Previously in this series, I have discussed the evidence linking lifestyle factors, such as diet, with eye diseases, such as age-related macular degeneration (AMD), and shown how advances in clinical investigation, such as adaptometry, are enabling eye care practitioners (ECPs) to better screen for disease affecting retinal function.
- Lifestyle eye care 1; Overview of lifestyle on systemic and eye health. Optician 11.12.20
- Lifestyle eye care 2; A deeper dive into some food components and dietary habits. Optician 22.01.21
- Lifestyle eye care 3; A move to the dark side: The role of dark adaptation. Optician 26.03.21
The body works as a dynamic integrated system and not as a series of compartmentalised organs working in isolation. From the previous articles in this series, this holistic approach clearly has implications for optometrists. In this article, I will attempt to consolidate what we know into some clinical actions with a view to help prevent disease.
As part of our comprehensive evaluation of our patients, we dutifully document history and symptoms and conduct thorough examinations resulting in, from the patient’s perspective, recommendations largely about the correction of refractive error.
A growing number of ECPs are now embracing the notion that the patient’s ocular health is a reflection of their general health and wellbeing. This opens up the possibility of ECPs having conversations based around lifestyle and diet, linking this to a wide range of systemic issues. For example obesity is a known risk factor for a variety of health problems that may impact upon ocular health.
AMD prevalence – or estimate?
AMD, the disease which has featured prominently in the literature focused upon in this series, is more prevalent than glaucoma and diabetic retinopathy combined,1 affecting more than 25% of people over 60 years of age in Europe,2 a number that is estimated to have doubled by the year 2050.3
It is worth asking ourselves, does this figure represent the number of AMD patients within my practice? If not, does my AMD screening process need reviewing? And with the prevalence of AMD being greater than many systemic diseases, it is also worth remembering the impact AMD can have upon quality of life.4
Worldwide, the number of people with AMD is predicted to increase from 196 to 288 million by 2040.5,6 After accounting for age differences in different countries, the ethnic origin of the populations also has an impact and, within any multicultural population, variation in AMD prevalence remains between different ethnicities. AMD prevalence has been estimated at 12.3% in people with European ancestry, versus 10.4% (Hispanic), 7.5% (African) and 7.4% (Asian).5 Europeans have substantially higher prevalence of early AMD, but only slightly higher prevalence of late AMD. This suggests Europeans are partially protected from progression from early to late disease, unlike those of Asian or African ancestry who are more likely to show disease characterised by a more direct progression to late AMD. Notably, beyond the European Union, a shift in global patterns of AMD is ongoing. Asia is predicted to have over half of the world’s late AMD cases. AMD can no longer be considered primarily a European disease.6
AMD patients report that the condition has a significant impact upon their daily activities,7,8 particularly in low light conditions.9 With the examination methods most commonly used at present, many believe that ophthalmologists and optometrists are underdiagnosing AMD, potentially missing almost 25% of patients with already showing clinically significant signs.10
So, what can be done to address this under-detection? There would appear to be two approaches which will be the focus of the remainder of this article:
- A better understanding of the influence of the various risk factors
- Access to developments in instrumentation allowing earlier diagnosis
Understanding risk factors
Family history and genetics
Research shows us that, when a parent has AMD, the increased odds that their offspring will develop the disease over their life-span is 28 times, while the odds are 12 times greater when a sibling is affected. These odds ratios are even higher when factors such as age and smoking are included in the risk calculation.11
As discussed in part two of this series, AMD has numerous genetic links, most notably the CFH and ARMS2 genes. Research is continuing, showing future potential for using genetic testing to improve early diagnosis in those likely to develop and be at greater risk of progression to late AMD, as well as in helping to differentiate those patients that may or may not benefit from nutritional supplement intervention.
The use of genetic testing for AMD research is well established. However, in a clinical setting, it is not currently advocated for aiding supplementation recommendation by a number of professional bodies and many researchers.12-15 Genetic testing of AMD risk is now a familiar part of the macular degeneration care arsenal for US optometrists. Interestingly, some patient groups acknowledge the benefits this genetic testing service has offered in modifying their lifestyles as a result of genetic testing.16 The general consensus seems to be that, regardless of genetics and stage of AMD, the recommendation of nutritional supplements makes sense.17,18
However, a word of caution. Although genetic testing as an aid in guiding supplementation recommendations is not currently advocated, some recent research suggests that certain specific genetic combinations respond better to AREDS formulations than others. Indeed, it has even been found that for some genetic variations, the use of an AREDS/AREDS2 formulation actually increases the likelihood of progression to CNV by three to four times.19
Once the exact role of genetic testing for eye care is established, incorporating it into clinical practice will offer an additional benefit in the UK as we enter an era of customised patient care. It should improve our understanding of the likelihood of a patient developing AMD and potentially help to pinpoint which supplements might offer the greatest benefit. Such testing could either be offered through NHS care pathways or as a private lab test offered by the practice.
Modifiable risk factors
There are a great many influences upon the expression and progression of AMD. Some key ones are summarised in table 1. A good number of these are modifiable as indicated.20
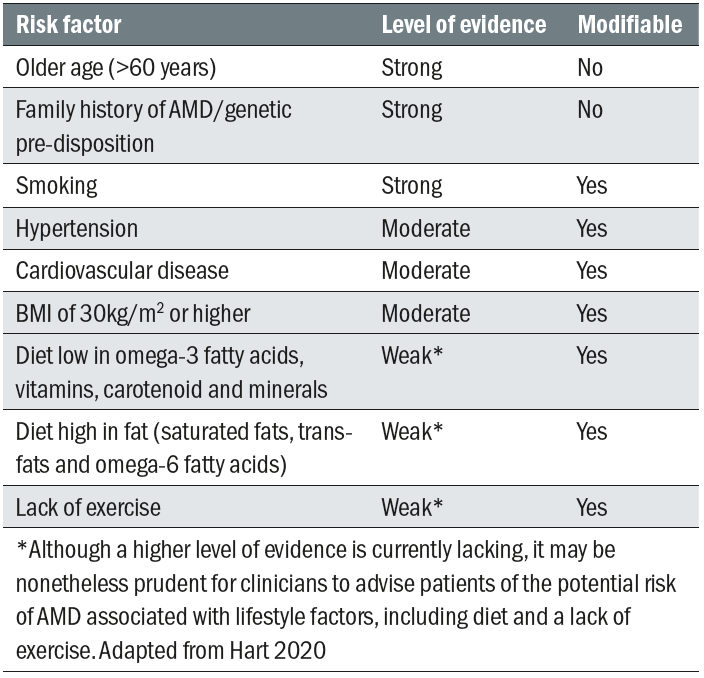 Table 1: Some important risk factors for AMD and their modifiability20
Table 1: Some important risk factors for AMD and their modifiability20
As was discussed in the first article in this series, the majority of the microbiota for the entire body resides in the gastrointestinal trac, which is home for almost 70% of the human body’s immune system and they play an active role in modulating the immune response. Thus, it is not surprising that a disruption of the intestinal microbiota, known as intestinal dysbiosis, has been associated with various diseases known to have an inflammatory component.21 The various influences thought to have an impact upon retinal health are summarised in figure 1.
