skin
- Photosensitivity
- Oral ulcers
- Non-erosive arthritis
- Serositis; inflammation of serous membranes
- Renal disorders
- Neurological disorders; including seizures or psychosis
- Haematological disorders; including anaemia, leucopoenia and thrombocytopaenia
- Immunological disorders; anti-DNA antibody, anti-Sm antibody and false positive Venereal Disease Research Laboratory testing
- Presence of antinuclear antibodies
At the ocular level, SLE could manifest through a wide range of signs, including keratitis, episcleritis, scleritis, uveitis, retinal vasculitis, immune complex deposition in the choriocapillaris, vascular occlusions, optic neuritis, ischaemic optic neuropathy, eye movements abnormalities, visual hallucinations, visual field defects, nystagmus and cortical blindness.48
Dry eye can also be a manifestation of SLE and is found in about one third of all patients, even in the absence of a concurrent Sjögren’s syndrome. Therefore, SLE patients should be closely monitored for various ocular conditions, including DED, and managed accordingly.
Dry eye and other inflammatory conditions
Scleroderma, ‘skin thickening’ or progressive systemic sclerosis, is a chronic autoimmune condition that is characterised by fibrosis of the skin and internal organs along with vasculopathy.49 It affects the skin, but can also impact upon the lungs, the heart, the kidneys, the gastrointestinal tract, the nervous system and the musculoskeletal system.50
Systemic sclerosis is classified into two subsets based on the extent of skin involvement:51
- Limited systemic sclerosis (lcSSc)
- Diffuse systemic sclerosis (dsSSc)
It occurs more often in women. However, the most severe cases are encountered in men.
The association between scleroderma and dry eye has previously been suggested by a number of case reports. However, a recent study of 60 patients with scleroderma found that DED was present in 84% of cases, and the lipid tear dysfunction was related to the severity and duration of the disease that caused inflammation and the subsequent atrophy of the MG.52 The authors recommended that early ophthalmological examination may be necessary in all cases of patients with scleroderma, whether or not they present with ocular symptoms.
Dry eye and systemic infections
Some systemic viral infections can trigger generalised autoimmune reactions that result in Sjögren-like disease.53 These viruses are:
- Human T-cell lymphotropic virus (HTLV)
- Human immunodeficiency virus (HIV)
- Epstein-Barr virus (EBV)
- Hepatitis C virus (HCV)
HTLV is endemic in Japan, the Caribbean basin, Central and South America and Africa. It is transmitted through blood fusion, breast-feeding and sexual intercourse. Although the vast majority of patients are asymptomatic, in some cases the infection with this virus can result in adult leukaemia or lymphoma, or in a spastic paraparesis (paralysis), which can be fatal.
At the ocular level, infection with HTLV can result in uveitis, dry eye, keratitis and retinal vasculitis.54
HIV infection causes acquired immune deficiency syndrome (Aids) allowing various opportunistic infections due to a depressed immune system. At the ocular level, these infections can result in blindness. Aids is also associated with aqueous tear deficiency.55
EBV is a herpes virus that infects epithelial cells located in oropharyngeal tissues, salivary glands and B-lymphocytes.53 It has been suggested that infection with EBV results in lacrimal gland lymphocytic proliferation, which leads to decreased aqueous tear production and severe DED.56
Infection with HCV can result in retinopathy, scleritis and keratitis, and also DED. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been linked to various ocular manifestations, including dry eye, foreign body sensation, itching, blurring of vision, conjunctivitis, chemosis and photophobia.57 However, the mechanism of dry eye is unclear in Covid-19 patients and may be related to other factors, such as wearing face masks and increased screen-time. These behaviours can exacerbate the dry eye-related symptoms, especially in patients with pre-existing DED.58
Dry eye and neurological and psychiatric conditions
Neuropathic pain is a pathological manifestation of peripheral and central sensitisation promoted by peripheral nerve injury. It can include dysaesthesia (unpleasant abnormal sensations), spontaneous pain, allodynia (pain response to innocuous stimuli such as light and wind) and hyperalgesia (exaggerated pain to noxious stimuli). These can also occur at the ocular level.59 It has been shown that severe and persistent dry eye symptoms are associated with symptoms of neuropathic ocular pain.60
Chronic pain syndrome (CPS) is a common chief complaint in an adult ambulatory care setting61 and has a high impact on patients’ daily activities and can result in isolation and depression.62 CPS has also been associated with DED. In addition, patients with more severe symptoms of neuropathic ocular pain report more frequent and severe chronic overlapping pain conditions and psychiatric disease.63
Other neurological conditions associated with DED include migraine64 and Parkinson’s disease.65 Mental health status has also been linked to DED in various directions. Patients suffering from depression or post-traumatic stress disorders have demonstrated a significant association with dry eye symptom scores.66 These patients are also recalcitrant to DED treatment.
