In part one of this two-part series, we considered the definition of the term pharmacovigilance and focused upon the legislative framework and organisations responsible for ensuring it is maintained at the required standard throughout clinical practice (Optician 27.08.21). This second article takes a closer look at clinical trials, which play an integral role in the development of clinical strategies, and in the evolution of our understanding of eye health and how that is best communicated to our patients.
Clinical Trials
One of the best definitions of the clinical trial (CT) is the following, suggested by Paul Varner in a recent paper offering a useful overview of the topic (and available as a free download1): ‘Clinical trials are medicine’s quest to provide scientific evidence and identify optimal strategies for the healing arts. Evidence-based medicine demands this process, and increasing health care costs provide an additional financial impetus to make these distinctions.’1 The ultimate goal of the CT is to improve the quality of our patients’ care.
The World Health Organisation defines the CT as ‘a type of research that studies new tests and treatments and evaluates their effects on human health outcomes. People volunteer to take part in clinical trials to test medical interventions including drugs, cells and other biological products, surgical procedures, radiological procedures, devices, behavioural treatments and preventive care.’2
Clinical trials are developed in a number of designs, or phases, which define their make-up and aims. These are:
- Phase 0; this phase is not the norm, as the earliest phase usually referred to is phase I. However, sometimes (and relevant to a very small number of people) it is important to find out if, in its general usage, a drug behaves the same way as in laboratory studies. This is something that has been particularly useful in cancer research.
- Phase I studies; these test new drugs within a small group (typically between 20 and 50 healthy people) to evaluate a safe dosage range and to identify side effects. Blood concentration of the drug, pharmacokinetics and pharmacodynamics of the drugs are also tested and established at this stage.
- Phase II studies; these test treatments that have been found to be safe in phase I. They are run on a larger group of human subjects with a known disease, typically several hundreds, to determine the optimal dose of a drug candidate.
- Phase III studies; these are conducted on patients for whom the medicine is eventually intended to be used. Participants are assigned to receive either the medication being evaluated or a placebo. This is to demonstrate whether or not a drug candidate offers a treatment benefit to a specific population, to provide more detailed safety data, and to serve as the basis for product labelling.3 In some phase III clinical trials, the new treatment is compared to standard treatment for the disease. Most phase III trials are randomised.
- Phase IV studies; these take place after a drug has been licenced. They are undertaken when there is a need for yet further testing within a wider population and over a longer period of time.
Ethical Considerations
As stated by Nardini: ‘The general problem with the ethics of clinical trials stems from the fact that those who stand to gain from the trial results are not the same that bear the risk and burden of trial participation.’4
Clinical trials are lengthy, costly and depend on the recruitment and retention of human subjects. In addition, there is a higher risk compared to standard care. ‘These risks are actually not offset by a prospective clinical benefit, since the primary end of the trial is not that of treating trial participants but rather that of producing generalisable medical knowledge.’4 Therefore, many check points should be put in place to ensure that ethical principles are considered and followed in a CT. Moreover, before any enrolment, subjects need to sign a declaration of their informed consent, usually after the issue of a patient information sheet, or PIS, as shown in figure 1. This is a necessary and important step for any ethical research. These documents should give the patient a clear understanding of the diagnostic or therapeutic procedure as well as their risks and prospective benefits.
