Few areas of optometry evolve with the same rapidity as the world of contact lenses. Yet, despite the apparent speed of change, as signalled by growing product portfolios, behind the scenes developmental pipelines are often protracted with a need to overcome both technical and regulatory hurdles.
In this article we consider, in brief, the recent and expected future changes to contact lens categories, the supportive evidence and future challenges.
Drug delivery through contact lenses
Eye drops can be an ineffectual vehicle for ocular drug delivery. Immediately upon instillation, drops will enter the lacrimal drainage system, dilute in the tears, and disperse across the ocular surface and inner eye lids. Thus, the amount of drug reaching the intended site of action, ie the bioavailability, can be limited.1-2
To obviate the risk of drug loss, an intuitive response may be to simply increase eye drop volume, however, the rich vasculature and larger surface area of the conjunctiva and surrounding ocular adnexa offer a diversion away from the cornea and a route into systemic circulation. Consequently, the risk of systemic adverse effects and interactions with other drugs may be increased.2
Another common obstacle is poor patient compliance, which may present a concern especially where repeated doses or multiple types of eye drops are required throughout the day.
Despite their shortcomings, eye drops remain the main method for ocular drug delivery; they are minimally invasive and can be self-administered unlike, for example, intraocular injections. Alternative topical drug delivery methods, such as ocular ointments, are beneficial for drug retention but can lead to temporary visual annoyance due to their high viscosity.3 Systemic approaches have difficulty crossing the blood-aqueous barrier, and so higher drug doses are required to achieve the desired concentrations within the eye; a process which also increases risk of systemic toxicity.4
A potentially more efficacious approach could be in the form of drug releasing contact lenses. The proximity of the lens to the cornea could help improve bioavailability, while affording additional benefits such as dosage modulation and, if the patient is already a contact lens wearer, a potential for improved compliance.
Methods of drug release through contact lenses
Drug delivery through contact lenses has, until recently, proven elusive. Success depends upon lens properties (eg ionic charge, hydrophilicity), features of the drug (eg molecular weight, solubility) and how they interact with one another.2 In theory, the drug will be released from the contact lens into the post lens tear film, where minimal tear exchange should prevent the drug from washing away and thereby improve drug retention times.5 In actuality, impregnating a contact lens with a specific drug, with a slow-release mechanism, which has neither a deleterious effect upon optical performance, oxygen permeability or comfort is a challenging feat.
Some of the past and current approaches for contact lens drug release are summarised below.
- Soaking: Commercially available contact lenses are soaked in the drug solution. Depending on the drug/lens material affinity there is potential for a ‘burst release’ effect for low affinity combinations, alternatively, a prolonged drug release delay for high affinity combinations. Other factors, such as drug concentration, will also affect drug release kinetics.6-7
- Molecular imprinting: A process by which memory sites that mimic the drug’s natural receptors are formed (imprinted) within the lens.6,8 Soaking the lens in the drug saturates these sites.9 Benefits may include greater loading and a slower drug release.10 With reusable lenses, to maintain similar levels of daily drug doses, the lens must be thoroughly cleaned to remove any residual drug solution before being reloaded for the next wear.
- Drug reservoirs: Biodegradable polymers containing the drug, that can be tailored to modulate drug release, are incorporated into the lens. Typically, these are positioned in the lens periphery to avoid interfering with the central lens optics.6
- Drug release barriers: A barrier, such as vitamin E, is added to extend the path that a drug must follow prior to release, thus extending the drug release time.6,11-12 Some barriers are added as layers within which the drug is held, this can also help increase drug loading. Interactions with the tear film and blinking lead to drug release from these layers, with the slowest release originating from the innermost layers and the most rapid from the outer layers.13
- Nanoparticle integration: Nanoparticles, that have a high affinity for a specific drug, are incorporated throughout the contact lens. Some of these nanocarriers are biodegradable, therefore degradation will lead to drug release, others make use of external stimuli such as light or temperature changes to elicit drug release.14
- Material ionic charge: The ionic charges of a drug and lens influence drug loading and release. The lens material charge can be manipulated to align with the drug of choice.13,15
Challenges
Drug release profiles continue to be one of the fundamental issues with drug eluting contact lenses. If a drug that typically requires multiple timed doses is released too rapidly (a burst release), then the individual may need to replace their contact lens in time for their second dose, clearly negating any convenience benefits afforded by drug release lenses. A summary of some of the obstacles encountered by researchers is listed in figure 1.
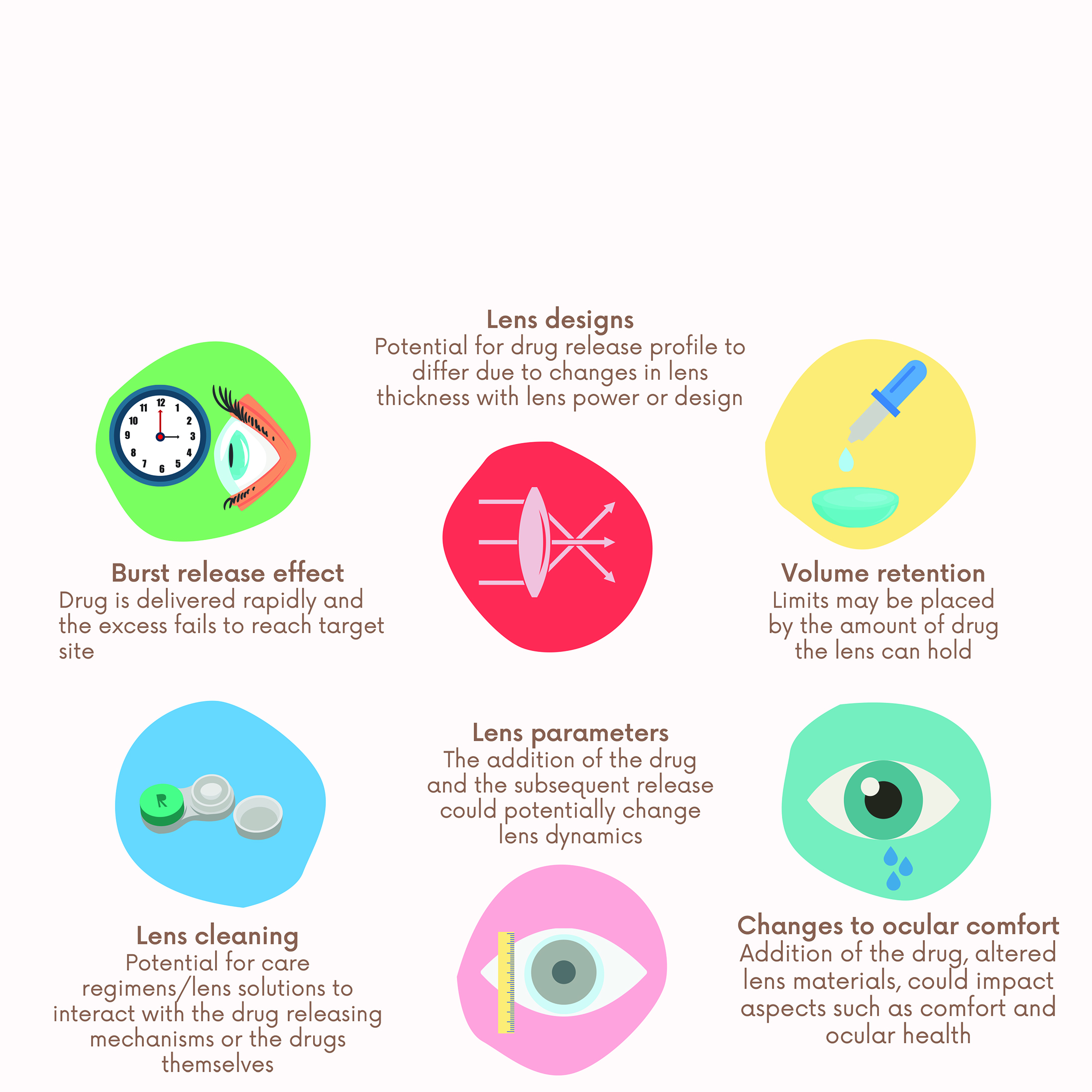 Figure 1: Obstacles faced by researchers investigating drug eluting lenses include issues relating to drug release kinetics, storage, drug stability, potential changes to lens dynamics, ocular comfort and visual experience2,6,7,13,14
Figure 1: Obstacles faced by researchers investigating drug eluting lenses include issues relating to drug release kinetics, storage, drug stability, potential changes to lens dynamics, ocular comfort and visual experience2,6,7,13,14
The present
Several different drugs, and many commercially available contact lenses,16 have been assessed for their viability in contact lens drug release. Applications have been proposed for both anterior and posterior eye diseases,17-19 each with differing levels of success. Not all attempts have been futile, products have reached late-stage clinical trials, but commercialisation has largely proven elusive. One product of note includes the antihistamine (ketotifen) releasing lens,16 which has demonstrated a positive impact on mean ocular itching scores compared to standard contact lenses.20-21
