Have you ever wondered what it would be like to live in a world in which every person is a stranger? A world in which faces cannot be identified? A world in which other people seem to instantly recognise people just by looking at their face, but you are unable to do so? A world in which other people use a language that you have no access to, namely the language of facial expressions? This is not a fictional world. This is reality for people who have impaired face recognition due to vision impairment (VI) or prosopagnosia. This article explores causes, challenges and compensation strategies for impaired recognition of faces and facial expressions.
Causes of prosopagnosia
Face recognition is dependent on three main factors:
- The face needs to be clearly seen
- There has to be a visual memory of the face from a previous encounter
- The visual memory of the face needs to match the face that is currently perceived
For people with ocular VI, the problem is primarily that the face is not clearly seen. In prosopagnosia, depending on the underlying cause, the face may be clearly seen and the patient may correctly describe specific facial features of the faces seen. However, the matching process is impaired, which means that faces are not instantly recognised. Prosopagnosia and reduced clarity of vision can also co-exist, for example in neurological sight loss.
Ocular vision impairment
Intact visual functioning is essential for the effective recognition of faces and facial expressions. Impairment of visual acuity, contrast sensitivity, reduced colour vision and visual fields can negatively affect this ability.1-4 Some examples are now described.
One of the most common causes of reduced vision is uncorrected or inadequately corrected refractive error. Myopes often explain that they can hear better when wearing their glasses. This is because the hitherto unseen lip movements synchronise with the sound and so complement perception.
Patients with AMD typically develop central vision impairment, affecting the ability to process the high spacial frequencies necessary for face recognition and detection of facial expressions and emotions. AMD leads to particular difficulties in recognising internal facial features. Full-face discrimination impairment is associated with reduced contrast sensitivity and visual acuity.3 In normally sighted people, the eye region of the face plays an important role in understanding the emotional state of other people.5 When the eye region can no longer be discriminated, patients tend to compensate for this by looking at the lower part of the face (mouth) instead.1
Face recognition impairment in patients with glaucoma has been associated with reduced contrast sensitivity and visual field impairments2,6 as well as increased sensitivity to central vision crowding.7,8 These effects render it difficult to recognise faces when they are viewed at a distance.
Diabetic retinopathy (DRP) can lead to reduced visual acuity and visual fields which tends to become more severe as the disease progresses.9,10 Reduced contrast sensitivity is associated with DRP, even from very early stages.11-15 Facial recognition is, therefore, often affected in patients with DRP (figure 1). There may also be a neurological underpinning for impaired face recognition in patients with impaired glucoregulation.16,17
 Figure 1: Facial recognition is often affected in patients with diabetic retinopathy
Figure 1: Facial recognition is often affected in patients with diabetic retinopathy
In summary, any eye condition that leads to impairment of visual acuity, contrast sensitivity or visual fields has the potential to lead to difficulties in recognising faces and facial expressions.
Prosopagnosia
The term prosopagnosia is reserved for a specific face recognition problem due to impaired function of the face area in the brain, the fusiform gyrus,18-21 where memories of faces and facial expressions are stored. The problem is not the clarity of vision, but rather the recognition of familiar faces.
The congenital form is referred to as developmental prosopagnosia and it is thought that there is a genetic component.22 Dutton reported a combination of prosopagnosia and topographic agnosia within families with different degrees and combinations of these features between family members.23 In developmental prosopagnosia, face recognition skills and facial emotion processing skills are not developed despite otherwise normal vision and normal brain images.19,22 The estimated prevalence of developmental prosopagnosia is at least 2%.24,25
One can also acquire prosopagnosia after brain injury. When no visual memories of faces are stored, there is no instant recognition of faces when the same face subsequently presents. Some people with prosopagnosia also struggle to interpret facial expressions: If facial expressions are not rooted in the visual memory store, they are not automatically understood. Interestingly, the memory store for routes is in an area adjacent to the one for faces. Therefore, people with prosopagnosia may also struggle with route finding.23 When people are encountered in an unexpected place, this can be particularly challenging for someone with prosopagnosia, but this happens to people without prosopagnosia too. When someone is encountered in an unexpected place, it can be difficult to place them, which can lead to embarrassment. Rather than admitting the face recognition failure, one tends to use different strategies for identification, such as listening to the voice or seeking conversational cues. People with prosopagnosia rely entirely on strategies like these in social situations.
Although prosopagnosia can have a profound effect on social interaction, not everyone with prosopagnosia is aware of their condition. For some people, compensation strategies have become second nature. For others, prosopagnosia is a daily challenge.
Neurological vision impairment
Neurological VI can be divided into the following two categories:
- Cerebral visual impairment (CVI); this develops during pregnancy, birth or early years
- Acquired brain injury; this develops later in life
Basic visual functions as described in the first section can be affected as well as higher visual processing functions. Ventral stream networks are referred to as the ‘what pathway’. This is the area where recognition takes place, including face recognition. 26,27 The dorsal stream network is referred to as the ‘where pathway’. This brain area provides a map in three-dimensional space and controls visual attention. One can imagine that impaired motion perception (dyskinetopsia) can render it difficult to detect fast moving facial expressions and mouth patterns (figure 2). In integrative agnosia the individual facial features can be distinguished, but the facial features are not integrated and therefore not perceived as a whole. Visual field inattention or neglect leads to only seeing or processing part of a face. Crowding of facial features can also pose problems for people with dorsal stream dysfunction.