 Figure 1: Examples of patient information sheets concerning clinical trials
Figure 1: Examples of patient information sheets concerning clinical trials
Good Clinical Practice (GCP) guidelines (as discussed later) and the International Conference on Harmonisation (ICH) recommendations are fundamental for writing the informed consent. In addition, extra care should be taken when enrolment involves vulnerable subjects. GCP also aims to protect human subject rights, integrity and confidentiality. GCP became obligatory in the UK and Europe in 2004. In 1964, the Declaration of Helsinki was drafted and adopted by the World Medical Association. This served as a backbone for the ethical principles that were then formulated into the current International Conference on Harmonization (ICH)-GCP guidelines.5
In 1996, the ICH Guideline was issued by the International Conference for Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH-GCP guidelines recommend that ‘all clinical trials should be conducted in compliance with ethical standards, clear scientific proof’. They also require a clear well-documented protocol as well as obtaining an informed consent and affirming confidentiality. In order to be able to be involved in a CT, staff should be appropriately qualified, as well as receive adequate training, such as GCP. CT data should be documented accurately and be easily accessible.5
Bias in CT
In order to ensure highest standards for CTs, there are many quality control points in place. There are many designs of research study that have been developed carefully over the years. Nevertheless, each of them could suffer of a certain degree of bias. Bias in CTs represents ‘systematic errors that encourage one outcome over others.’6
Where there is bias, it is very likely that the wrong conclusions may be drawn with potential disastrous effects. Bias in CTs may be of different types, including the following:
- Extrapolation; a potential problem in experimental models
- Recall bias; a concern for retrospective studies
- Confounding by indication; when patients are allocated to the intervention or control group on the basis of patient and investigator preferences, patient characteristics, and clinical history. This is a concern for prospective and retrospective studies.7
- Selection bias; when patients in the control group have less favourable outcomes than patients in the experimental group
- Allocation bias; a concern for randomised CTs
- Bias related to patients and investigators’ expectations, competing interests and financial bias; typically affects industry-funded CTs
In addition, most clinical trials have historically been undertaken using subjects of white ethnicity along with just a few other specific selected groups. As a result, there has been an under-representation of the following subjects which might have had some influence upon the outcomes and their significance:
- Very young, paediatric patients
- Elderly patients
- Women; especially those pregnant or breast-feeding who are generally excluded
- Patients with significant renal or hepatic impairment
- Patients from a wide range of ethnic minorities.
This cohort constraint is another form of selection bias (figure 2). When bearing in mind the already known influence of age, ethnicity and gender on eye health, there is a strong argument for some redress of the selection bias in future eye research.
 Figure 2: Clinical trials are susceptible to selection bias, which may have great significance for our understanding of eye care
Figure 2: Clinical trials are susceptible to selection bias, which may have great significance for our understanding of eye care
Under-reporting and Hidden Data
The Health Research Authority (HRA) issued a report titled Research Transparency (last updated on the July 29, 2021), which announced: ‘Research transparency is central to ethical research practice. Research studies should be registered and the results made public, so that participants are protected from unnecessary studies and research funding maximised. When research is carried out openly and transparently, everyone will be able to see what research is happening and the outcomes from finished studies.’8
CTs are costly and have high stakes. Therefore, when the results are not those expected, there is the possibility that the related industry will fail to report, mis-report or delay the announcement of some or all results. Publication bias, where there is the selective publication of only the findings of trials with positive results or delayed publications with negative results, have, indeed, been reported.6 Nevertheless, ‘publication of clinical trials, especially those with negative results, remains vital, helping to improve future trials, steering resources away from fruitless therapies, and informing patients about better clinical approaches.’9
Covid-19 and CT
‘The ongoing pandemic is affecting how clinical trials are managed, not just in terms of patient recruitment and immediate patient care but also in terms of data collection, analysis and safety reporting.’10 As a result, the EMA, the MHRA and the FDA have all issued guidance on the conduct of CTs and post-marketing surveillance during the Covid-19 pandemic.
Electronic safety reporting was not affected by the pandemic. However, those that require courier or hand delivery were severely affected. The pandemic has taught us a few lessons about security, safe data transfer and avoidance of data corruption or influence, and the drug safety reporting systems have been quick to respond.
The EMA has also produced a new document concerning this matter, called the Questions and Answers on Regulatory Expectations for Medicinal Products for Human Use during Covid-19 Pandemic. This is available to download at: https://ec.europa.eu/health/sites/default/files/human-use/docs/guidance_regulatory_covid19_en.pdf.
In addition, the MHRA published a guidance document on how to manage CT during Covid-19 pandemic. This guidance is available to download at: https://www.gov.uk/guidance/managing-clinical-trials-during-coronavirus-covid-19.
Pharmacovigilance in Eye care Practice
In clinical disciplines related to eye health and eye care, pharmacovigilance is important in addressing the following areas:
- Ocular adverse effects of ophthalmic medicines
- Ocular adverse effects of systemic medicines (see later)
- Medication errors
- Lack of drug efficacy reporting
- Use of medicines for ophthalmic indications that are not approved and for which there is an inadequate scientific basis
- Adverse interactions of medicines with chemicals or other medicines11
Nevertheless, from the pharmacovigilance point of view, ophthalmology is a special area. Indeed, pharmacovigilance refers here, not only to the ocular adverse effects of ophthalmic drugs, but also to the ocular adverse effects of systemic drugs and systemic side effects of ocular medication. This is an area that is not very well covered. Indeed, more than 50% of any ophthalmic drug will be absorbed systemically, either via the conjunctiva or through the lacrimal drainage system (figure 3).