The future
As contact lenses that mediate drug release become more widely available, eye care practitioners (ECPs) may begin to consider the clinical and commercial implications of this novel lens category on their own practice. As yet, data on practitioner and patient views towards drug eluting lenses remains limited and the early evidence is mixed.22-25 Further work investigating safety, tolerability and efficacy could help in understanding the likely uptake by patients and practitioners.26
Smart contact lenses
Smart contact lenses have captivated the attention of audiences both within and outside the optometric profession. While part of the enthusiasm may be attributed to the involvement of technology conglomerates such as Google, it is also likely part of a broader trend towards wearable health technologies.27
A paucity of published scientific literature can make it difficult to look beyond the press releases, but patents and clinical trial registrations can offer some clues to the direction in which the field is moving.
Methods by which smart contact lenses work
Contact lens developers can make use of various photometric, optical and bio-sensors to detect changes in the eye or visual environment. For example, piezoresistive sensors or strain gauges, can be used to detect pressure changes,28 ie a potential application for measuring intraocular pressure; and microfluidic sensors to detect changes in biological components such as enzymes or antibodies within the tears, ie a potential application in monitoring ocular infections and allergies.29 Multifunctional lenses that can monitor more than one disease are also in development, and the use of newer materials30 such as graphene is helping to move this field forwards.31
There are several lens subcategories that fall under the umbrella of the ‘smart lens’, a brief overview is provided below.
Disease monitoring lenses
Diabetes
Compared to blood testing, disease detection through tears and contact lenses offers a relatively less invasive approach, with the added potential of providing more comprehensive clinical data, eg diurnal variations.32
To overcome the need for home finger-prick blood tests, companies such as Microsoft and Google have tried developing contact lenses for monitoring tear glucose levels.335
Several approaches have been proposed34-35 including use of graphene sensors;302 and boronic acid based attempts to develop lenses that fluoresce in the presence of raised glucose.36-37 Thus far, there seems to be little in the way of commercially available products.
Intraocular Pressure (IOP)
The Sensimed Triggerfish contact lens is a FDA approved and CE marked device for measuring diurnal intraocular pressure fluctuations for periods of up to 24 hours, intended for use in individuals at risk of glaucoma.38 A strain gauge sensor, embedded within the soft disposable contact lens, detects changes in corneoscleral shape. Information is then sent wirelessly to an adhesive external antenna, attached near the eye, before being passed onto a wearable portable recorder.39 At the end of the recording period, the recorder data can be transferred to the practitioner. The intention is to monitor fluctuations in IOP; these are recorded in millivolts, unlike conventional IOP measurements which are recorded in millimetres of mercury, thus making it difficult to draw direct comparisons with conventional tonometry readings.40
Other novel proposals to measure IOP, using contact lenses, have included development of materials that change colour in response to pressure and moisture changes.41
Dry eye
Dry eye related conditions are linked to the presence of various biomarkers within the tears.42-43 There is potential to both detect and treat dry eye conditions using smart contact lenses.41,44
There have also been reports that biomarkers for Parkinson’s disease, multiple sclerosis and possibly for some types of cancers may be present in the tears,45-58 thereby extending the diagnostic potential of smart contact lenses to beyond ocular conditions.49
Enhancing vision
Accommodative
In addition to disease detection, smart contact lenses have been used to enhance vision, including proposals to develop
accommodating contact lenses for presbyopes.
While several approaches have been put forth50-52 one which is supported by growing research interest is the use of liquid crystal (LC) cells.51-53 A small change in voltage brings about large changes in refractive index and thus lens power. Activation could potentially be via an external device, eg a smartwatch, or possibly through deliberate blinking.54-56
Digital smart lenses
Augmented reality, mixed reality and virtual reality, are terms found more often among tech blogs than the optical press (see figure 2), but this has gradually been changing over the past decade. Frequent reports of smart glasses and contact lenses detail prospective features that include displaying of digital alerts such as text messages, weather information and the potential to capture images and video recording. Several tech giants such as Sony and Samsung have been granted patents relating to smart contact lenses;57-58 with the most recent press releases reporting on a collaboration between Menicon and Mojo Vision.59
 Figure 2: Augmented, virtual and mixed reality definitions62
Figure 2: Augmented, virtual and mixed reality definitions62
In addition to the entertainment and novelty aspects, smart lenses also possess the potential to help aid navigation, object detection and to magnify aspects of the visual environment; all potentially useful applications for individuals with visual impairment.
The delays in these, sometimes theoretical, products reaching the market are a likely mix of technical and regulatory challenges. The microelectronics need to be small and lightweight enough to not impede oxygen permeability, positioned to avoid negative impact on vision, while retaining lens dynamics and comfort. An added challenge is displaying the digital images in such a way that is both visible, but not obstructive.60-61
The future
Most smart contact lenses remain in a developmental stage. While newer materials and technological advances may overcome any technical hurdles,31 wider discussions about privacy, safety, and the role ECPs might play, will all need to take place.