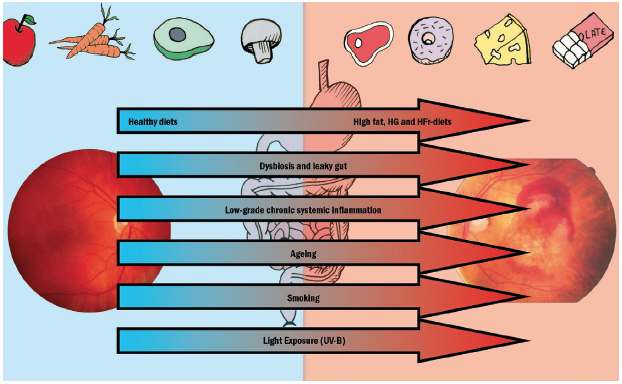 Figure 1: Altered dietary habits, dysbiosis and leaky gut, and low grade inflammation, together with aging, smoking and light exposure may influence the risk and progression of AMD. (HG = high glucose; HFr = high fructose)2
Figure 1: Altered dietary habits, dysbiosis and leaky gut, and low grade inflammation, together with aging, smoking and light exposure may influence the risk and progression of AMD. (HG = high glucose; HFr = high fructose)2
The link between AMD and dietary habits is well established. More recently, the impact of processed, nutrient-deficient food gradually displacing whole, unprocessed, nutrient dense food, which has occurred in recent decades, upon the incidence of AMD has become apparent.22 This is all the more concerning as studies based in the US suggest that 63% of the food consumed there is processed, with just 12% from plant sources and 25% from animal sources.23
POINT TO REMEMBER: Unprocessed food has a beneficial effect on AMD incidence.
Evidence-based recommendations of dietary supplements and discussions of the importance of a healthy diet, along with lifestyle changes such as smoking cessation, have been considered the standard of care for optimal AMD care for a number of years.8,24 The reality is, however, that clinician-led recommendations are not consistently carried out.8 Discussions concerning supplementation, although widely undertaken, often result in recommendations that do not comply with the current best research evidence. Also, even though most ECPs ask about smoking habit as a matter of course, aware of its significance as a potential contributor to ocular disease, it is still the case that all too many express a lack of confidence or too little familiarity with the knowledge base when making recommendations.8
Educating patients about diet
A comprehensive history, taking into account all the risk factors listed in table 1, is essential to the clinical recommendation. This should highlight all areas that may benefit from further discussion.
Hypertension is a known risk factor for a variety of systemic and ocular health concerns (figure 2). Hypertension affects 28% (15 million people) of the adult UK population according to the British Heart Foundation and, worryingly, over 50% (some eight million people) are either undiagnosed or have poorly controlled blood pressure. This raises the opportunity for ECPs to play a role in educating patients about hypertension and to consider incorporating blood pressure screening into their assessments (figure 3).
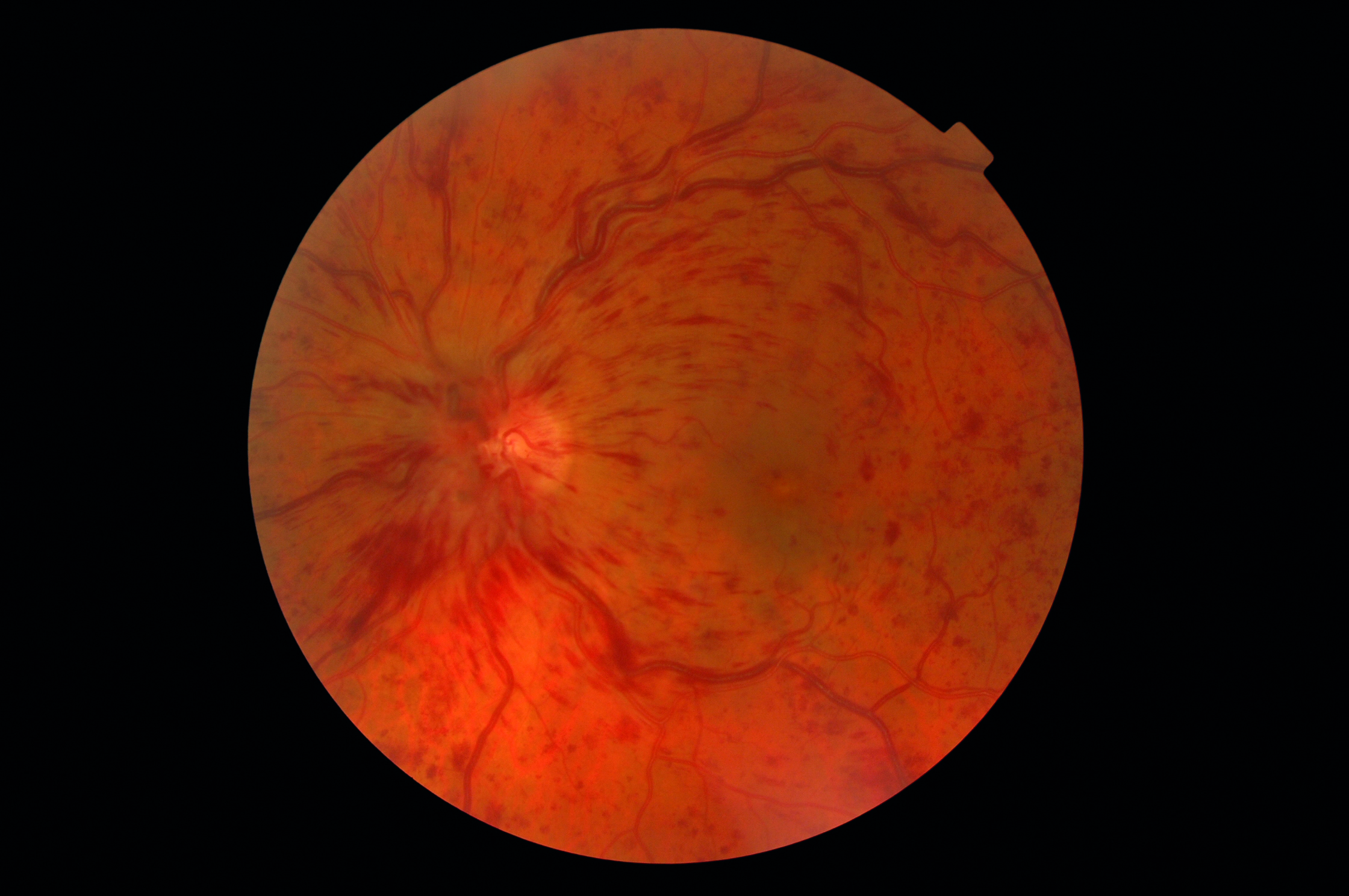 Figure 2: Hypertension has a strong association with a range of ocular diseases, such as retinal vein occlusion
Figure 2: Hypertension has a strong association with a range of ocular diseases, such as retinal vein occlusion
 Figure 3: Eye care practitioners could usefully include blood pressure monitoring into their care protocol
Figure 3: Eye care practitioners could usefully include blood pressure monitoring into their care protocol
There are various initiatives set up locally to address the role of optometrists in establishing Healthy Living Optical Practices.25 Assessing a patient’s cholesterol status might also be a useful service offered by ECPs. In general, low levels of high density lipoprotein (HDL) and high levels of low density lipoprotein (LDL) increase the risk of atherosclerosis and the potential sequelae of coronary heart disease and stroke.26,27 Raising HDL levels and lowering LDL will, therefore, have a beneficial effect.