 Figure 2: Impaired face recognition due to dyskinetopsia
Figure 2: Impaired face recognition due to dyskinetopsia
Cerebral visual impairment
Impaired face recognition has been associated with periventricular leukomalacia and altered brain development in pre-term children,28,29 children born small for gestational age,30 cerebral palsy31 and perinatal stroke with suspected parietal lobe involvement.32 It has also been suggested that there may be a link between face recognition and word processing mechanisms.33 Children and adults with dyslexia appear to have difficulties with recognising words, faces and visually complex objects and it is thought that this may be due to ventral stream dysfunction.33 Impaired face recognition has also been associated with autism,34 although one needs to be careful not to consider problems with face recognition as autism-specific, but to consider the possibility of underlying visual processing dysfunction.
Acquired Brain Injury
Acquired brain injury occurs secondary to brain damage and structural lesions are usually identifiable.22 Impaired facial emotion recognition and impaired face recognition have been described in adult stroke patients, irrespective of the affected hemisphere.35-38 The incidence could be as high as 50% after a right hemisphere stroke.39 Different emotions appear to be mapped in specific brain areas.38
Alzheimer’s disease can affect both low-level and high-level visual perception. Basic processing issues include visual acuity, contrast sensitivity, visual fields and colour vision and higher processing issues include motion perception, face recognition, recognising facial emotional expressions40 and recognising inferred personality traits.41
Another cause for acquired brain injury is a brain tumour. Recognition of faces42 and facial expressions43 can be affected in patients who have had treatment for brain lesions.
Challenges
Impaired face perception can have a detrimental effect on social interactions and quality of life (QoL). See table 1.
 Table 1: Self-reported challenges in patients with age-related macular degeneration leading to impaired perception of faces. Adapted from Lane et al44
Table 1: Self-reported challenges in patients with age-related macular degeneration leading to impaired perception of faces. Adapted from Lane et al44
As with other aspects of VI, the inability to perceive faces affects the way people interact within their visual world. Facial recognition renders it difficult to identify people, even friends and family. It is therefore more difficult to initiate conversations. This can give people the impression that the affected person does not care about them. However, once the person has been identified, the memories associated with that person are completely intact. Many people with impaired face recognition also have difficulty interpreting the language communicated by facial expressions. This affects the ability to make appropriate judgements in terms of other people’s emotions while the subtle nuances of the meaning of fleeting facial expressions can be completely missed resulting in awkward social situations. This can lead to withdrawal or reduced confidence.
Interventions
In situations where ocular pathology is the main cause for impaired face recognition, one needs to consider treating the eye condition if this is possible. In cases where treatment is not possible, rehabilitation may be more effective. The same is applicable to neurological VI and prosopagnosia. For example, stroke patients may experience recovery or improvement of face recognition ability after treatment, although the majority of these patients would still benefit from rehabilitation.39
The aim of rehabilitation in the context of face recognition impairment is to reduce the impact of this impairment on everyday living through compensatory strategies and remedial training.45 Remedial training seeks to restore normal face processing mechanisms through visuo-cognitive training. For this, one relies on the potential neuroplasticity of the face area of the brain.
Holistic face processing systems emerge early in life with evidence of this ability in children as young as 3 months of age. However, further development of this mechanism is thought to continue in early years and into adolescence with evidence of some plasticity later in life.46 Remedial versus compensatory training is an ongoing subject of debate with compensatory techniques, such as verbalising distinctive facial features, showing more effectiveness.47
Compensation strategies
The effectiveness of compensatory strategies depends on the underlying mechanism. Is it ocular VI, neurological VI or developmental prosopagnosia?