Myopia management with contact lenses
It is reported that almost half the world’s population will be myopic by the year 2050;63 but a statistic of greater significance to practitioners in the UK and Ireland is that Western Europe is expected to reach this unfortunate milestone around a decade earlier. In fact, by the year 2030, an estimated ~45% of the population in Western Europe will have already become myopic.63
Axial myopia is attributed to a discord between increasing axial length and the refractive capabilities of the cornea and crystalline lens.64 Typically, a 1mm increase in axial length equates to approximately ~2.5 to 3DS of axial myopia,65-66 but differences can exist between different demographics.
Earlier onset is often linked to greater risk of progression to higher levels of myopia.67 For most individuals, progression will stabilise by the late teens,68-69 however, an unlucky minority may continue to progress beyond this point.68-69 As myopia and axial length increase, so too does the risk of developing sight threatening disorders.70 Hence, inhibiting the onset of myopia, or at least minimising the risk of high myopia, could yield significant benefits.
The reasons underlying escalating myopia prevalence are believed to be multifactorial. Exposure to a myopigenic environment (a lack of time outdoors, near work, pursuit of higher education),71-73 may increase an individual’s risk of developing myopia, elements of which may be further compounded by an existing genetic predisposition.74
Theories of myopia development and progression are wide-ranging, but the design of many myopia inhibiting contact lenses relies, in some way, on the concepts of relative peripheral hyperopia (see figure 3) and/or accommodative lag.
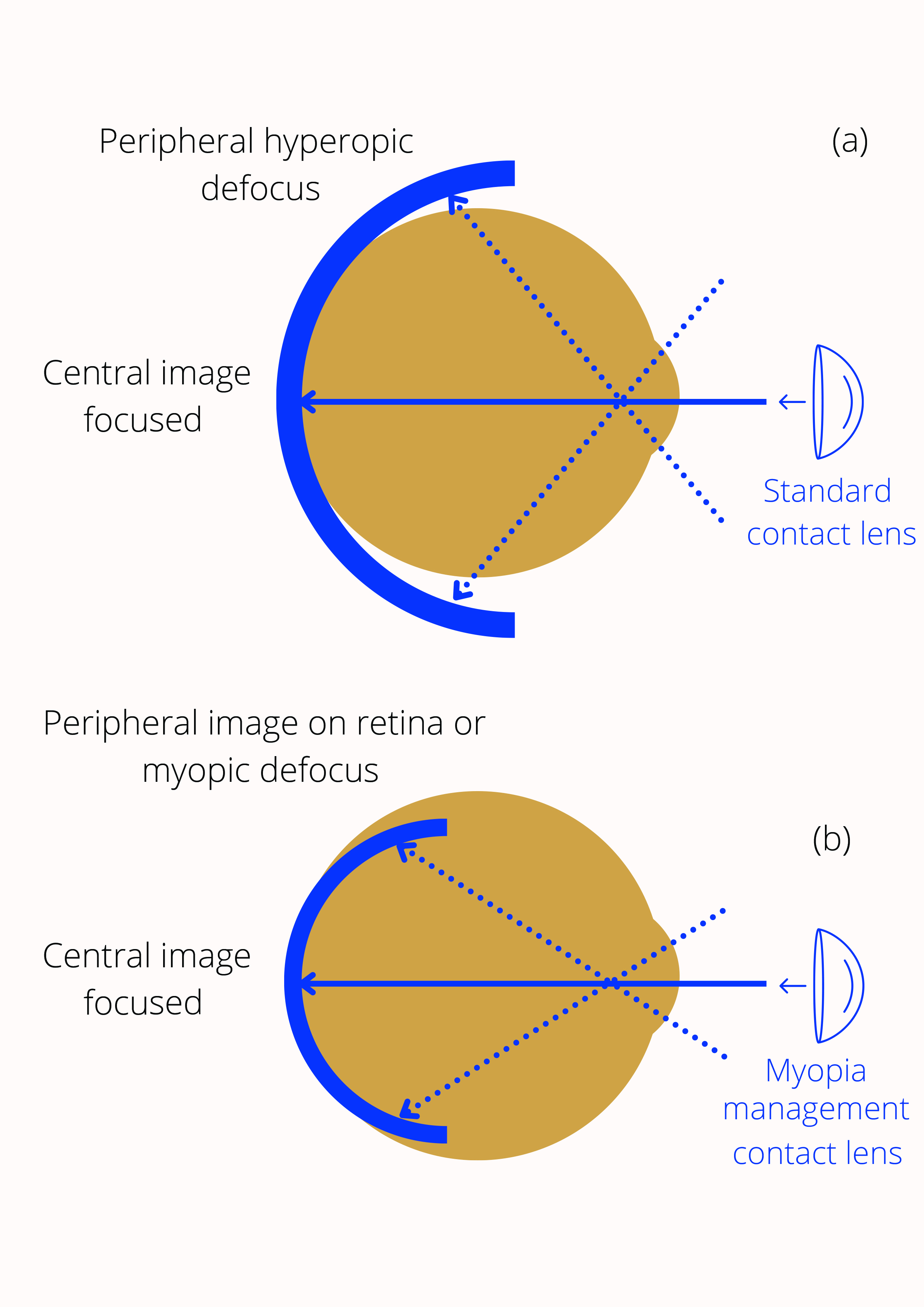 Figure 3: (a) Peripheral hyperopic defocus, relative to the central retina, is believed to influence central axial growth. Image shell falls behind retina for the peripheral region (b) Reducing this hyperopic defocus is believed to inhibit myopia progression
Figure 3: (a) Peripheral hyperopic defocus, relative to the central retina, is believed to influence central axial growth. Image shell falls behind retina for the peripheral region (b) Reducing this hyperopic defocus is believed to inhibit myopia progression
Methods of myopia management using contact lenses
While time outdoors may deter the onset of myopia, for individuals who are already myopic various myopia inhibiting solutions are gradually making their way to market. Current solutions include spectacle lenses, contact lenses and on the horizon is the potential for myopia specific pharmaceutical preparations.
Rigid gas permeable corneal lenses
Some older texts advocated the use of rigid corneal lenses to manage myopia, while this practice has now largely fallen out of favour it still persists in some parts of the world.75 There is, however, limited evidence of a genuine treatment effect.76 Any positive impact is thought to be linked to a mechanical flattening of the cornea rather than a slowing of axial elongation.76
Orthokeratology in myopia
Orthokeratology (OK) has long been used as a reversible non-surgical alternative to refractive surgery. Lenses are worn at night to temporarily reshape the cornea providing temporary relief from refractive error during the day.77-78 While in this article OK is referred to in the context of correcting myopic refractive error, the technique has also been used to correct hyperopic, presbyopic and astigmatic errors.