This can be achieved through use of the Mediterranean or Asian diets28 and is worth discussing with patients with raised blood cholesterol (see table 2).29 Additionally, when one considers that HDL carries lutein to the retina,30,31 there is all the more reason for recommending Med/Asian diets to those patients who would benefit from increased lutein absorption in their gut. Exercise also helps in raising serum HDL and so is also worthy of discussion with patients identified as having greater disease risk.
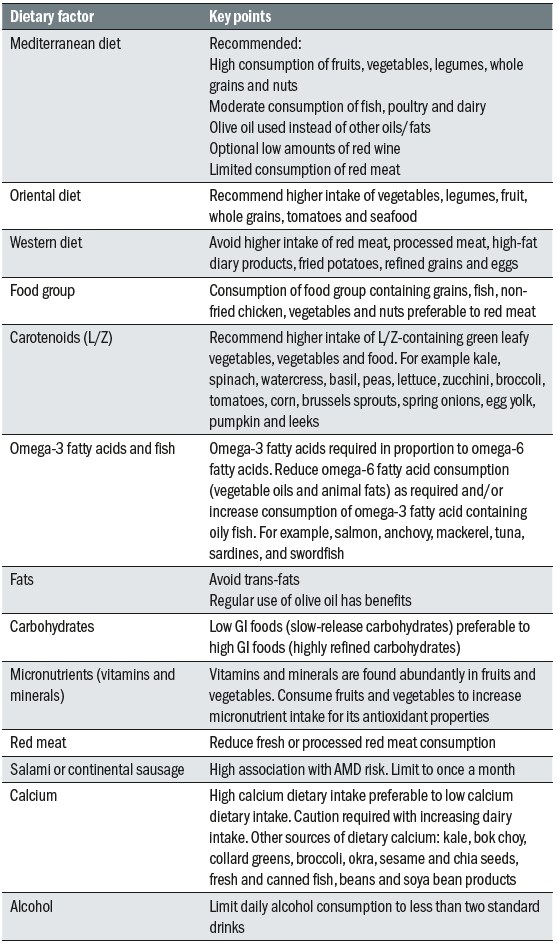 Table 2: Key points about different diets and their constituents
Table 2: Key points about different diets and their constituents
POINT TO REMEMBER: Full medical and lifestyle history is important to the final recommendation.
POINT TO REMEMBER: Mediterranean diet helps raise HDL/lowers LDL so is good for lutein transport, is anti-inflammatory in its effect and improves gut health.
POINT TO REMEMBER: Find out if your LOC has established a Healthy Living Optical Practices, or similar, protocol.
In addition to the reduced risk of cardiovascular disease and stroke through lipid lowering, additional benefits of the Mediterranean diet include a degree of protection against oxidative stress, inflammation, cancer, the improvement of gut health and an increased life expectancy.28
Age and diet
The elderly have greater challenges achieving a healthy diet as well as suffering higher levels of both obesity and undernourishment.34 This can be linked to ‘breaking point’ events that disrupt lifestyle and eating habits. Having an awareness of any social breaking points, such as retirement or bereavement, and medical breaking points, such as a recent diagnosis of dementia, may help to identify and explain any inappropriate newly adopted eating habits, which may be the beginning of an unbalanced diet.35 Medications can also impact upon nutrient absorption, so exacerbating the situation further.36
Recent studies highlight the tendency among many elderly patients towards the eating of smaller ‘meals’ more frequently. This ‘snacking’ tendency, when not guided, can also further reduce nutrient intake among this population.37
Understanding a patient’s personal circumstances are particularly relevant when considering nutrition and lifestyle and should form part of the history. Men living alone are less likely to eat appropriately, tending to prefer a convenience food diet deficient in adequate amounts of fruit and vegetable, than female patients that live alone.37,38 In these situations, where a dietary concern is raised, the opportunity exists for the clinician to discuss the importance of a regular intake of dried fruit and vegetables and remind the patient that these are readily available and require little preparation time. This promotes a better intake of not just micronutrients, but also fibre (known to assist the microbiome) and antioxidants.
POINT TO REMEMBER: The history should include personal circumstances. Identify any breaking points that could negatively impact upon eating habits and nutrient absorption.
POINT TO REMEMBER: Recognise the potential for advising patients about the benefits of fruit (dried or fresh) and vegetables in assisting overall nutrient intake and acting as a prebiotic.
Age is obviously a major risk factor for many systemic and ocular conditions, including AMD. Since the elderly make up a significant proportion of our patient base, it is important to recognise that aging impacts the gastrointestinal (GI) tract. This has a knock-on effect on the pro-inflammatory mechanism influencing nutrients reaching the retina. As one ages, a variety of processes, including reduced stomach hydrochloric acid secretion and a loss of the beneficial Bifidobacteria in the gut occur.21 This, in turn, diminishes the ability to breakdown and absorb nutrients.