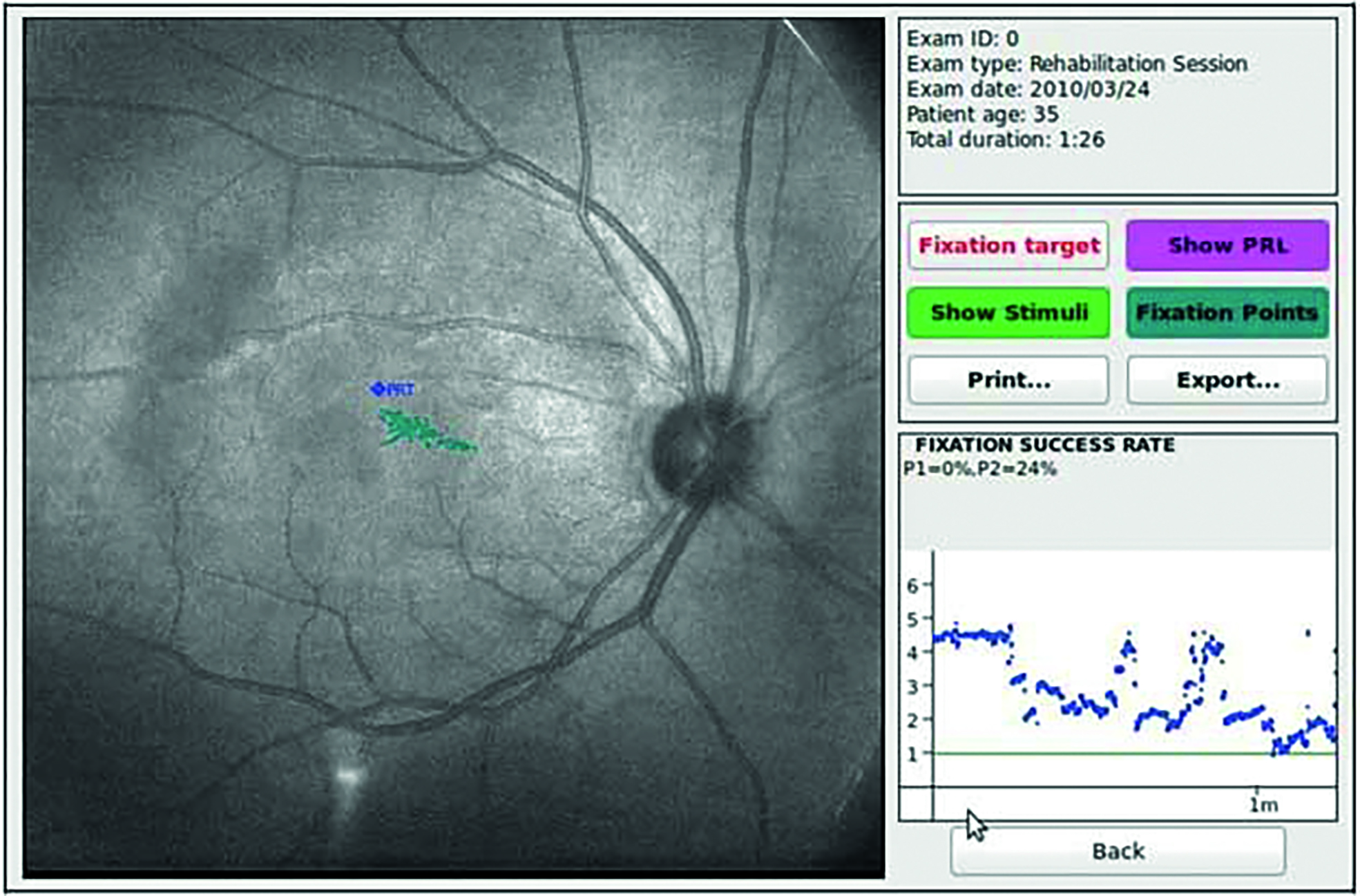
Patients with a central scotoma due to macular pathology often use a preferred retinal locus for viewing faces and objects (figure 3). This is referred to as eccentric viewing. This technique can develop naturally or it can be taught by identifying the preferred retinal locus and then training the patient to use this ‘island of vision’ by looking above, below or to the side of the object of interest, in this case the face.
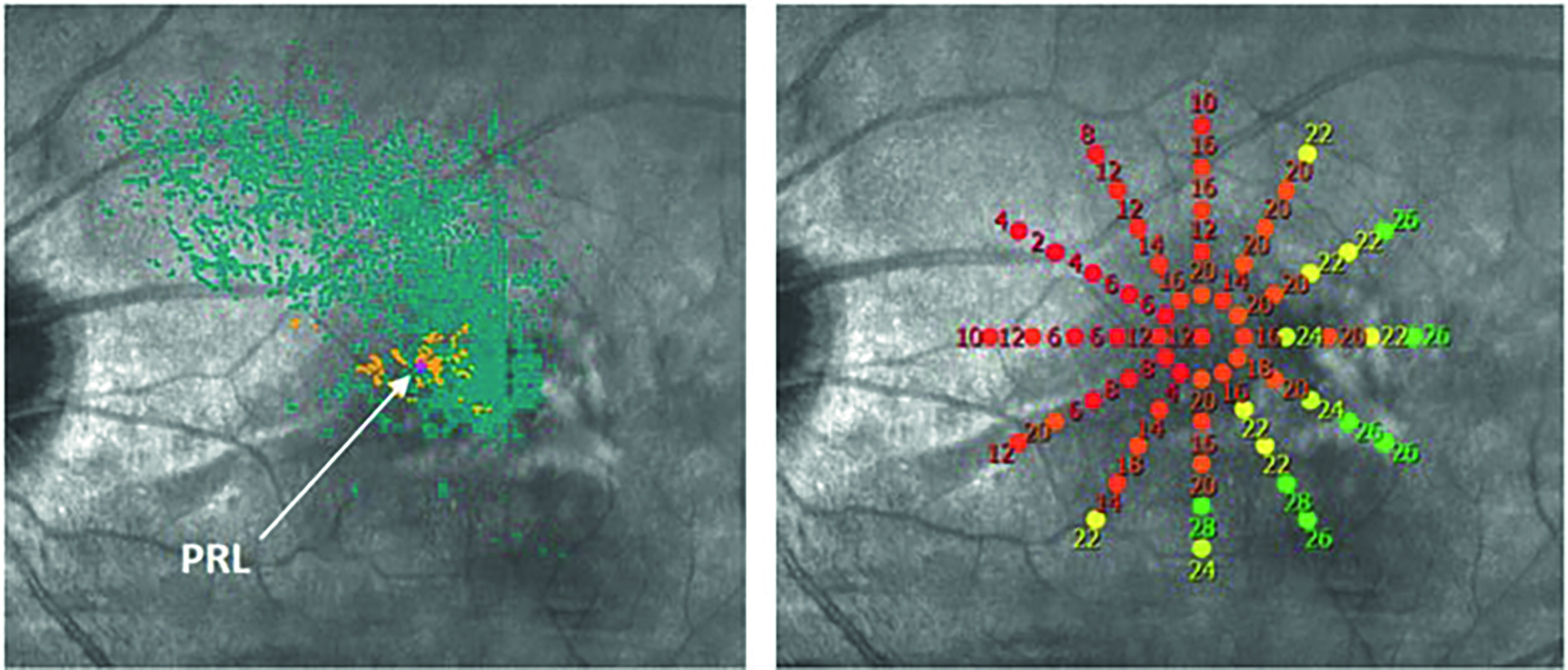
Figure 3 (above) The preferred retinal locus (PRL) established by microperimetry in a patient with maculopathy and unstable fixation, as seen by the spread of blue fixation points. (below) With training, the patient is able to adopt a more stable PRL relocation target (or PRT), with much less fixation spread
The severity of acquired face recognition impairment in neurological conditions depends on the lesion size and location and the timing of the injury.46 Contextual cues48 and presentation also contribute to the ability to recognise faces. Different strategies may be required in different situations. For example, a small study showed improved face recognition skills for patients with developmental prosopagnosia if the faces were presented in motion as opposed to a still image.49
As with other functional aspects in patients with VI, there is a lot to be learned from those who are living with the condition. Patients with face recognition impairment report a number of effective strategies that they employ to improve functioning (see table 2).