OK affords freedom from spectacles and contact lenses during most waking hours, however, towards the end of the day corneal shape may begin to recover, causing a regression effect76-77 of around ~0.50-0.75D. To counter this, a small amount of overtreatment is often incorporated.78
OK (or reverse geometry) lenses are unlike the rigid corneal lens designs intended for daily wear. There is a greater need for high oxygen transmissibility; stability, to ensure the same part of the cornea is treated consistently and, of course, a requirement to temporarily reshape corneal geometry.79 The latter is achieved through use of a flat central base curve and the incorporation of a secondary lens curve that is relatively steeper than both the central and peripheral curves. This arrangement creates a tear reservoir or ‘reverse zone’ that facilitates lens stability while permitting rapid changes to corneal shape.
During lens fitting and aftercare appointments the cornea is closely monitored. Corneal staining and lens binding are common complications associated with OK,79,82 but among the most serious potential complications is microbial keratitis; the risk of which increases due to overnight lens wear.
It is now well established that use of OK can inhibit axial length increase and therefore impede myopia progression.76,81 While the mechanisms underlying this inhibitory effect are unclear, some researchers have attributed it to a redistribution of corneal cells, in which there is central corneal flattening, compared to relative steepening more peripherally.82 In line with the theories of peripheral hyperopia; changes to corneal morphology could, therefore, both reduce central myopia and induce peripheral myopic defocus.83 Others suggest an increase in higher order aberrations84 or changes to accommodative response may also play a role.85
The introduction of OK lenses specifically for myopia management may signify the growing interest of manufacturers in this area of practice. Yet questions remain, including a need for further clarity and consensus around a potential rebound effect, the underlying mechanisms for the myopia inhibition effect and a need for longer-term data in some population groups.79
Soft contact lenses for myopia management
Until recently a lack of licensed soft lens products, for myopia management, led some ECPs to off-label prescribing of multifocal lenses intended for presbyopia. While these lenses generated pockets of impressive data, a published review of myopia management, that was limited to randomised controlled trials (RCTs), concluded bifocal soft contact lenses were of little benefit.76 Nevertheless, since this landmark review was undertaken, several key RCTs of soft lenses for myopia management have now been published, demonstrating an impressive slowing of both axial elongation and myopia using dual focus and extended depth of focus lenses.86-87
Evidence is still emerging and recent work has shown how manipulation of the lens add can lead to further improvements in outcomes.76 Future developments may give rise to a broader range of myopia management options including greater soft lens provisions for myopic astigmats.
The future
Myopia management is an area of active development and while many of the previous barriers to uptake have now been addressed, gaps in the knowledge base remain. Not all practitioners will wish to undertake myopia control, but it is important for all to remain informed of the options so that patients can be appropriately advised.
In the future, there may be potential for contact lens ECPs to expand myopia management offerings through combination treatments, such as combined contact lens and low-dose atropine use.
Photochromic contact lenses
Visual discomfort or impaired visual performance attributable to a bright light source is referred to as glare. The impact of glare depends upon several factors including the intensity of the light source, the angular distance, ocular adaptive state and opacities in the optical media.88-89
Glare may be broadly classified as either disability or discomfort glare; the former impairs an individual’s ability to see (eg the glare from oncoming headlights) and the latter causes discomfort compelling the individual to avert their gaze (ie photoaversion).89 The presence of glare and loss of contrast sensitivity is one of the reasons why despite demonstrably good visual acuities in the test room, some patients may experience poorer visual performance when undertaking real-life tasks.90
Since their initial introduction in the late 1960s, photochromic spectacle lenses have become an integral part of the ophthalmic dispensing options on offer to patients. It has, however, taken a further ~60 years for photochromic contact lenses to become commercially available.
While the inception of photochromic contact lens designs can be traced back to at least the mid-1980s,91-93 it was in 2018 that Johnson & Johnson Vision Care, Inc (in collaboration with Transitions Optical), secured FDA and latterly CE approval for their Acuvue Oasys with Transitions contact lens.94-95
The lens works through the incorporation of a photochromic additive throughout the lens matrix.96 Changes in ambient lighting, specifically exposure to ultraviolet (UV) or high energy visible (HEV) light, bring about changes in the light transmitted by the lens. When fully activated (ie a darkened state) only 35±5% of visible light across the range 380-780nm will be transmitted; and 85±5% when minimally activated (ie a clear lens state).97 Product literature claims it can take less than one minute for the lens to activate in situ and around one-and-a-half minutes to return to its non-activated state.95
Visual function with the lenses
Early studies have shown the lens can be successfully fitted to most neophyte contact lens wearers and compares favourably, or at least is demonstrably comparable to alternative standard optical corrections.97-101 The data also suggest the lens could be beneficial in both outdoor and,98 to a lesser degree, within indoor environments96,101 (see figure 4), ie beneficial even when in a minimally activated state.101 The improvements to indoor vision have, in part, been attributed to the lens facilitating adaptation to changes in perceived brightness.