Healthy lifetime
Though the elderly are particularly susceptible to some of the negative impacts of external influences such as a poor diet, there are many external adverse influences acting throughout a patient’s life. These have been outlined by Richer et al and are summarised in figure 4.39
The evidence suggests that positive habits (green factors at the centre of figure 4) promoting both general and ocular health include:
- A predominantly plant-based Med/Asian-type diet
- Physical activity
- Protection/maintenance of the GI microbiome against unnecessary dysbiosis, infection, inflammation and malabsorption
- Protection from reactive oxygen species generated by exposure to short wavelength blue and ultraviolet light
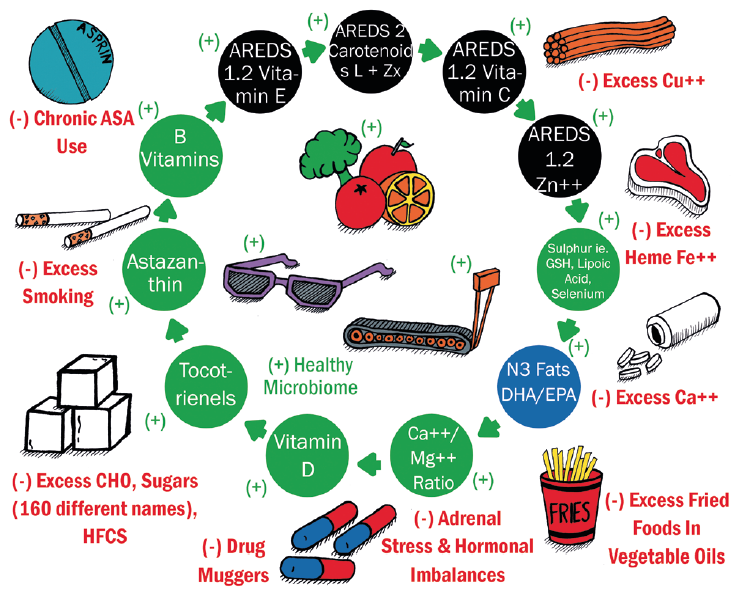
Figure 4: Positive (in green) and negative (in red) health habits and nutritional factors for lifetime ocular health39
Other positive factors for systemic and ocular health that are based on the preponderance of scientific evidence are shown as the shaded circles around the centre. These include a recognition of the AREDS I and AREDS II micronutrients (black circles) and the importance of omega-3 fats, particularly DHA (blue circles). The green shaded circles describe potential micronutrients that appear to maintain retinal health.39
The outermost, negative environmental factors (with red labelling in figure 4) are thought to have a negative impact upon the health of the retina, but their significance may be underestimated by clinicians and researchers alike. One factor often little known is that an excessive accumulation of calcium, iron and copper can lead to degeneration.39 Ageing eyes, and those with AMD in particular, present with an over-mineralisation of retinal tissue to Fe, Ca and Cu, the latter often due to long exposure to copper from water piping in modern houses. Excess iron impairs RPE phagocytosis and is associated with retinal neurodegeneration,40,41 while a general calcification of Bruch’s membrane impairs the effectiveness of the visual cycle in the RPE with a subsequent impact upon dark adaptation.
The amount of absorbable Fe can be reduced through lifestyle choices. Red and processed meat contains significantly more absorbable Fe compared to chicken and is associated with a higher risk of early AMD, whereas chicken appears to have an inverse association with late AMD. This is something that can be addressed when considering lifestyle modification with patients.42
POINT TO REMEMBER: Red and processed meats are high in absorbable iron, and a dietary intake of Fe-containing products increases risk of early AMD. Substitution with chicken reduces the Fe intake and reduces risk of AMD.
Obesity
Discussion of a healthy weight should be considered within the scope of our practice, both from an ocular as well as a public health perspective. Obesity is a major contributor to the chronic inflammatory response and linked with hypertension43 and insulin resistance.44
Obesity accounted for over one million hospital admissions in the UK last year and represents a major risk factor for various systemic diseases,45 in addition to being a risk factor for AMD46,47 by virtue of its associated with pro-inflammatory processes.48
Abdominal obesity is a key risk factor for AMD risk, with waist-hip ratio (WHR) being a more reliable indicator than body mass index (BMI).49 A higher WHR is associated with a greater risk of AMD progression.50,51 To further strengthen the argument, a reduction in WHR is associated with reduced odds of AMD development.52
Adipose tissue is a storage site for carotenoids, in particular abdominal adipose tissue.53 The implication is, therefore, that the greater the abdominal obesity, the fewer carotenoids will be available to supply the macula.55 Of particular interest, it would seem that those with a high BMI (>25) had less lutein reaching the retina than those with lower BMI.55
Adipose tissue consists of white and brown adipose tissue and both abdominal and visceral fat are composed primarily of white adipose tissue. Brown adipose tissue actively promotes energy expenditure55 and is considered desirable in slowing down age-related metabolic pathologies, such as insulin resistance and obesity. The ability to covert white adipose to brown, known as browning, declines with age.56 There are certain foods groups that actively promote browning and specific dietary components, including capsaicin, resveratrol, curcumin, green tea, menthol and fish-derived omega-3 fatty acids, that have this property also appear to be beneficial for promoting weight loss.57 Collaboration with a GP or nutritionist regarding weight management and nutritional advice may be the most appropriate action for the ECP to consider for some patients.
The concept of intermittent fasting has gained popularity recently (figure 5). It has been associated with a beneficial effect on numerous systemic conditions58 and has been linked with a reduction of abdominal obesity59 as well as improvement of the gut microbiome.60 Dietary restriction through intermittent fasting, coupled with the Mediterranean diet, strengthens a variety of biologic processes resulting in reduced inflammation, promotion of mitochondrial function and tissue repair, improved organ function and resistance to stress.61
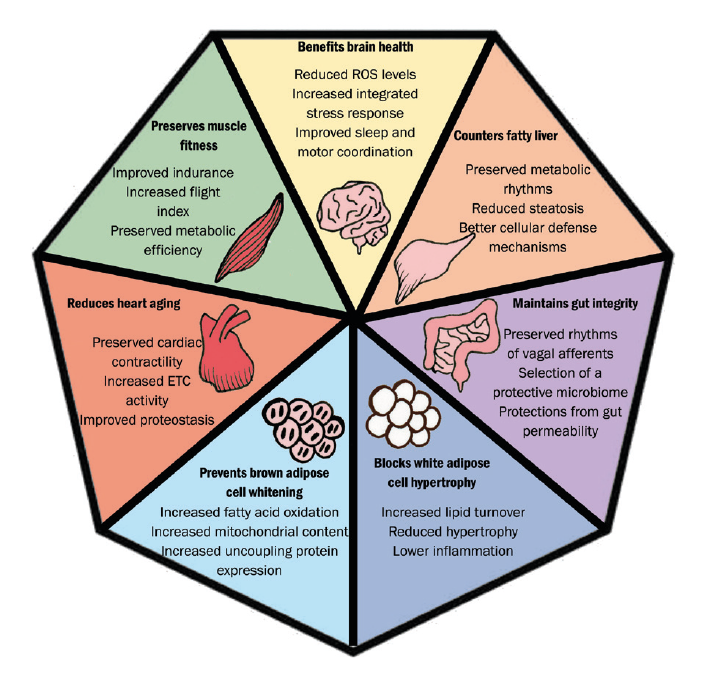
Figure 5: Potential benefits from time-restricted feeding
POINT TO REMEMBER: Obesity is a strong risk factor for AMD. Lutein is stored in adipose tissue and this can limit the efficacy of ingested lutein. Whether through diet or supplements.