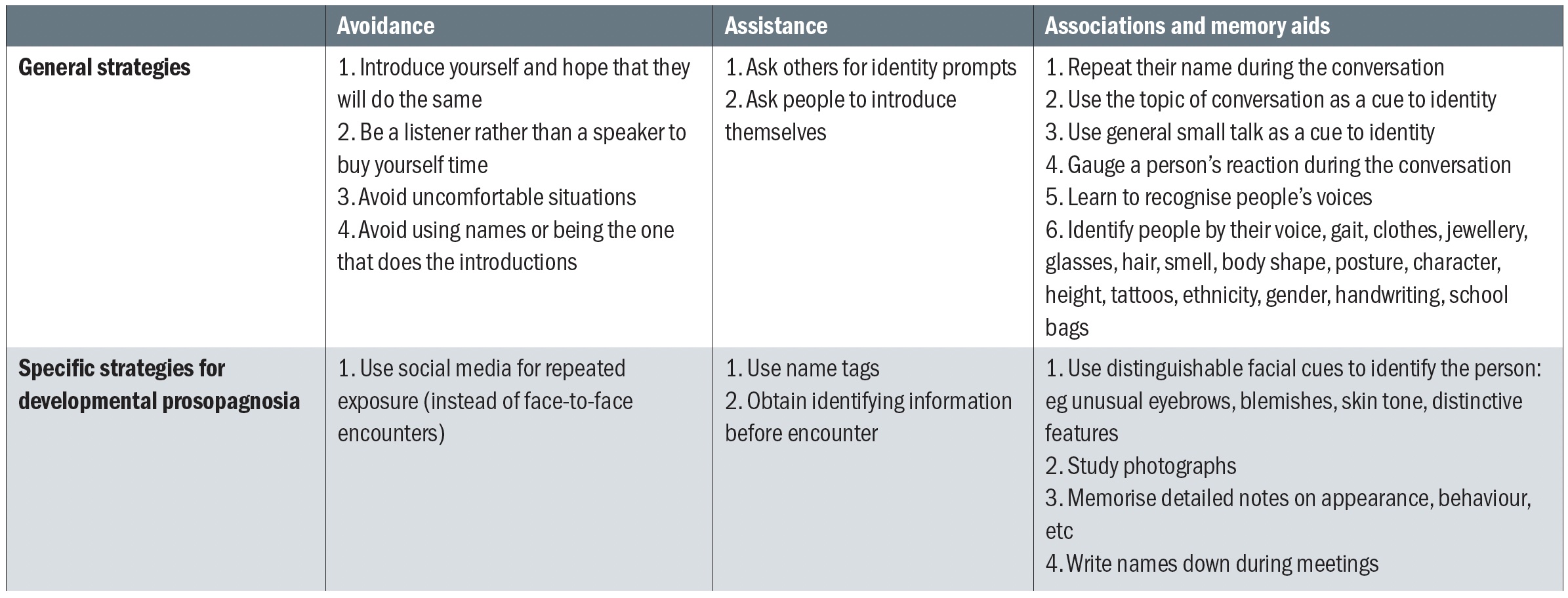 Table 2: Strategies that are employed by people with impaired face recognition. Adapted from Adams et al48
Table 2: Strategies that are employed by people with impaired face recognition. Adapted from Adams et al48
Assistive technology
For patients with ocular VI, one can improve face recognition using enhancement and enlargement. Face recognition software can assist anyone with face recognition problems. A number spectacle-mounted and head-mounted devices can be used to achieve this. Some of these devices are listed in table 3 and shown in figure 4.