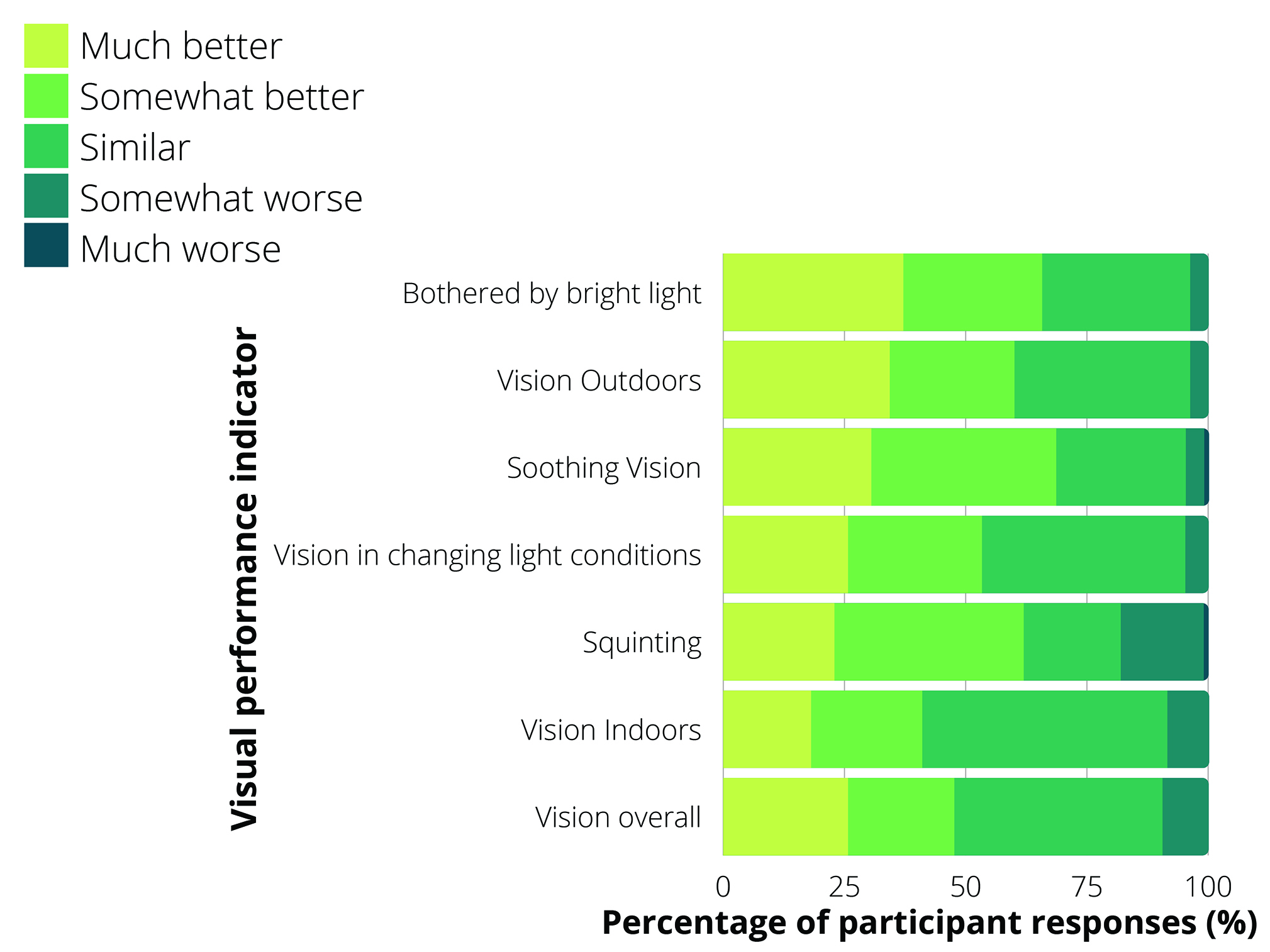 Figure 4: Subjective assessment of visual performance with the photochromic contact lens compared to spectacles by neophyte contact lens wearers at five weeks follow up (four weeks of contact lens wear, followed by one week of spectacle wear, n=105) (based on data from Buch et al 2020)99
Figure 4: Subjective assessment of visual performance with the photochromic contact lens compared to spectacles by neophyte contact lens wearers at five weeks follow up (four weeks of contact lens wear, followed by one week of spectacle wear, n=105) (based on data from Buch et al 2020)99
While objective visual performance with the lens is impressive, subjective assessment is also reasonable.96 Figure 4 shows the subjective responses of more than 100 neophyte contact lens wearers to photochromic contact lens wear over a four-week period, followed by a resumption of their habitual spectacle lenses. The largest positive gains were made for ‘soothing vision’, which was described by the authors as the ability to see comfortably under bright light. The least positive gains were made for ‘vision indoors’, but ~41% of respondents still noticed an improvement and around half reported vision to be similar.96
Driving
To date, only one study has investigated driving with the commercially available photochromic contact lenses.98 Driving performance, in both day and night-time conditions, was deemed non-inferior compared to standard clear contact lenses and to photochromic spectacles. While most parameters assessed showed no significant difference (eg lap time, hazard avoidance), one parameter that did show significant improvement with the lenses was the distance at which road signs were recognised; by an estimated 7.5m and 17.8m for both day and night driving respectively.98
Summary
The field of contact lenses continues to be an active area of development. In addition to this brief overview of new contact lens applications, additional opportunities and efforts to broaden contact lens practice are gradually reaching fruition, for example, a growing interest in telehealth,102 dry eye treatments103 and innovations in ocular imaging.104-105

When keeping abreast of changes in the field, ECPs should be cognisant of relevant professional guidance. Regulatory steps to bring a product to market exist, among other reasons, to establish product and patient safety.
- Dr Manbir Nagra is an optometrist, educator and researcher and a paid consultant for the Johnson & Johnson Institute, UK. Dr Rachel Hiscox is a Professional Education & Development Manager, UK & Ireland for Johnson & Johnson Vision Care.
- This article is part of a revised and updated ‘Essential Contact Lens Practice’ series, originally authored by Jane Veys, John Meyler and Ian Davies. This article was produced without further input or review from the original authors.
References
- Lux A, Maier S, Dinslage S, Süverkrüp R, Diestelhorst M. A comparative bioavailability study of three conventional eye drops versus a single lyophilisate. British journal of ophthalmology. 2003 Apr 1;87(4):436-40.
- Fan X, Torres-Luna C, Azadi M, Domszy R, Hu N, Yang A, David AE. Evaluation of commercial soft contact lenses for ocular drug delivery: a review. Acta Biomaterialia. 2020 Aug 25.
- Baranowski P, Karolewicz B, Gajda M, Pluta J. Ophthalmic drug dosage forms: characterisation and research methods. The Scientific World Journal. 2014 Jan 1;2014.
- Lee J, Pelis RM. Drug transport by the blood–aqueous humor barrier of the eye. Drug Metabolism and Disposition. 2016 Oct 1;44(10):1675-81.
- Grassiri B, Zambito Y, Bernkop-Schnürch A. Strategies to prolong the residence time of drug delivery systems on ocular surface. Advances in Colloid and Interface Science. 2020 Dec 13:102342.
- Toffoletto N, Saramago B, Serro AP. Therapeutic Ophthalmic Lenses: A Review. Pharmaceutics. 2021 Jan;13(1):36.
- Maulvi FA, Soni TG, Shah DO. A review on therapeutic contact lenses for ocular drug delivery. Drug delivery. 2016 Oct 12;23(8):3017-26.
- Gote V, Sikder S, Sicotte J, Pal D. Ocular drug delivery: present innovations and future challenges. Journal of Pharmacology and Experimental Therapeutics. 2019 Sep 1;370(3):602-24.
- White CJ, McBride MK, Pate KM, Tieppo A, Byrne ME. Extended release of high molecular weight hydroxypropyl methylcellulose from molecularly imprinted, extended wear silicone hydrogel contact lenses. Biomaterials. 2011 Aug 1;32(24):5698-705.
- White CJ, Byrne ME. Molecularly imprinted therapeutic contact lenses. Expert opinion on drug delivery. 2010 Jun 1;7(6):765-80.
- Kim J, Peng CC, Chauhan A. Extended release of dexamethasone from silicone-hydrogel contact lenses containing vitamin E. Journal of Controlled Release. 2010 Nov 20;148(1):110-6.
- Sekar P, Chauhan A. Effect of vitamin-E integration on delivery of prostaglandin analogs from therapeutic lenses. Journal of colloid and interface science. 2019 Mar 15;539:457-67.
- Hui A. Contact lenses for ophthalmic drug delivery. Clinical and Experimental Optometry. 2017 Sep;100(5):494-512.