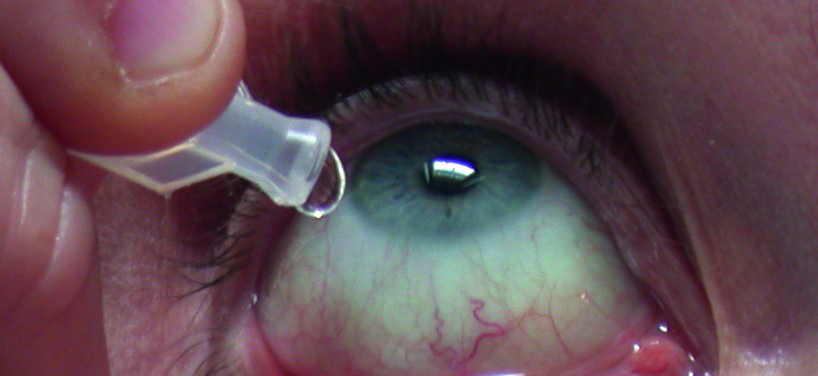 Figure 3: More than 50% of any ophthalmic drug will be absorbed systemically, either via the conjunctiva or through the lacrimal drainage system
Figure 3: More than 50% of any ophthalmic drug will be absorbed systemically, either via the conjunctiva or through the lacrimal drainage system
In addition, most of our patients, especially older adults, suffer from systemic pathologies that also require various drug treatments. A large number of these substances, however, besides their intended actions, also induce side effects and some of these are expressed at the ocular level. Due to its highly vascularised structure, the eye is particularly vulnerable to any substances that enter the blood stream (figure 4). Eye care practitioners should, at the very least, be aware of the most commonly presenting of these effects.
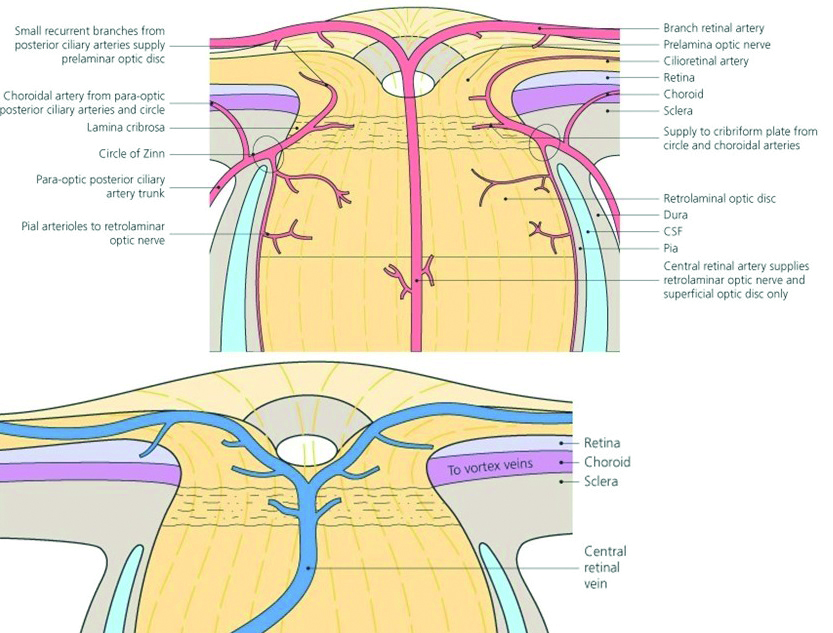 Figure 4: Due to its highly vascularised structure, the eye is particularly vulnerable to any substances that enter the blood stream
Figure 4: Due to its highly vascularised structure, the eye is particularly vulnerable to any substances that enter the blood stream
Alongside maintaining a good working knowledge of systemic pathologies for appropriate positive and differential diagnoses, eye care practitioners should also know when to follow up more closely those patients taking certain specific systemic medications. This is especially important when one remembers that some of the side effects of these medications can be toxic and may produce irreversible ocular changes. More details on ocular side effects of systemic drugs and systemic side effects of ocular drugs have been published by the author in Optician.12-19
Only a very few systematic reviews have looked at the link between ocular side effects and systemic drugs.20,21 These effects are, in general, mild. In addition, they mainly occur in certain sub-populations of patients, such as in children and the elderly who are exposed to polypharmacy. Blindness has been reported on occasion, however, with relation to the administration off several systemic drugs. In addition, ocular drugs and treatments, such as the use of anti-VEGF injections, have been linked to serious adverse systemic circulatory events. As both visual disability and cardiovascular disease represent a huge burden on our patients’ well-being and increase their risk of mortality, a greater awareness and reporting of such serious adverse responses (SAR) is important.