Diabetic patients
Although medication to control blood glucose levels is the mainstay of type 2 diabetes therapy, lifestyle modification should also be introduced. Changes in lifestyle have been shown to be as effective as medication in many cases62,63 and without the side effects,64 and it is well within the ECP’s ability to raise awareness of this. Specifically, improved glycaemic control through diets formulated for diabetics are well known. However, plant-based diets, rich in fruit, vegetables, whole grains, beans, nuts and seeds, have a superior effect in reducing HbA1c.65-67
A reduction in red and processed meats reduces the prevalence of type II diabetes. Substituting as little as 5% of the calories from meat to plant protein reduced the risk by some 23%.68 Adequate physical activity and normalising weight decreases the chronic inflammatory reaction of the body69,70 and improves HbA1c to levels comparable to those achieved with medication.71
POINT TO REMEMBER: Exercise can be as effective as medication in type 2 diabetic patients.
Developments in instrumentation
Using emerging technology to complement current methods offers the potential for early diagnosis and management as well as serving as a useful practice builder. The role of dark adaptation and macular pigment density in AMD assessment has been discussed previously in this series and a detailed description of these and other techniques able to assist in the early diagnosis of retinal disease is outside the scope of this article. However, a brief description of some key techniques follows.
Macular pigment measurement
Macular pigment (MP) is a yellow pigment concentrated around the macular region of the retina in a thin band in front of the central photoreceptors. MP is composed of the xanthophyll carotenoids lutein (L), zeaxanthin (Z) and meso-zeaxanthin, the first two of which are fully derived from diet. MP appears to have an anti-oxidative effect and so helps limit the processes that over time would degrade retinal tissue. MP also helps filter out short wavelength visible and longer wavelength UV light that might otherwise damage retinal tissues through a photo-oxidative effect.
There is a general consensus and also extensive evidence to suggest a link between reduced levels of MP and increased risk of AMD.72 MP levels have also been linked with aspects of visual function, such as dark adaptation and contrast sensitivity.73 It is not surprising, therefore, that clinicians have sought to measure MP as a way of indicated retina and macular integrity and also monitoring the impact of any intervention, such as MP supplementation.
The majority of practice-based tests have incorporated a subjective testing strategy, which uses the patient’s ability to detect a flickering blue target to correlate with MP optical density (MOPD). Objective tests do exist and the more commonly found are an adapted form of imaging with a blue light source to look for absorption of light by the MP, the latter often employing use of software run on an OCT or a scanning laser ophthalmoscope with autofluorescence capability.
Despite a solid theoretical underpinning, MP testing still proves somewhat controversial with disagreement over repeatability, accuracy and relevance. That said, most would argue some value in MP testing as exemplified by this concluding remark from a study published this year; ‘While further investigation is warranted, this finding should prompt clinicians to consider MPOD evaluation in healthy patients with specific complaints.’74
Recently, there has been some claims made about a correlation between skin carotenoid levels, serum and macular carotenoid levels,75-77 opening up the possibility for the clinician to establish a baseline reading and future monitoring as needed.78 Indeed, some are now looking at the use of skin carotenoid sensor instruments (figure 6).
 Figure 6: A skin carotenoid sensor
Figure 6: A skin carotenoid sensor
OCT
The ability to accurately assess small changes in retinal structure over time and to view retinal tissue in cross-section has revolutionised the way clinicians can screen the eye for signs of retinal disease at an early stage (figure 7).
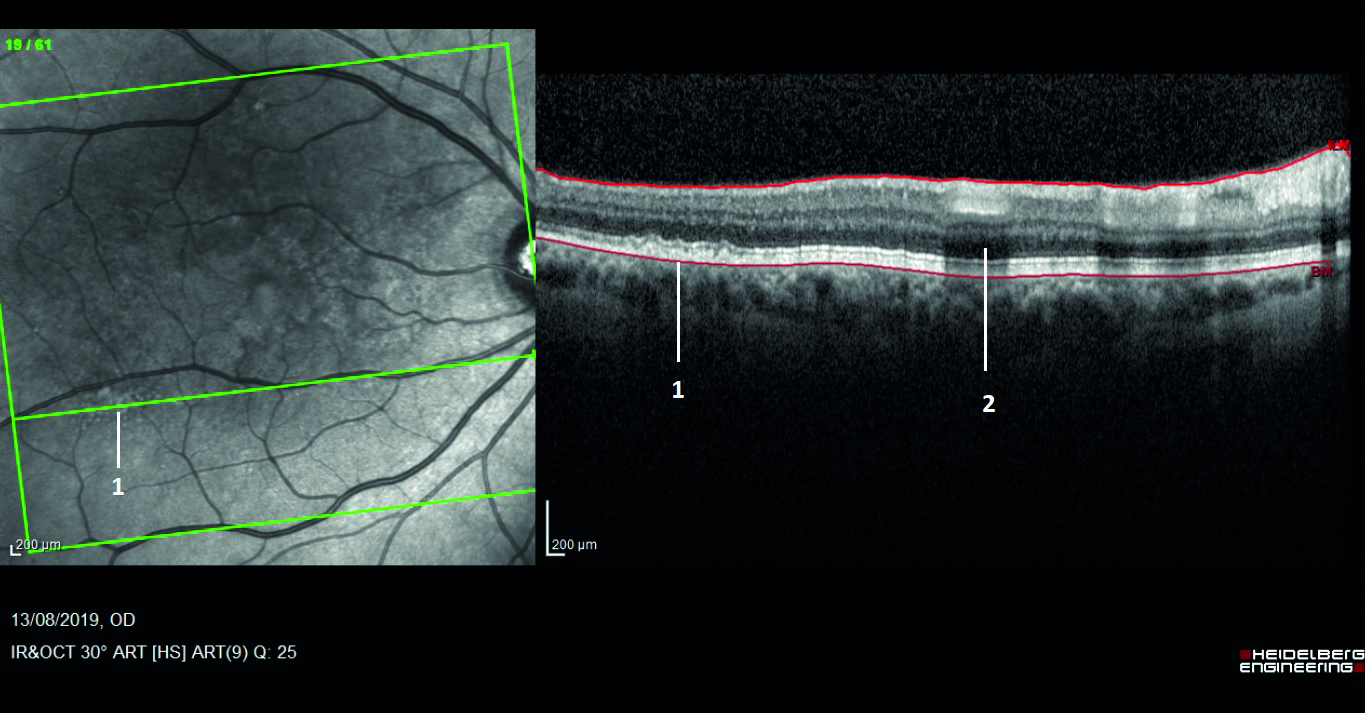 Figure 7: The infrared view on the left clearly shows small white lesions that were not obvious on standard ophthalmoscopy (labelled as 1). In cross-section, they are confirmed as drusen. Label 2 is shadowing from the overhead blood vessel.
Figure 7: The infrared view on the left clearly shows small white lesions that were not obvious on standard ophthalmoscopy (labelled as 1). In cross-section, they are confirmed as drusen. Label 2 is shadowing from the overhead blood vessel.