- Choi SW, Kim J. Therapeutic contact lenses with polymeric vehicles for ocular drug delivery: a review. Materials. 2018 Jul;11(7):1125.
- Kakisu K, Matsunaga T, Kobayakawa S, Sato T, Tochikubo T. Development and efficacy of a drug-releasing soft contact lens. Investigative ophthalmology & visual science. 2013 Apr 1;54(4):2551-61.
- Soluri A, Hui A, Jones L. Delivery of ketotifen fumarate by commercial contact lens materials. Optometry and Vision Science. 2012 Aug 1;89(8):1140-9.
- Bengani LC, Kobashi H, Ross AE, Zhai H, Salvador-Culla B, Tulsan R, Kolovou PE, Mittal SK, Chauhan SK, Kohane DS, Ciolino JB. Steroid-eluting contact lenses for corneal and intraocular inflammation. Acta Biomaterialia. 2020 Oct 15;116:149-61.
- Li Y, Huang C, Yang X, Zhang X. Ofloxacin laden microemulsion contact lens to treat conjunctivitis. Journal of Biomaterials Science, Polymer Edition. 2020 Aug 12;31(12):1566-79.
- Maulvi FA, Singhania SS, Desai AR, Shukla MR, Tannk AS, Ranch KM, Vyas BA, Shah DO. Contact lenses with dual drug delivery for the treatment of bacterial conjunctivitis. International journal of pharmaceutics. 2018 Sep 5;548(1):139-50.
- Pall B, Gomes P, Yi F, Torkildsen G. Management of ocular allergy itch with an antihistamine-releasing contact lens. Cornea. 2019 Jun;38(6):713.
- Johnson & Johnson Vision’s Investigational Antihistamine-Releasing Contact Lens Demonstrates Positive Phase 3 Results. Johnson and Johnson. www.jjvision,com [internet]. Available from: https://www.jjvision.com/press-release/johnson-johnson-visions-investigational-antihistamine-releasing-contact-lens, publication date 26th March 2019 (accessed 28.02.21)
- Ghazal H, Ahmadouk J, Dhanji S, El-Bushra A, Kayyali R, ElShaer A. Patients’ and prescribers’ perception of contact lenses as a potential ocular drug delivery system. Contact Lens and Anterior Eye. 2019 Apr 1;42(2):190-5.
- Taniguchi EV, Kalout P, Pasquale LR, Kohane DS, Ciolino JB. Clinicians’ perspectives on the use of drug-eluting contact lenses for the treatment of glaucoma. Therapeutic delivery. 2014 Oct;5(10):1077-83.
- Varadaraj V, Kahook MY, Ramulu PY, Pitha IF. Patient acceptance of sustained glaucoma treatment strategies. Journal of glaucoma. 2018 Apr 1;27(4):328-35.
- Wang BB, Lin MM, Nguyen T, Turalba AV. Patient attitudes toward novel glaucoma drug delivery approaches. Digital journal of ophthalmology: DJO. 2018;24(3):16.
- Lanier OL, Christopher KG, Macoon RM, Yu Y, Sekar P, Chauhan A. Commercialization challenges for drug eluting contact lenses. Expert Opinion on Drug Delivery. 2020 Aug 2;17(8):1133-49.
- Loncar-Turukalo T, Zdravevski E, da Silva JM, Chouvarda I, Trajkovik V. Literature on wearable technology for connected health: scoping review of research trends, advances, and barriers. Journal of medical Internet research. 2019;21(9):e14017.
- Yang X, Cheng H. Recent developments of flexible and stretchable electrochemical biosensors. Micromachines. 2020 Mar;11(3):243.
- Moreddu R, Elsherif M, Adams H, Moschou D, Cordeiro MF, Wolffsohn JS, Vigolo D, Butt H, Cooper JM, Yetisen AK. Integration of paper microfluidic sensors into contact lenses for tear fluid analysis. Lab on a Chip. 2020;20(21):3970-9.
- Kim J, Kim M, Lee MS, Kim K, Ji S, Kim YT, Park J, Na K, Bae KH, Kim HK, Bien F. Wearable smart sensor systems integrated on soft contact lenses for wireless ocular diagnostics. Nature communications. 2017 Apr 27;8(1):1-8.
- Lee S, Jo I, Kang S, Jang B, Moon J, Park JB, Lee S, Rho S, Kim Y, Hong BH. Smart contact lenses with graphene coating for electromagnetic interference shielding and dehydration protection. ACS nano. 2017 Jun 27;11(6):5318-24.
- Phan CM, Subbaraman L, Jones LW. The use of contact lenses as biosensors. Optometry and Vision Science. 2016 Apr 1;93(4):419-25.
- Senior, Melanie. “Novartis signs up for Google smart lens.” (2014): 856-856.
- Badugu R, Reece EA, Lakowicz JR. Glucose-sensitive silicone hydrogel contact lens toward tear glucose monitoring. Journal of biomedical optics. 2018 May;23(5):057005.
- Lin YR, Hung CC, Chiu HY, Chang PH, Li BR, Cheng SJ, Yang JW, Lin SF, Chen GY. Noninvasive glucose monitoring with a contact lens and smartphone. Sensors. 2018 Oct;18(10):3208.
- Badugu R, Lakowicz JR, Geddes CD. Ophthalmic glucose monitoring using disposable contact lenses—a review. Journal of fluorescence. 2004 Sep;14(5):617-33.
- Geddes C. Ophthalmic glucose monitoring using disposable contact lenses. InThe 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society 2004 Sep 1 (Vol. 2, p. 5122). IEEE.
- Sensimed Triggerfish. https://www.sensimed.ch/ [internet]. Available from: https://www.sensimed.ch/sensimed-triggerfish/ (accessed 28.02.21)
- Dunbar GE, Shen BY, Aref AA. The Sensimed Triggerfish contact lens sensor: efficacy, safety, and patient perspectives. Clinical Ophthalmology (Auckland, NZ). 2017;11:875.
- The SENSIMED Triggerfish contact lens sensor for continuous 24-hour recording of ocular dimensional changes in people with or at risk of developing glaucoma. National Institute for Health and Care Excellence. www.nice.org.uk [internet]. Available from: https://www.nice.org.uk/advice/MIB14, publication date 19th Nov 2014 (accessed 28.02.21).
- Wang Y, Zhao Q, Du X. Structurally coloured contact lens sensor for point-of-care ophthalmic health monitoring. Journal of Materials Chemistry B. 2020;8(16):3519-26.
- Zhou L, Beuerman RW, Chan CM, Zhao SZ, Li XR, Yang H, Tong L, Liu S, Stern ME, Tan D. Identification of tear fluid biomarkers in dry eye syndrome using iTRAQ quantitative proteomics. Journal of proteome research. 2009 Nov 6;8(11):4889-905.
- Fong PY, Shih KC, Lam PY, Chan TC, Jhanji V, Tong L. Role of tear film biomarkers in the diagnosis and management of dry eye disease. Taiwan journal of ophthalmology. 2019 Jul;9(3):150.