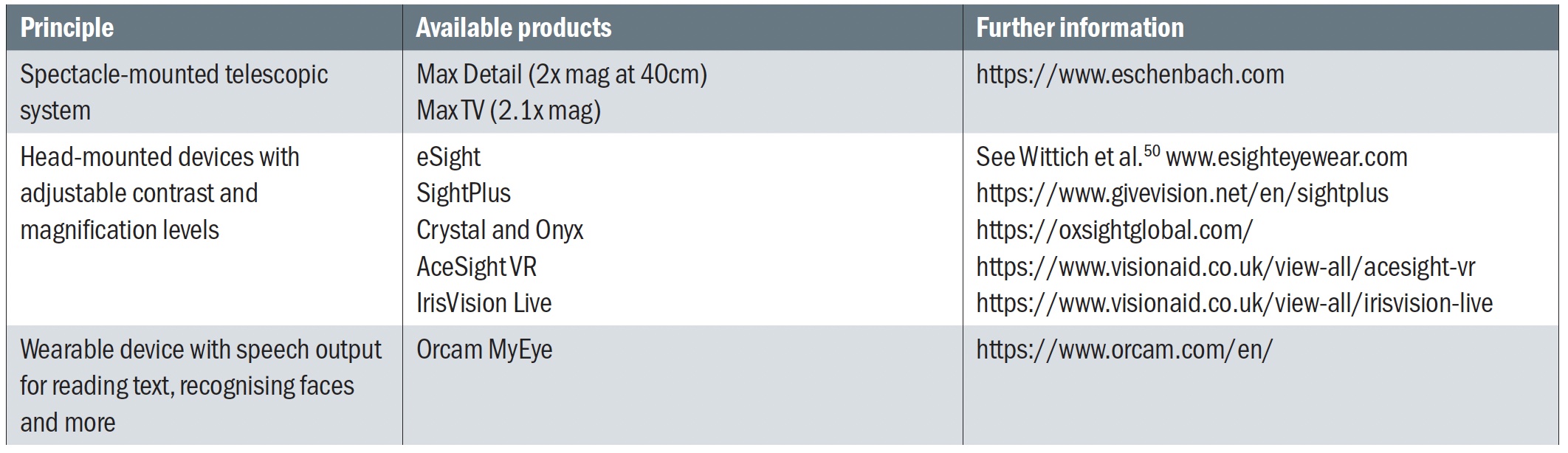 Table 3: Available wearable devices that can be used for face recognition
Table 3: Available wearable devices that can be used for face recognition

Case scenarios
Age-related macular degeneration (AMD)
A female in her eighties, with a history of bilateral wet AMD treated with anti-VEGF, attended the low vision clinic. She had reduced vision (6/36 in the better eye), significantly reduced contrast sensitivity (Mars 19%) and colour vision impairment (Ishihara 2/14 plates).
Furthermore, she had a relative central scotoma, with lines appearing ‘wriggly and foggy’ on the Amsler grid. When asked what she found difficult now that her eye sight was not as good as it used to be, she responded that she was no longer able to see her friends, even if they were close-up. She worried that she might be perceived as being rude for not recognising people and she also struggled to see people’s facial expressions when she was in conversation.
This is a common scenario and it can be difficult to address this issue satisfactorily. However, some of the aforementioned strategies could be employed. For example, she could pay more attention to the voice, gait, posture, height and clothes of people to improve recognition. She could also consider telling her friends that she has difficulties recognising them due to her eye-sight and ask them to introduce themselves when they meet.
In terms of holding conversations, it can be useful to think carefully about the patient’s and the other person’s position in relation to light sources, in order to reduce back-light scatter and to improve the contrast to enhance facial features. On the odd
occasion, people find the Eschenbach MaxDetail (figure 5) helpful when talking to close friends or relatives, although most people perceive a spectacle-mounted or head-mounted device as another barrier. The Orcam My Eye (figure 4a) can assist with face identification.
 Figure 5: The Eschenbach MaxDetail
Figure 5: The Eschenbach MaxDetail
Cerebral visual impairment and potential developmental prosopagnosia
A nine-year-old girl with a history of CVI attended for an eye examination. She had accommodative esotropia which was controlled with glasses. Her vision was around 6/12 in both eyes, with normal colour vision, contrast sensitivity and visual fields. However, after careful history taking, she appeared to struggle in crowded environments and found it difficult to hold a conversation whilst talking. This led to her bumping into objects and people and tripping over objects on a regular basis. She also reported difficulties with facial recognition. She could not recognise her own relatives on photographs or in real life, and found it difficult to find her parents when they were standing in a group. She tended to recognise her parents by their perfume. At school and in play parks, she made friends with everyone. It was not clear if this was a strategy that she employed in order to compensate for the fact that she cannot recognise who her friends are. After all, it is better to be friends with everyone than to have no friends at all. Her mum also reported that she could not interpret emotions on people’s faces. In clinic, she only recognised very basic facial expressions (happy and sad), but could only do this within two metres distance.
The problems with recognition of faces and facial expressions may be attributable to her dorsal stream dysfunction, but could also be a case of developmental prosopagnosia.
What advice could be given to this girl and her family? First of all, the parents could pre-arrange a precise location where they always stand at school pick-up times. They can also wear a specific bright scarf or coat that the girl can easily recognise from a distance. At school she would benefit from sitting at the front of the classroom in order to reduce distraction from classmates and in order to see the teacher better. The other children could wear name tags for instant identification and the parents and teachers could give identification prompts when necessary. It is worth considering teaching the whole class about the way the child sees in order to improve understanding and create an atmosphere of support and protection. She can also be taught some of the strategies as outlined in table 1.