Autofluorescence
The natural emission of light by biological structures, subsequent to the absorption of light is termed autofluorescence. Some organelles, specialist subunits within cells with their own specific function, such as lysosomes possess this characteristic. For retinal assessment this is helpful as it is known that an indicator of inflammatory response, lipofuscin, will fluoresce and indicate an active disease process (figure 8).
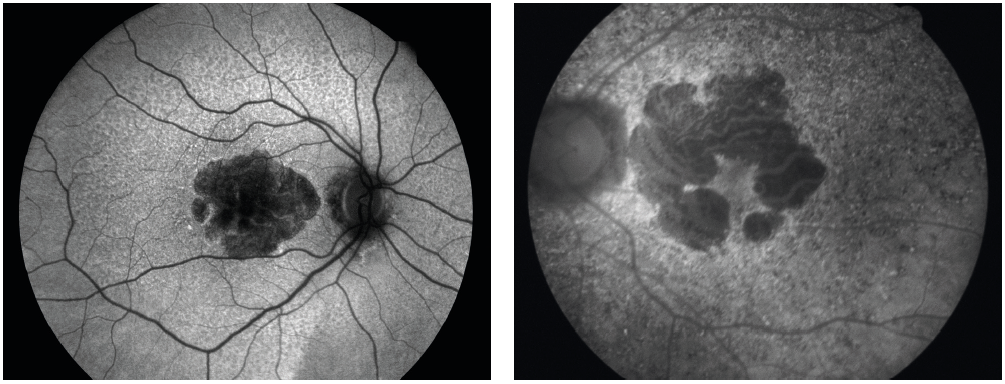 Figure 8: (Left) Longstanding geographic atrophy where the diseased atrophic tissue appears black or hypofluorescent. (Right) Areas of light, hyperfluorescence outside the margins of a geographic atrophy imply further inflammatory process and expected future tissue damage
Figure 8: (Left) Longstanding geographic atrophy where the diseased atrophic tissue appears black or hypofluorescent. (Right) Areas of light, hyperfluorescence outside the margins of a geographic atrophy imply further inflammatory process and expected future tissue damage
Dark adaptometry
As explained in detail in part three of this series, dark adaptometry has the potential to detect functional effects that are subclinical and aid in predicting patients at risk of developing AMD.
Clinical communication
The importance of effective clinical communication is key to patient engagement. The majority of patients are comfortable, and have the expectation, of discussing diet and smoking status during their eye exam.79 A significant percentage of AMD patients express dissatisfaction with their clinicians largely around a lack of education regarding nutrition and lifestyle choices.80
Most clinicians ask about smoking status. However, follow-up may be lacking, losing the opportunity to discuss the importance of smoking cessation or sign posting patients appropriately. This has the potential to leave over 60% of patients feeling their clinician had not been given adequate advice about AMD, often feeling a general lack of support.81 Most worrying for the profession is that the clinical recommendation may be diluted by the public perception of optometry prioritising the retail business needs over their healthcare role, potentially degrading the profession’s credibility and people’s trust in future optometrist recommendations.
There are a variety of options for the clinician to gain confidence when managing a patient with AMD, one such is the ‘Nutrition advice for people with, or at risk of AMD clinical decision-making tool,’ developed at Aston University82 and previously reported in Optician 17.02.17. This decision tree (figure 9) can form a valuable aid in conjunction with discussion around modifying risk factors where applicable.
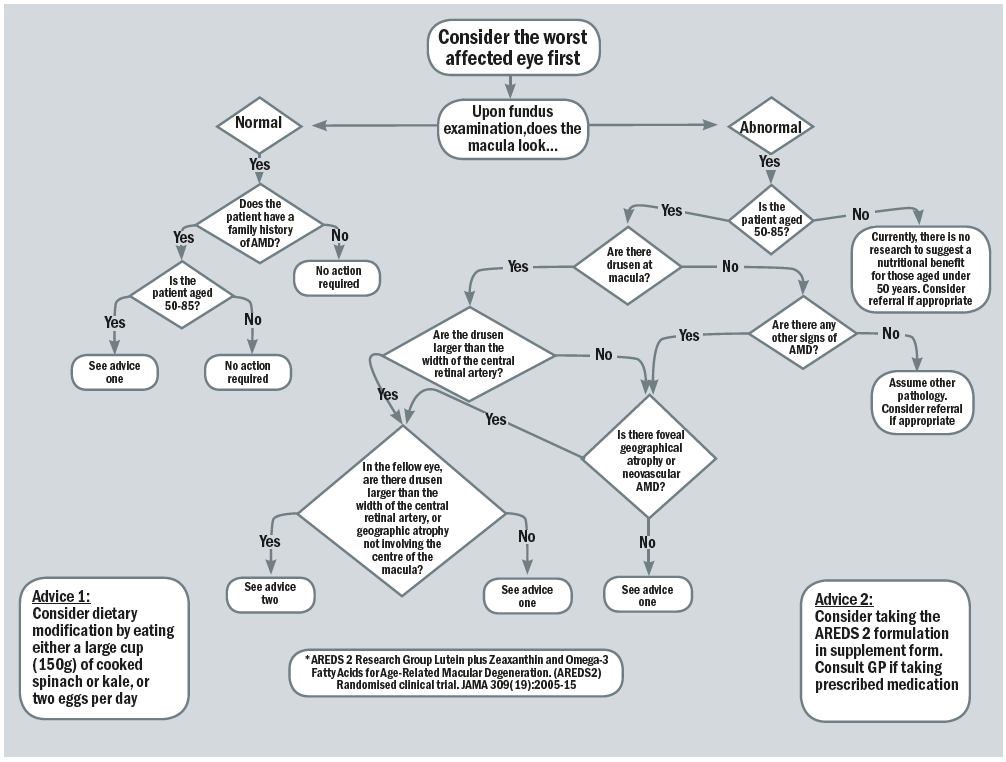 Figure 9: The clinical decision-making flowchart82
Figure 9: The clinical decision-making flowchart82
The use of educational material can help initiate and guide the discussion. The patient’s understanding of the advice given is important and knowing that changing their diet can slow the progression of their AMD allows patients to feel more supported and also better involved in their own health.80
Conclusion
As there is currently no cure for AMD, the importance of trying to integrate new technologies and lifestyle recommendations to halt, or at least slow the disease progression cannot be understated. Preventing degenerative diseases through a blend of the frequent and regular monitoring of eye and vision health, coupled with educating patients that disease processes occur not just as a function of age, but through unhealthy lifestyle choices. These can be addressed through wellness and nutritional strategies and allow the clinician to offer a more personalised health care plan for patients.
Dr Rohit Narayan is a therapeutic optometrist and visiting clinician at Aston University.