- Kusama S, Sato K, Yoshida S, Nishizawa M. Self-Moisturizing Smart Contact Lens Employing Electroosmosis. Advanced Materials Technologies. 2020 Jan;5(1):1900889.
- Salvisberg C, Tajouri N, Hainard A, Burkhard PR, Lalive PH, Turck N. Exploring the human tear fluid: D iscovery of new biomarkers in multiple sclerosis. PROTEOMICS–Clinical Applications. 2014 Apr;8(3-4):185-94.
- Edman MC, Janga SR, Kakan SS, Okamoto CT, Freire D, Feigenbaum D, Lew M, Hamm-Alvarez SF. Tears–more to them than meets the eye: why tears are a good source of biomarkers in Parkinson’s disease. Biomarkers in medicine. 2020 Feb;14(2):151-63.
- Evans V, Vockler C, Friedlander M, Walsh B, Willcox MD. Lacryglobin in human tears, a potential marker for cancer. Clinical & experimental ophthalmology. 2001 Jun;29(3):161-3.
- Hagan S, Martin E, Enríquez-de-Salamanca A. Tear fluid biomarkers in ocular and systemic disease: potential use for predictive, preventive and personalised medicine. Epma Journal. 2016 Dec;7(1):1-20.
- Tseng RC, Chen CC, Hsu SM, Chuang HS. Contact-lens biosensors. Sensors. 2018 Aug;18(8):2651.
- Ghosh C, Mastrangelo A, Karkhanis MU, Deshpande AP, Banerjee A, Kim H, Mastrangelo C. Low-Profile Induced-Voltage Distance Ranger for Smart Contact Lenses. IEEE Transactions on Biomedical Engineering. 2020 Nov 24.
- Tuan KM, inventor; Tectus Corp, assignee. Dynamic presbyopia correction in electronic contact lenses. United States patent US 10,775,643. 2020 Sep 15.
- Lemoff BE, inventor; Tectus Corp, assignee. Dynamic presbyopia correction in electronic contact lenses. United States patent US 10,871,660. 2020 Dec 22.
- Charman WN. Developments in the correction of presbyopia I: spectacle and contact lenses. Ophthalmic and Physiological Optics. 2014 Jan;34(1):8-29.
- Bailey J, Morgan PB, Gleeson HF, Jones JC. Switchable liquid crystal contact lenses for the correction of presbyopia. Crystals. 2018 Jan;8(1):29.
- Milton HE, Morgan PB, Clamp JH, Gleeson HF. Electronic liquid crystal contact lenses for the correction of presbyopia. Optics express. 2014 Apr 7;22(7):8035-40.
- Lin YH, Chen HS. Electrically tunable-focusing and polarizer-free liquid crystal lenses for ophthalmic applications. Optics express. 2013 Apr 22;21(8):9428-36.
- Sako Y, Iwasaki M, Hayashi K, Kon T, Nakamura T, Onuma T, Tange A, inventors; Sony Corp, assignee. Contact lens and storage medium. United States patent application US 14/785,249. 2016 Apr 7.
- Kim T, Hwang S, Kim S, Ahn H, Chung D, inventors; Samsung Electronics Co Ltd, assignee. Smart contact lenses for augmented reality and methods of manufacturing and operating the same. United States patent US 10,359,648. 2019 Jul 23.
- Mojo Vision and Menicon Announce Joint Development Agreement on Smart Contact Lens Products. Menicon. www.menicon.com [internet]. Available from: https://www.menicon.com/corporate/news/view/90 , publication date 9th Dec 2020 (accessed 28.02.21).
- Chen J, Mi L, Chen CP, Liu H, Jiang J, Zhang W. Design of foveated contact lens display for augmented reality. Optics express. 2019 Dec 23;27(26):38204-19.
- Wu Y, Chen CP, Mi L, Zhang W, Zhao J, Lu Y, Guo W, Yu B, Li Y, Maitlo N. Design of retinal-projection-based near-eye display with contact lens. Optics express. 2018 Apr 30;26(9):11553-67.
- Rokhsaritalemi S, Sadeghi-Niaraki A, Choi SM. A review on mixed reality: current trends, challenges and prospects. Applied Sciences. 2020 Jan;10(2):636.
- Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, Wong TY, Naduvilath TJ, Resnikoff S. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016 May 1;123(5):1036-42.
- Harb EN, Wildsoet CF. Origins of refractive errors: environmental and genetic factors. Annual review of vision science. 2019 Sep 15;5:47-72.
- Li T, Jiang B, Zhou X. Axial length elongation in primary school-age children: a 3-year cohort study in Shanghai. BMJ open. 2019 Oct 1;9(10):e029896.
- Hartwig A, Charman WN, Radhakrishnan H. Baseline peripheral refractive error and changes in axial refraction during one year in a young adult population. Journal of optometry. 2016 Jan 1;9(1):32-9.
- Gifford KL, Richdale K, Kang P, Aller TA, Lam CS, Liu YM, Michaud L, Mulder J, Orr JB, Rose KA, Saunders KJ. IMI–clinical management guidelines report. Investigative ophthalmology & visual science. 2019 Feb 28;60(3):M184-203.
- Polling JR, Klaver C, Tideman JW. Myopia progression from wearing first glasses to adult age: the DREAM Study. British Journal of Ophthalmology. 2021 Jan 24.
- COMET Group. Myopia stabilization and associated factors among participants in the Correction of Myopia Evaluation Trial (COMET). Investigative ophthalmology & visual science. 2013 Dec;54(13):7871.
- Flitcroft DI. The complex interactions of retinal, optical and environmental factors in myopia aetiology. Progress in retinal and eye research. 2012 Nov 1;31(6):622-60.
- Mirshahi A, Ponto KA, Hoehn R, Zwiener I, Zeller T, Lackner K, Beutel ME, Pfeiffer N. Myopia and level of education: results from the Gutenberg Health Study. Ophthalmology. 2014 Oct 1;121(10):2047-52.
- Huang HM, Chang DS, Wu PC. The association between near work activities and myopia in children – a systematic review and meta-analysis. PloS one. 2015 Oct 20;10(10):e0140419.
- Xiong S, Sankaridurg P, Naduvilath T, Zang J, Zou H, Zhu J, Lv M, He X, Xu X. Time spent in outdoor activities in relation to myopia prevention and control: a meta-analysis and systematic review. Acta ophthalmologica. 2017 Sep;95(6):551-66.
- Tedja MS, Haarman AE, Meester-Smoor MA, Kaprio J, Mackey DA, Guggenheim JA, Hammond CJ, Verhoeven VJ, Klaver CC. IMI–myopia genetics report. Investigative ophthalmology & visual science. 2019 Feb 28;60(3):M89-105.