DED itself has been shown to be associated with higher symptom scores of anxiety and depression, as well as with an increased prevalence of the psychiatric conditions.67 Moreover, it has also been suggested that DED and depression share a common mechanism, highlighted by the fact that both diseases are more common in women, occur most often after menopause and are associate with an increased omega-6:omega-3 ratio.68
Anti-depressants are also linked to DED. Indeed, selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs) have antagonistic effects on muscarinic receptors, which can cause adverse effects including DED.69 Antipsychotics also have anticholinergic effects, therefore resulting in dry eye. Lithium carbonate and sodium valproate, prescribed for bipolar disorder, have been associated with decreased tear film breakup time.70
Conclusion
When talking about DED, there is more than meets the eye.
Particular attention should be paid to those patients with inconsistent dry eye symptoms and signs, and those with a poor response to conventional treatments. ECPs should always undertake a careful history and assessment in all patients presenting with dry eye symptoms or disease, and always be aware that the cause might not be a simple ocular problem.
A good knowledge of those systemic diseases that can result in or be associated with DED is imperative and ECPs should always consider a more complex background to the condition, especially among the elderly population who commonly have multiple co-morbidities and their treatments. ECPs should also be aware that an assessment of both physical and mental health may be necessary for patients with DED, and be ready to refer appropriately and timely in order to ensure an early diagnosis and personalised management as required.
- Dr Doina Gherghel is an academic ophthalmologist with a special interest in inter-professional learning for optometrists.
References
- The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye Workshop (2007) Ocular Surface, 2007;5(2):75–92
- https://www.nature.com/articles/s41433-018-0149-5?platform=hootsuite
- Craig JP, Nichols KK, Akpek EK et al. (2017): TFOS DEWS II definition and classification report. Ocular Surface, 5: 276–283.
- https://www.bhf.org.uk/what-we-do/news-from-the-bhf/contact-the-press-office/facts-and-figures#:~:text=There%20are%20around%207.6%20million,of%20heart%20and%20circulatory%20disease
- Bu J, Wu Y, Cai X et al. (2019): Hyperlipidemia induces meibomian gland dysfunction. Ocular Surface, 17: 777–786.
- Butovich IA, Millar TJ & Ham BM (2008). Understanding and analyzing meibomian lipids – a review. Current Eye Research, 33: 405–420.
- Mudau M, Genis A, Lochner A & Strijdom H (2012): Endothelial dysfunction: the early predictor of atherosclerosis. Cardiovascular Journal of Africa, 23: 222–231
- https://pubmed.ncbi.nlm.nih.gov/33576186/
- Chen W, Batawi HIM, Alava JR et al. (2017). Bulbar conjunctival microvascular responses in dry eye. Ocular Surface 15: 193–201.
- LeDoux MS, Zhou Q, Murphy RB, Greene ML & Ryan P (2001). Parasympathetic innervation of the meibomian glands in rats. Investigative Ophthalmology and Vision Science, 42: 2434–2441.
- Bohlman H. Communicating the ocular and systemic complications of obesity to patients. Optometry, 2005;76:701–12
- Rathnakumar K, Ramachandran K, Baba D, Ramesh V, Anebaracy V, Vidhya R, et al. Prevalence of dry eye disease and its association with dyslipidemia. Journal of Basic Clinical and Physiological Pharmacology, 2018;29:195–9. https://doi.org/10.1515/jbcpp-2017-0001.
- Erdur SK, Aydin R, Ozsutcu M, Olmuscelik O, Eliacik M, Demirci G, et al. The Relationship between Metabolic Syndrome, Its Components, and Dry Eye: A Cross-Sectional Study. Current Eye Research, 2017;42:1115–7
- Tang YL, Cheng YL, Ren YP, Yu XN, Shentu XC. Metabolic syndrome risk factors and dry eye syndrome: A Meta-analysis. International Journal of Ophthalmology, 2016;9:1038–45.
- Socorro Sánchez-Sánchez A, Rodríguez-Murguía N, Martinez-Cordero C, Chávez-Cerda S. Protein Diet in Bariatric Patients Could Modify Tear Film 2019. https://doi.org/10.1007/s11695-019-04310-8
- Alexopoulos N, Katritsis D, Raggi P. Visceral adipose tissue as a source of inflammation and promoter of atherosclerosis. Atherosclerosis, 2014;233:104–12.