In eye health practice, pharmacovigilance might be susceptible to a number of pitfalls. These including the following:
- Under-reporting or selective reporting
- Missing data from large datasets
- False positive results
- Results skewed by the voluntary nature of the reports
- Failure to recognise ocular SAR in the context of a systemic drug clinical trial (and vice versa)
- Difficulties in recognising a SAR linked to a specific drug increase when polypharmacy is present
- Efficacy and adverse reactions can be different in different ethnicities. As most of the CTs are carried out using samples comprised primarily of white ethnic individuals, this is a significant concern and needs to be resolved.
- Randomised CTs (RCTs) are designed to detect the efficacy of a drug and are not sufficiently powered to detect any rare SAR.22
- CTs in eye care suffer from limitations of bias, such as patient selection restriction, small sample size and limited follow-up
- Safety reporting is requested, but still remains at the practitioner’s discretion. This is a major limitation.
- Trials are unlikely to uncover all adverse effects, especially those that are rare
- Only continuous monitoring for adverse events after the medicine has been marketed and given to many more patients might a more comprehensive pattern of adverse effects be established
In order to increase the awareness and reporting of various adverse reactions to any specific CT drug, a few recommendations have been suggested.22 These advise that the eye care practitioner should:
- Assess a patient’s risk profile in more detail prior to treatment and/or study enrolment. Patients with ocular diseases are often elderly and suffer from various systemic comorbidities and there is a risk that this can be overlooked by eye care providers (figure 5).
- Involve patients in the reporting of any suspected adverse effects. To facilitate this process, good patient communication is key. In addition, the use of social media for monitoring such reports from patients is also being explored and may improve matters significantly.
- Use specific software and electronic data collection methods. This will also allow improved data verification and good reporting quality.
 Figure 5: Elderly people are likely to have systemic co-morbidity alongside ocular disease
Figure 5: Elderly people are likely to have systemic co-morbidity alongside ocular disease
The Royal College of Ophthalmologists underlines this with their stated ambition to ‘promote quality and safety in all aspects of ophthalmology and has developed ophthalmic safety alerts based upon the changing aspects of eye care delivery.’23 These alerts are designed to inform members of the need to always improve the quality and safety of services they provide and are based on a full and maintained awareness of the following:
- Changes to drug safety
- Updated licencing of therapeutics
- Updated awareness of adverse incidents
They publish various safety alerts related to ophthalmic drugs periodically. Recent examples include those related to retinal vein occlusion post Covid-19 vaccination (2021), risk to future fertility from boron-containing excipients in chloramphenicol eyedrops, (2021), cataract related cystic fibrosis conductance regulator gene (CTFR) for cystic fibrosis (2019) and so on. All these reports can be found at https://www.rcophth.ac.uk/standards-publications-research/quality-and-safety/.
The College of Optometrists is also concerned with the use and supply of drugs and medicine in the optometric practice; the A458 section of this report outlines the reporting schemes in case of adverse drug reactions.24 Nevertheless, this information is still limited and many optometrists will struggle to understand the best way of dealing with such occurrences.
Conclusions
More and more optometrists will be qualified as independent prescribers and, inevitably, will be involved in various CTs. In addition, they are also at the frontline of patient monitoring and could detect and report various side effects of ocular and systemic drugs. Therefore, further training in PV is a modern requirement for this category of healthcare providers.
- Dr Doina Gherghel is an academic ophthalmologist with a special interest in inter-professional learning for optometrists.