Useful links
- Smoking cessation services; www.nhs.uk/better-health/quit-smoking
- Dietary advice; www.hsph.harvard.edu/nutritionsource/healthy-weight/diet-reviews/mediterranean-diet
- Nutrition and nutrient intake; www.nutrition.org.uk/attachments/article/234/Nutrition%20Requirements_Revised%20Oct%202016.pdf
- Lifestyle advice for optometrists; www.college-optometrists.org/membership/free-patient-resources/patient-leaflets.html
- Healthy life advice; www.aop.org.uk/ot/in-practice/business-management/2018/06/25/healthy-me-healthy-you
References
- Klein R et al. Prevalence of age-related macular degeneration in the US population. Archives of Ophthalmology. 2011 Jan;129(1):75-80
- Li JQ et al. Prevalence and incidence of age-related macular degeneration in Europe: a systematic review and meta-analysis. British Journal of Ophthalmology, 2020,104(8), pp.1077-1084
- Corbin, G.S. Diagnose AMD earlier with dark adaptation testing. Primary Care Optometry News. 2019.www.healio.com/news/optometry/20190826/diagnose-amd-earlier-with-dark-adaptation-testing
- Taylor DJ et al. How does age-related macular degeneration affect real-world visual ability and quality of life? A systematic review. BMJ Open 2016;6: e011504.
- Keenan TD et al. Study the past if you would define the future (Confucius). British Journal of Ophthalmology, 2020, Apr;104(4):449-450
- Wong, WL et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. The Lancet Global Health, 2(2), pp. e106-e116
- Gopinath B et al. Age-related macular degeneration and 5-year incidence of impaired activities of daily living. Maturitas, 2014 Mar;77(3):263-6
- Jalbert I et al. A qualitative exploration of Australian eyecare professional perspectives on Age-Related Macular Degeneration (AMD) care. PLoS One. 2020 Feb 11;15(2):e0228858
- Owsley C et al. Delays in rod-mediated dark adaptation in early age-related maculopathy. Ophthalmology. 2001 Jul;108(7):1196-202
- Neely DC et al. Prevalence of undiagnosed age-related macular degeneration in primary eye care. JAMA Ophthalmology, 2017, 135(6), pp.570-575
- Shahid, H et al. Genetic Factors in AMD Study Group. Age-related macular degeneration: the importance of family history as a risk factor. British Journal of Ophthalmology, 2012, 96(3), pp.427-431
- Assel M.J. et al. Genetic polymorphisms of CFH and ARMS2 do not predict response to antioxidants and zinc in patients with age-related macular degeneration: independent statistical evaluations of data from the age-related eye disease study. Ophthalmology. 2018; 125: 391-397
- Flaxel CJ et al. Age-related macular degeneration preferred practice pattern. Ophthalmology, 2020, 127(1), pp.P1-P65
- Stone EM et al. Clinically Focused Molecular Investigation of 1000 Consecutive Families with Inherited Retinal Disease. Ophthalmology. 2017;124(9):1314-1331
- Warwick A, Lotery A. Genetics and genetic testing for age-related macular degeneration. Eye (London). 2018 May;32(5):849-857
- McCarty CA et al. How Do Patients Respond to Genetic Testing for Age-related Macular Degeneration? Optometry and Vision Science. 2018, Mar;95(3):166-170
- Carneiro  et al. Nutritional and lifestyle interventions for age-related macular degeneration: a review. Oxidative Medicine and Cellular Longevity, 2017:6469138
- Hobbs RP et al. Nutrient supplementation for age-related macular degeneration, cataract, and dry eye. Journal of Ophthalmic and Vision Research. 2014;9(4):487-493
- Kaufman SR et al. Genetics and age-related eye disease study formulation interaction in neovascular age-related macular degeneration. Journal of Vitreoretinal Diseases, 2020, p.2474126420941713
- Hart KM et al. Optometry Australia’s chairside reference for the diagnosis and management of age‐related macular degeneration. Clinical and Experimental Optometry, 2020, 103(3), pp.254-264
- Rinninella E et al. Food components and dietary habits: Keys for a healthy gut microbiota composition. Nutrients, 2018, 11(10), p.2393
- Islam AFM et al. Dietary patterns and their associations with age-related macular degeneration: the Melbourne collaborative cohort study. Ophthalmology, 2014, Jul;121(7):1428-1434.e2
- Knobbe CA et al. The ‘displacing foods of modern commerce’ are the primary and proximate cause of age-related macular degeneration: A unifying singular hypothesis. Medical Hypotheses, 2017, 109, pp.184-198
- Evans JR, Lawrenson JG. Antioxidant vitamin and mineral supplements for preventing age-related macular degeneration. Cochrane Database Systemic Reviews, 2017, Jul 30;7(7): CD000253
- Cartwright C. Looking ahead: Lifestyle advice and disease prevention in community practice. Optician, 27.11.2020, pp31-36
- Chapman MJ. Therapeutic elevation of HDL-cholesterol to prevent atherosclerosis and coronary heart disease. Pharmacology & therapeutics, 2006, 111(3), pp.893-908
- Yeh PS et al. Low levels of high-density lipoprotein cholesterol in patients with atherosclerotic stroke: a prospective cohort study. Atherosclerosis, 2013, 228(2), pp.472-477
- Tosti V et al. Health benefits of the Mediterranean diet: metabolic and molecular mechanisms. The Journals of Gerontology: Series A, 2018, 73(3), pp.318-326
- Rondanelli M et al. Mediterr-Asian diet products that could raise HDL-cholesterol: A systematic review. Biomedical Research International, 2016:2025687
- Renzi LM et al. The relation between serum lipids and lutein and zeaxanthin in the serum and retina: results from cross-sectional, case-control and case study designs. Lipids in Health and Disease, 2012,11(1), p.33
- Thomas SE et al. Mechanisms of selective delivery of xanthophylls to retinal pigment epithelial cells by human lipoproteins. Journal of Lipid Research, 2016, 57(10), pp.1865-1878
- Ruiz-Ramie JJ et al. Effects of exercise on HDL functionality. Current Opinion in Lipidology, 2019, 30(1), p.16
- Juhas I et al. Effects of an eight-week exercise program on parameters of the lipid profile of female students. Journal of Medical Biochemistry, 2020, 39(1), p.40
- Jones N et al. Comparison of the eating behaviour and dietary consumption in older adults with and without visual impairment. British Journal of Nutrition, 2020,123(6), pp.712-720
- Sulmont-Rossé C. Eating in the Elderly. Handbook of eating and drinking: Interdisciplinary perspectives, 2020, pp.433-457
- Mohn ES et al. Evidence of drug–nutrient interactions with chronic use of commonly prescribed medications: an update. Pharmaceutics, 2018, 10(1), p.36
- Belice,T et al. Importance of dried fruits and vegetables in the older adults. European Journal of Geriatrics and Gerontology, 2020, 2(2), pp.28-35
- Donkin AJ et al. Gender and living alone as determinants of fruit and vegetable consumption among the elderly living at home in urban Nottingham. Appetite, 1998, 30(1), pp.39-51