- Shikama Y, Kurosawa M, Furukawa M, Ishimaru N, Matsushita K. Involvement of adiponectin in age-related increases in tear production in mice. Aging (Albany NY) 2019;11:8329–46
- Gilbert C. The eye signs of vitamin A deficiency. Community Eye Health Journal, 2013;26:66–7.
- Malm E, Ghosh F. Chronic conjunctivitis in a patient with folic acid deficiency. Acta Ophthalmologica Scandinavia, 2006;85:226–226.
- Ozen S, Ozer MA, Akdemir MO. Vitamin B12 deficiency evaluation and treatment in severe dry eye disease with neuropathic ocular pain. Graefe’s Archives of Clinical and Experimental Ophthalmology, 2017;255:1173–7
- https://www.diabetes.org.uk/professionals/position-statements-reports/statistics
- Hom M, De Land P. Self-reported dry eyes and diabetic history. Optometry, 2006 Nov; 77(11):554-8
- Research in dry eye: report of the Research Subcommittee of the International Dry Eye WorkShop (2007). Ocular Surface, 2007 Apr; 5(2):179-93
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4861815
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4287254
- Iskeleli G, Karakoc Y, Abdula A. Tear film osmolarity in patients with thyroid ophthalmopathy. Japanese Journal of Ophthalmology, 2008 Jul-Aug; 52(4):323-326
- Gilbard JP, Farris RL. Ocular surface drying and tear film osmolarity in thyroid eye disease. Acta Ophthalmologica (Copenhagen), 1983 Feb; 61(1):108-16.
- Khalil HA, de Keizer RJ, Kijlstra A.Analysis of tear proteins in Graves’ ophthalmopathy by high performance liquid chromatography. American Journal of Ophthalmology, 1988 Aug 15; 106(2):186-90.
- https://www.hindawi.com/journals/joph/2014/754923/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5410683/
- Kassan SS, Moutsopoulos HM. Clinical manifestations and early diagnosis of Sjögren syndrome. Archives of International Medicine, 2004 Jun 28; 164(12):1275-84.
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5410683/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5410683/
- Goto E, et al. Tear evaporation rates in Sjogren syndrome and non-Sjogren dry eye patients. American Journal of Ophthalmology, 2007;144:81–85
- https://www.nature.com/articles/s41433-018-0149-5?platform=hootsuite
- Shiboski CI, Shiboski SC, Seror R, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: A consensus and data-driven methodology involving three international patient cohorts. Annals of Rheumatic Disease, 2017 Jan;76(1):9-16.
- https://www.ophthalmologytimes.com/view/getting-root-dry-eye-know-clues-mgd-sjgrens-diagnosis
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5499014/
- https://pubmed.ncbi.nlm.nih.gov/33544639/
- Dogru M, Gunay M, Celik G, Aktas A. Evaluation of the tear film instability in children with allergic diseases. Cutaneous and Ocular Toxicology, 2016 Mar; 35(1):49-52.
- Bielory L. Ocular toxicity of systemic asthma and allergy treatments. Current Allergy and Asthma Reports, 2006 Jul; 6(4):299-305
- https://www.sciencedirect.com/science/article/pii/S0738081X19301439?via%3Dihub
- Mizoguchi, H. Iwanishi, R. Arita, et al. Ocular surface inflammation impairs structure and function of meibomian gland Experimantal Eye Research, 163 (2017), pp. 78-84
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7429800/
- https://iovs.arvojournals.org/article.aspx?articleid=2334381
- https://www.tandfonline.com/doi/full/10.1080/25785826.2020.1729597
- https://www.healio.com/news/ophthalmology/20120331/dry-eye-disease-associated-with-presence-severity-of-juvenile-rheumatoid-arthritis
- Yu C, Gershwin ME, Chang C. Diagnostic criteria for systemic lupus erythematosus: a critical review. Journal of Autoimmunity, 2014;48–49:10–13
- https://www.hindawi.com/journals/ad/2012/290898/
- https://www.thelancet.com/pdfs/journals/lancet/PIIS0140-6736(17)30933-9.pdf
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6753596/
- Barsotti S, Bellando Randone S, Guiducci S, Della Rossa. A Systemic sclerosis: a critical digest of the recent literature. Clinical and Experimental Rheumatology, 2014 Nov-Dec; 32(6