Important contacts
- Association of the British Pharmaceutical Industry (ABPI): regulatory@abpi.org.uk
- British Generic Manufacturers Association (BGMA): info@britishgenerics.co.uk
- BioIndustry Association (BIA): regulatory@bioindustry.org
- Clinical & Contract Research Association (CCRA): mail@ccra.org.uk
- Ethical Medicines Industry Group (EMIG): info@emig.org.uk
- European Medicine Agency https://www.ema.europa.eu/en/human-regulatory/post-authorisation/pharmacovigilance/good-pharmacovigilance-practices
- Health Food Manufacturers’ Association (HFMA): pennyviner@btconnect.com
- The National Pharmacy Association (NPA): independentsvoice@npa.co.uk
- Proprietary Association of Great Britain (PAGB): regulatory@pagb.co.uk
- MHRA : gpvpinspectors@mhra.gov.uk
References
- Varner, Paul. (2015). Ophthalmic pharmaceutical clinical trials: design considerations. Clinical Investigation. 5. 457-475 (available as a free download at; www.openaccessjournals.com/articles/ophthalmic-pharmaceutical-clinical-trials-design-considerations.pdf
- https://www.who.int/health-topics/clinical-trials#tab=tab_1
- https://www.concertpharma.com/understanding-clinical-trial-terminology-whats-a-phase-1-2-or-3-clinical-trial/
- Nardini C. (2014). The ethics of clinical trials. Ecancermedicalscience, 8, 387. https://doi.org/10.3332/ecancer.2014.387
- Ahmad W, Al-Sayed M. Human subjects in clinical trials: Ethical considerations and concerns. Journal of Translational Science, 4: 2018
- Gluud LL. Bias in clinical intervention research. American Journal of Epidemiology, Volume 163, Issue 6, 2006, 493–501.
- Deeks JJ et al. Evaluating non-randomised intervention studies. Health Technology and Assessment. 2003; 7: 1–173.
- www.hra.nhs.uk/planning-and-improving-research/policies-standards-legislation/research-transparency (accessed after 29.07.2021)
- Karsy, M et al. Hiding in plain sight: underreporting of clinical trial results in neurology. Neurosurgery, 2018, Volume 83, Issue 3, E96
- https://www.iconplc.com/insights/blog/2020/04/29/the-impact-of-covid-19-on-pharmacovigilance/index.xml.
- Dubey A, Handu S. Are we pharmacovigilant enough in ophthalmic practice? Indian Journal of Ophthalmology: May 2013, Volume 61, Issue 5, 226-229
- Gherghel D. Ocular side effects of systemic drugs 1; cholesterol lowering, anti-hypertensive and cardiac drugs. Optician, 27.03.2020
- Gherghel D. Ocular side effects of systemic drugs 2; hypoglycaemic, hormonal and anti-rheumatic drugs. Optician, 24.04.2020
- Gherghel D. Ocular side effects of systemic drugs 3; central nervous system agents. Optician, 22.05.2020
- Gherghel D. Ocular side effects of systemic drugs 4; anti-infectives, chemotherapy and vitamins and herbal supplements. Optician, 26.06.2020
- Gherghel D. Systemic side effects of ocular medication part 1: diagnostic drugs –Optician, 31.07.2021
- Gherghel D. Systemic side effects of ocular medication part 2: topical therapeutic drugs. Optician, 28.08.2020
- Gherghel D. Systemic side effects of ocular medication part 3; intravitreal and intravenous drugs. Optician, 18.09.2020
- Gherghel D. Systemic side effects of ocular medication part 4; intravitreal anti-infectives and anti-metabolites, and peri-operative drugs. Optician, 30.10.2020
- Miguel A, Henriques F, Azevedo LF, Pereira AC. Ophthalmic adverse drug reactions to systemic drugs: a systematic review. Pharmacoepidemiology and Drug Safety. 2014;23(3):221–33;
- Karrer JE et al. Pharmacovigilance in ophthalmology in Switzerland: an analysis of the most frequently reported ocular adverse drug reactions within the last 25 years. Swiss Medicine Weekly, 2019, Jun 30; 149
- Ziemssen F et al, OCEAN Study Group. Reporting of safety events during anti-VEGF treatment: pharmacovigilance in a non-interventional trial. Journal of Ophthalmology, 2020, Article ID 8652370, 13, 2020
- www.rcophth.ac.uk/standards-publications-research/quality-and-safety
- https://guidance.college-optometrists.org/guidance-contents/knowledge-skills-and-performance-domain/use-and-supply-of-drugs-or-medicines-in-optometric-practice