- Richer S et al. Age-related macular degeneration beyond the age-related eye disease study II. Advances in Ophthalmology and Optometry, 2016, 1(1), pp.335-369
- Picard E et al. Targeting iron-mediated retinal degeneration by local delivery of transferrin. Free Radical Biology and Medicine, 2015, 89, pp.1105-1121
- Picard E et al. From rust to quantum biology: the role of iron in retina physiopathology. Cells, 2020, 9(3), p.705
- Chong E et al. Red meat and chicken consumption and its association with age-related macular degeneration. American Journal of Epidemiology, 2009, 169(7), pp.867-876
- Aronow WS. Association of obesity with hypertension. Annals of Translational Medicine, 2017, 5(17)
- Kahn B et al. Obesity and insulin resistance. The Journal of Clinical Investigation, 2000, 106(4), pp.473-481
- Gherghel D et al. Obesity and oculr health. Optician, 19.04.2019, pp 26-31
- Adams MK et al. Abdominal obesity and age-related macular degeneration. American Journal of Epidemiology, 2011, 173(11), pp.1246-1255
- Singh N et al. Prevention of age-related macular degeneration. The Asia-Pacific Journal of Ophthalmology, 2017, 6(6), pp.520-526
- Zhang QY et al. Overweight, obesity, and risk of age-related macular degeneration. Investigative Ophthalmology & Visual Science, 2016, 57(3), pp.1276-1283
- Noble RE. Waist-to-hip ratio versus BMI as predictors of
cardiac risk in obese adult women. Western Journal of Medicine, 2001;174(4):240-241 - Seddon JM et al. Progression of age-related macular degeneration: association with dietary fat, trans-unsaturated fat, nuts, and fish intake. Archives of Ophthalmology, 2003, 121(12), pp.1728-1737
- Peeters A et al. Changes in Abdominal Obesity and Age-Related Macular Degeneration: The Atherosclerosis Risk in Communities Study. Archives of Ophthalmology. 2008;126(11):1554–1560
- Peeters A et al. Changes in the rates of weight and waist circumference gain in Australian adults over time: A longitudinal cohort study. BMJ Open, 2014, 4. e003667
- Chung HY et al. Site-specific concentrations of carotenoids in adipose tissue: relations with dietary and serum carotenoid concentrations in healthy adults. The American Journal of Clinical Nutrition, 2009, 90(3), pp.533-539
- Obana A et al. Effect of an antioxidant supplement containing high dose lutein and zeaxanthin on macular pigment and skin carotenoid levels. Scientific Reports, 2020, 10(1), pp.1-12
- Cannon B et al. Brown adipose tissue: function and physiological significance. Physiology Reviews, 2004 Jan;84(1):277-359
- Graja A et al. Aging of brown and beige/brite adipose tissue. In Brown Adipose Tissue, 2018 (pp. 55-72). Springer.
- El Hadi H et al. Food ingredients involved in white-to-brown adipose tissue conversion and in calorie burning. Frontiers in Physiology, 2019, 9, p.1954
- Chaix A et al. Time-restricted eating to prevent and manage chronic metabolic diseases. Annual Review of Nutrition, 2019, 39, pp.291-315
- Kesztyüs D et al. Adherence to time-restricted feeding and impact on abdominal obesity in primary care patients: Results of a pilot study in a pre-post design. Nutrients, 2019; 11(12):2854
- Frank J et al. Brain–gut–microbiome interactions and intermittent fasting in obesity. Nutrients, 2021, 13(2), p.584
- Fontana L et al. Promoting health and longevity through diet: from model organisms to humans. Cell, 2015, 161(1), pp.106-118
- Bodai BI et al. Lifestyle medicine: A brief review of its dramatic impact on health and survival. The Permanente Journal, 2018, 22
- Sami W et al. Effect of diet on type 2 diabetes mellitus: A review. International Journal of Health Sciences, 2017,11(2), p.65
- American Diabetes Association Pharmacologic approaches to glycemic treatment. Diabetes Care, 2017, 40(Supplement 1), pp.S64-S74
- Barnard ND et al. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes care, 2006, 29(8), pp.1777-1783
- McMacken M et al. A plant-based diet for the prevention and treatment of type 2 diabetes. Journal of Geriatric Cardiology: JGC, 2017, 14(5), p.342
- Wolfram T et al. Efficacy of high-fiber diets in the management of type 2 diabetes mellitus. Endocrine Practice, 2011, 17(1), pp.132-142
- Malik VS. Dietary protein intake and risk of type 2 diabetes in US men and women. American Journal of Epidemiology, 2016, 183(8), pp.715-728
- Knowler WC et al. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New England Journal of Medicine, 2002, 346(6), pp.393-403
- Wu H et al. The gut microbiota in prediabetes and diabetes: A population-based cross-sectional study. Cell Metabolism, 2020, 32(3), pp.379-390
- Rodríguez-Gutiérrez R et al. Glycemic control for patients with type 2 diabetes mellitus: Our evolving faith in the face of evidence. Circulation: Cardiovascular Quality and Outcomes, 2016, 9(5), pp.504-512
- Loane E et al. The rationale and evidence base for a protective role of macular pigment in age-related maculopathy. British Journal of Ophthalmology, 2008, 92, 1163–1168
- Loughman J et al. Macular pigment and its contribution to visual performance and experience. Journal of Optometry, 2010, 3, 74–90
- Wilson M. Macular pigment optical density and visual quality of life. Journal of Optometry, 2021, Volume 14, Issue 1, 92-99
- Conrady C et al. Correlations between macular, skin, and serum carotenoids. Investigative Ophthalmology & Visual Science, 2017, 58(9), pp.3616-3627
- Matsumoto M et al. Skin Carotenoid Level as an Alternative Marker of Serum Total Carotenoid Concentration and Vegetable Intake Correlates with Biomarkers of Circulatory Diseases and Metabolic Syndrome. Nutrients. 2020;12(6):1825
- Sauer L et al. Ocular carotenoid status in health and disease. Annual Review of Nutrition, 2019, 39, pp.95-120
- Mayne ST et al. Resonance Raman spectroscopic evaluation of skin carotenoids as a biomarker of carotenoid status for human studies. Archives of Biochemistry and Biophysics, 2013, 539(2), pp.163-170
- Downie LE et al. What do patients think about the role of optometrists in providing advice about smoking and nutrition? Ophthalmic and Physiological Optics, 2017; 37: 202–211
- Stevens R et al. Dietary analysis and nutritional behaviour in people with and without age-related macular disease. Clinical Nutrition ESPEN, 2015,10(3), pp.e112-e117
- Taylor DJ et al. Measuring dynamic levels of self-perceived anxiety and concern during simulated mobility tasks in people with non-neovascular age-related macular degeneration. British Journal of Ophthalmology 2020;104:529-534
- Stevens R et al. Testing the impact of an educational intervention designed to promote ocular health among people with age-related macular degeneration. British Journal of Visual Impairment, 2018, 36(2), pp.110-127