Autism spectrum disorder
A young man with a history of autism spectrum disorder (ASD) and dyslexia attended the LV clinic. He struggled with social interactions. Busy environments made him feel anxious and hesitant. When he was walking in a busy street, he was afraid that he might bump into people. He also struggled with uneven pavements, floor boundaries, steps and kerbs. His visual acuity was normal after spectacle correction (6/5 each eye) and he had normal contrast sensitivity and colour vision. Careful examination and history taking revealed that he had lower field inattention, simultanagnosia, dyskinetopsia and impaired facial perception.
When a patient has prosopagnosia, especially in the presence of other visual processing dysfunctions, it can be challenging to discern which of the symptoms are related to impaired visual processing and which ones are related to autistic behaviour.51 Visual and perceptive dysfunction are common features in ASD.52
Impaired performance of facial recognition53-55 and facial emotion56,57 have been associated with ASD. Can these characteristics be explained by underlying CVI or DP?
Recognition of objects, people and places can be affected in ventral stream dysfunction.58 This includes the perception of faces and facial expressions,59,60 which can affect cognitive development and social relationships. Dorsal stream dysfunction can lead to difficulties in mental parallel processing and simultanagnostic difficulties as explained by Chokron et al in table 4.51 A validated method for making the differential diagnosis between ASD and CVI would be a welcome aid in understanding the conditions and offering compensation strategies.
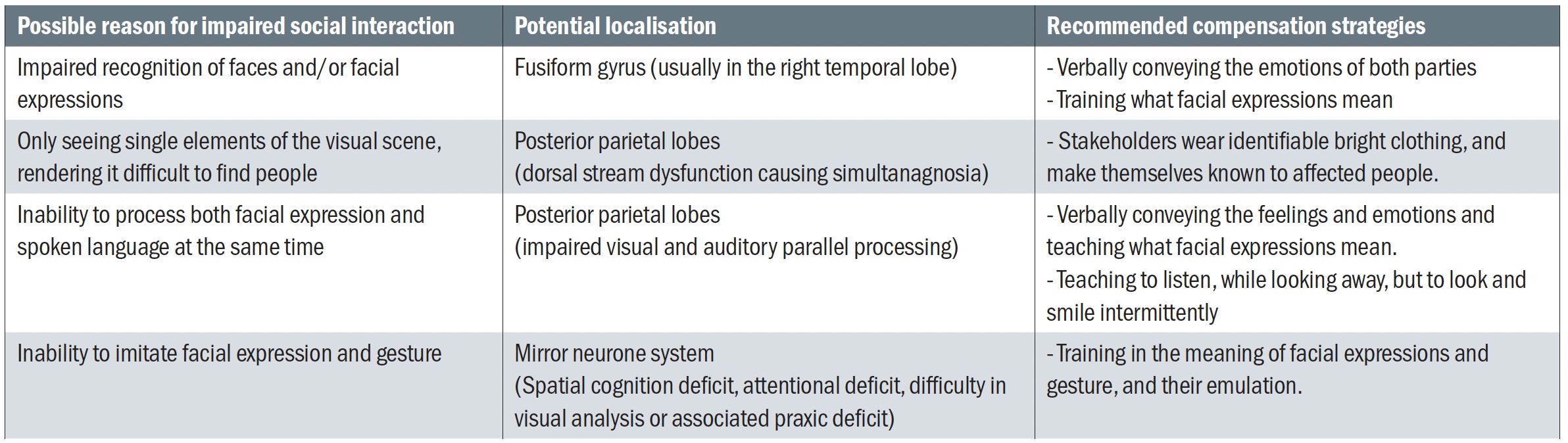 Table 4: Impaired social interaction and visual impairment. Adapted from Chokron et al51
Table 4: Impaired social interaction and visual impairment. Adapted from Chokron et al51
An artist’s eye
An artist with a history of aphakia and glaucoma related to congenital cataract attended for a LV assessment. She had excellent colour vision and contrast sensitivity and an eye for detail. Her vision measured 6/48 in her best eye. She undertook her painting using optical magnification, requiring a short working distance and reduced field of view. Overall, this worked reasonably well, but one thing had always been a stumbling block; the ability to paint portraits. She was unable to see the facial features sufficiently to enable her to paint a true likeness and capture the character of people’s faces. In social contexts, she often found it difficult to recognise people and to read facial emotions. She had developed a fine art of avoiding difficult situations and tended to introduce herself in the hope that others would do the same. She relied on others for identity prompts if possible, and she used small talk and social context to aid identification. She also recognised people by their clothes, their hair, body shape and height. The use of social media had been another helpful tool for social encounters. Overall, she felt that face recognition problems are poorly understood by people and lead to awkward social situations, but on the whole she had learned to adapt and cope very well.
Conclusion
Face recognition impairment is a relatively common phenomenon, that can exist in isolation, as part of neurological sight loss or as a result of reduced visual functions in a number of ocular conditions, including uncorrected or inadequately corrected refractive error. Patients do not always volunteer the information about their difficulties and it can be helpful to ask questions about face recognition as part of a routine assessment, especially when one is dealing with the groups of patients that are more frequently affected. Recognising the problem and offering strategies can impact on quality of life and functioning.