- Wolffsohn JS, Calossi A, Cho P, Gifford K, Jones L, Jones D, Guthrie S, Li M, Lipener C, Logan NS, Malet F. Global trends in myopia management attitudes and strategies in clinical practice–2019 Update. Contact Lens and Anterior Eye. 2020 Feb 1;43(1):9-17.
- Walline JJ, Lindsley KB, Vedula SS, Cotter SA, Mutti DO, Ng SM, Twelker JD. Interventions to slow progression of myopia in children. Cochrane Database of Systematic Reviews. 2020(1).
- Swarbrick HA. Orthokeratology review and update. Clinical and Experimental Optometry. 2006 May;89(3):124-43.
- Chan B, Cho P, Mountford J. The validity of the Jessen formula in overnight orthokeratology: a retrospective study. Ophthalmic and Physiological Optics. 2008 May;28(3):265-8.
- Cho P, Tan Q. Myopia and orthokeratology for myopia control. Clinical and Experimental Optometry. 2019 Jul;102(4):364-77.
- Liu YM, Xie P. The safety of orthokeratology – a systematic review. Eye & contact lens. 2016 Jan;42(1):35.
- Guan M, Zhao W, Geng Y, Zhang Y, Ma J, Chen Z, Peng M, Li Y. Changes in axial length after orthokeratology lens treatment for myopia: a meta-analysis. International ophthalmology. 2020 Jan;40(1):255-65.
- Sánchez-García A, Ariza MA, Büchler P, Molina-Martin A, Piñero DP. Structural changes associated to orthokeratology: A systematic review. Contact Lens and Anterior Eye. 2020 Oct 10.
- Bullimore MA, Johnson LA. Overnight orthokeratology. Contact Lens and Anterior Eye. 2020 Apr 22.
- Lau JK, Vincent SJ, Cheung SW, Cho P. Higher-order aberrations and axial elongation in myopic children treated with orthokeratology. Investigative ophthalmology & visual science. 2020 Feb 7;61(2):22-.
- Han X, Xu D, Ge W, Wang Z, Li X, Liu W. A comparison of the effects of orthokeratology lens, medcall lens, and ordinary frame glasses on the accommodative response in myopic children. Eye & contact lens. 2018 Jul 1;44(4):268-71.
- Chamberlain P, Peixoto-de-Matos SC, Logan NS, Ngo C, Jones D, Young G. A 3-year randomized clinical trial of MiSight lenses for myopia control. Optometry and Vision Science. 2019 Aug 1;96(8):556-67.
- Sankaridurg P, Bakaraju RC, Naduvilath T, Chen X, Weng R, Tilia D, Xu P, Li W, Conrad F, Smith III EL, Ehrmann K. Myopia control with novel central and peripheral plus contact lenses and extended depth of focus contact lenses: 2 year results from a randomised clinical trial. Ophthalmic and Physiological Optics. 2019 Jul;39(4):294-307.
- Millodot M. Dictionary of Optometry and Visual Science E-Book. Elsevier Health Sciences; 2014 Jul 30.
- Aslam TM, Haider D, Murray IJ. Principles of disability glare measurement: an ophthalmological perspective. Acta Ophthalmologica Scandinavica. 2007 Jun;85(4):354-60.
- Rubin GS, Adamsons IA, Stark WJ. Comparison of acuity, contrast sensitivity, and disability glare before and after cataract surgery. Archives of Ophthalmology. 1993 Jan 1;111(1):56-61.
- Nabais CR, Heron BM, De Sousa HC, Gil MH, Sobral AJ. Synthesis and characterization of co-polymers based on methyl methacrylate and 2-hexyl acrylate containing naphthopyrans for a light-sensitive contact lens. Journal of Biomaterials Science, Polymer Edition. 2011 Jan 1;22(1-3):139-52.
- Pek YS, Wu H, Chow EP, Ying JY. Transparent nanostructured photochromic UV-blocking soft contact lenses. Nanomedicine. 2016 Jun;11(12):1599-610.
- Lemelson JH, inventor; Lemelson Jerome H, assignee. Contact lens containing light sensitive material. United States patent US 4,681,412. 1987 Jul 21.
- FDA clears first contact lens with light-adaptive technology. FDA. www.fda.gov [internet]. Available from: https://www.fda.gov/news-events/press-announcements/fda-clears-first-contact-lens-light-adaptive-technology, published April 10th 2018 (accessed 28.02.21)
- Contact Lenses That Darken When Exposed to Light? Check Out the New Acuvue® Oasys With Transitions™ Light Intelligent Technology™. Johnson and Johnson. www.jnj.com [internet]. Available from: https://www.jnj.com/innovation/how-does-acuvue-oasys-with-transitions-light-intelligent-technology-work, published on April 1st 2019 (accessed 28.02.21).
- Renzi-Hammond L, Buch JR, Cannon J, Hacker L, Toubouti Y, Hammond BR. A contra-lateral comparison of the visual effects of a photochromic vs. non-photochromic contact lens. Contact Lens and Anterior Eye. 2020 Jun 1;43(3):250-5.
- Buch JR, Sonoda L, Cannon JL. Lens fitting and subjective acceptance of senofilcon A with photochromic additive on a neophyte population. Contact Lens and Anterior Eye. 2020 Oct 13.
- Buch JR, Toubouti Y, Cannon J. Randomized crossover trial evaluating the impact of senofilcon a photochromic Lens on driving performance. Optometry and Vision Science. 2020 Jan;97(1):15.
- Buch J, Hammond BR, Ruston D. The contact lens that knows light. Optician. 2019 May;2019(5):215711-1.
- Hammond Jr BR, Buch J, Hacker L, Cannon J, Toubouti Y, Renzi-Hammond LM. The effects of light scatter when using a photochromic vs. non-photochromic contact lens. Journal of optometry. 2020 Oct 1;13(4):227-34.
- Renzi-Hammond LM, Buch JR, Hacker L, Cannon J, Hammond Jr BR. The Effect of a Photochromic Contact Lens on Visual Function Indoors: A Randomized, Controlled Trial. Optometry and Vision Science. 2020 Jul;97(7):526.
- Nagra M, Vianya-Estopa M, Wolffsohn JS. Could telehealth help eye care practitioners adapt contact lens services during the COVID-19 pandemic? Contact Lens and Anterior Eye. 2020 Jun 1;43(3):204-7.
- Jones L, Downie LE, Korb D, Benitez-del-Castillo JM, Dana R, Deng SX, Dong PN, Geerling G, Hida RY, Liu Y, Seo KY. TFOS DEWS II management and therapy report. The ocular surface. 2017 Jul 1;15(3):575-628.
- Otero C, García-Porta N, Tabernero J, Pardhan S. Comparison of different smartphone cameras to evaluate conjunctival hyperaemia in normal subjects. Scientific reports. 2019 Feb 4;9(1):1-8.
- Huntjens B, Basi M, Nagra M. Evaluating a new objective grading software for conjunctival hyperaemia. Contact Lens and Anterior Eye. 2020 Apr 1;43(2):137-43.