- Cirta Tooth is a specialist low vision optometrist, working for a private practice and for the hospital eye service.
References
- Boucart M, Dinon J-F, Despretz P et al. 2008. Recognition of facial emotion in low vision: a flexible usage of facial features. Visual Neuroscience 25(4), pp.603-609
- Hirji SH, Liebmann JM, Hood DC et al. 2020. Macular damage in glaucoma is associated with deficits in facial recognition. American Journal of Ophthalmology 217, pp.1-9
- Logan AJ, Gordon GE and Loffler G. 2020. The effect of age-related macular degeneration on components of face perception. Investigative Ophthalmology and Vision Science 61(6): 38
- Tan KW and Stephen ID. 2013. Colour detection thresholds in faces and colour patches. Perception 42(7), pp.733-741
- Schmidtmann G, Logan AJ, Carbon C-C et al. 2020. In the blink of an eye: reading mental states from briefly presented eye regions. I-Perception 11(5)
- Glen FC, Crabb DP, Smith ND et al. 2013. Do patients with glaucoma have difficulties recognizing faces? Investigative Ophthalmology and Visual Science 53, p.3629-3637
- Schafer A, Rouland JF, Peyrin C et al. 2018. Glaucoma affects viewing distance for recognition of sex and facial expression. Investigative Ophthalmology and Vision Science 59(12), pp.4921-4928
- Stievenard A, Rouland JF, Peyrin C et al. 2021. Sensitivity to central crowding for faces in patients with glaucoma. Journal of Glaucoma 30(2), pp.140-147
- Bengtsson B, Heijl A and Agardh E. 2005. Visual fields correlate better than visual acuity to severity of diabetic retinopathy. Diabetologia 48(12), pp.2494-2500
- Henricsson M and Heijl A. 1994. Visual fields at different stages of diabetic-retinopathy. Acta Ophthalmologica, 72(5), pp.560-569
- Chande PK, Raman R, John P et al. (2020). Contrast-sensitivity function and photo stress-recovery time in prediabetes. Clinical Optometry 12, pp.151-155
- Eggers ED and Carreon TA. (2020). The effects of early diabetes on inner retinal neurons. Visual Neuroscience 37. Article Number E006
- Ferreira JT and Pinto L. (2017). New trends for early diabetic retinopathy diagnosis. Proceedings of the 5th International Conference on Photonics, optics and Laser Technology (Photoptics), Porto, pp.402-406
- Pramanik S, Chowdhury S, Ganguly U et al. (2020). Visual contrast sensitivity could be an early marker of diabetic retinopathy. Heliyon 6(10). Article Number e05336
- Rahman AA, Badarudin NE, Azemin MZC et al. (2018). Changes in contrast sensitivity in young adults with diabetes. Makara Journal of Health Research 22(1), pp.22-26
- Jones N, Riby LM and Smith MA. 2018. Glucose regulation and face recognition deficits in older adults: the role of attention. Aging Neuropsychology and Cognition 25(5), pp.673-694
- Jones N, Riby LM and Smith MA. 2016. Impaired word and face recognition in older adults with type 2 diabetes. Archives of Medical Research 47(5), pp.372-381
- Behrman M, Avidan G, Black S et al. 2007. Structural imaging reveals anatomical alterations in inferotemporal cortex in congenital prosopagnosia. Cerebral Cortex 17(10), pp.2345-2363
- Burns EJ, Martin J, Xu H et al. 2017. Impaired processing of facial happiness, with or without awareness in developmental prosopagnosia. Neuropsychologia 102, pp.217-228
- Furl N, Garrido L, Duchaine B et al. Fusiform gyrus face selectivity relates to individual differences in facial recognition ability. Journal of Cognitive Neuroscience 23(7), pp.1723-1740
- Zhao YF, Zhen ZL, Liu J et al. 2018. The neural network for face recognition: insights from an fMRI study on developmental prosopagnosia. Neuroimage pp.151-161
- Corrow SL, Dalrymple KA and Barton JJS. 2016. Prosopagnosia: current perspectives. Eye and Brain 8, p.165-175
- Dutton GN. 2003. Cognitive vision, its disorders and differential diagnosis in adults and children: knowing where and what things are. Eye 17, pp.289-304
- Bowles DC, McKone E, Dawel E et al. 2009. Diagnosing prosopagnosia: Effects of aging, sex, and participant-stimulus ethnic match on the Cambridge Face Memory Test and the Cambridge Face Perception Test. Cognitive Neuropsychology 26, pp.423-455
- Kennerknecht I, Yee-Ho N and Wong VCN. 2008. Prevalence of hereditary prosopagnosia (HPA) in Hong Kong Chinese population. American Journal of Medical Genetics Part A (22) 146A, pp.2863-2870
- Farivar R, Blanke O and Chaudhuri A. 2009. Dorsal-ventral integration in the recognition of motion-defined unfamiliar faces. Journal of Neuroscience 29(16), pp.5336-5342
- Grill-Spector K, Weiner KS, Gomez J et al. 2017. The functional neuroanatomy of human face perception. Annual Review of Vision Science 33, pp.167-196
- Carbajal-Valenzuela C, Santiago-Rodriguez E, Quirarte GL et al. 2017. Development of emotional face processing in premature and full-term infants: a quantitative EEG study. Clinical EEG and Neuroscience 48(2), pp.88-95
- Fazzi E, Bova S, Giovenzanna A et al. 2009. Cognitive visual dysfunctions in preterm children with periventricular leukomalacia. Developmental Medicine and Child Neurology 51(12), pp.974-981
- Perez-Roche T, Altemir I, Gimenez G et al. 2017. Face recognition impairment in small for gestational age and preterm children. Research in Developmental Disabilities 62, pp.166-173
- Jasper S and Philip SS. 2018. Profile of cerebral visual impairment in children with cerebral palsy at a tertiary care referral center in Southern India. Journal of Clinical and Diagnostic Research 12(3), pp.NC01-NC04
- Ballantyne AO and Trauner DA. 1999. Facial recognition in children after perinatal stroke. Neuropsychiatry Neuropsychology and Behavioral Neurology 12(2), pp.82-87
- Sigurgardottir HM, Ivarsson E, Kristjansson A et al. 2015. Impaired recognition of faces and objects in dyslexia: evidence for ventral stream dysfunction? Neuropsychology 29(5), pp.739-750
- Kovarski K, Caetta F, Mermillod M et al. 2020. Emotional face recognition in autism and in cerebral visual impairment: in search for specificity. Journal of Neuropsychology 15(2), pp.235-252
- Asperud J, Kuhn CD, Gerlach C et al. 2019. Word recognition and face recognition following posterior cerebral artery stroke: overlapping networks and selective contributions. Visual Cognition 27(1), pp.52-65
- Heutink J, Brouwer WH, Kums E et al. 2012. When family looks strange and strangers look normal: a case of impaired face perception and recognition after stroke. Neurocase 18(1), pp.39-49
- Tippett DC, Godin BR, Oishi K et al. 2018. Impaired recognition of emotional faces after stroke involving right amygdala or insula. Seminars in Speech and Language 39(1), pp.87-99
- Van den Berg NS, de Haan EHF, Huitema RB et al. 2021. The neural underpinnings of facial emotion recognition in ischemic stroke patients. Journal of Neuropsychology [Online ahead of print]. http//doi:10.1111/jnp.12240
- Cousins R. 2013. Prosopagnosia after stroke: potentials for impairment and treatment. Topics in Stroke Rehabilitation 20(6), pp.471-477
- Lenoir H, Sieroff E. 2019. Visual perceptual disorders in Alzheimer’s disease. Geriatrie et Psychologie Neuropsychiatrie de Vieillissement 17(3), pp.307-316
- Hirai M, Sakurada T, Muramatsu S. 2019. Face-to-trait inferences in patients with Parkinson’s disease. Journal of Clinical and Experimental Neuropsychology 41(2), pp.170-178
- Corrivetti F, Herbet G, Duffau H et al. 2017. Prosopagnosia induced by a left anterior temporal lobectomy following a right tempero-occipital resection in a multicentric diffuse low-grade glioma. World Neurosurgery 97
- Midorikawa A, Saito S, Shinoura N et al. 2019. Biased recognition of surprised facial expressions following awake craniotomy of a right temporal lobe tumor. Frontiers in Psychology 10
- Lane J, Rohan EMF, Sabeti F et al. 2018. Impacts of impaired face perception on social interactions and quality of life in age-related macular degeneration: A qualitative study and new community resources. Plos One 13(12)
- Wilson CE, Palermo R, Brock J et al. Specificity of impaired facial identity recognition in children with suspected developmental prosopagnosia. Cognitive Neuropsychology 27(1), pp.30-45
- Bate S and Bennetts RI. 2014. The rehabilitation of face recognition impairments: a critical review and future directions. Frontiers in Human Neuroscience 8
- DeGutis JM, Chiu C, Cohan S et al. 2014. Face processing improvements in prosopagnosia: successes and failures over the last 50 years. Frontiers in Human Neuroscience 8
- Adams A, Hills PJ, Bennetts RJ et al. 2020. Coping strategies for developmental prosopagnosia. Neuropsychological Rehabilitation 30(10), pp.1996-2015
- Bennetts, RJ, Butcher, N, Lander, K, Udale, R, & Bate, S. 2015. Movement cues aid face recognition in developmental prosopagnosia. Neuropsychology, 29(6), 855-860
- Wittich W, Lorenzi MC, Dagnelie G et al. 2018. The effect of a head-mounted low vision device on visual function. Optometry and Vision Science 95(9), pp.774-784
- Chokron S, Kovarski K, Zalla T and Dutton GN. 2020. The inter-relationships between cerebral visual impairment, autism and intellectual disability. Neuroscience and Biobehavioral Reviews 114, pp.201-210
- Little J-A. 2018. Vision in children with autism spectrum disorder: a critical review. Clinical and Experimental Optometry 101(4), pp.504-513
- Cygan HB, Okuniewska H, Nowicka A et al. 2018. Face processing in a case of high functioning autism with developmental prosopagnosia. Acta Neurobiologiae Experimentalis 78(2), pp.114-131
- Spencer J, O’Brien J, Riggs K et al. 2000. Motion processing in autism: evidence for a dorsal stream deficiency. Neuroreport 11, pp.2765–2767
