An understanding of the anatomy, physiology and potential impact of contact lens wear on the anterior eye is important. There have been numerous relevant publications on these topics, however, they have not been published consistently, and some of these aspects are still unknown. The objective of the BCLA CLEAR section by Downie et al1 was to review the relevant literature in this area, as a foundation to help shape our understanding of the potential relationship with contact lenses and to identify areas in need of further research. This article highlights the key findings and the relevance to contact lens practice. A more exhaustive discussion will be covered later in the series when reviewing the subsequent BCLA CLEAR sections.2-10
Terminology
For standardisation, the Federative Committee on Anatomical Terminology (FCAT) is generally favoured as this uses descriptors rather than the name of the person who typically discovered the structure. For example, in the context of describing the corneal anatomy, anterior and posterior limiting lamina are referenced instead of Bowman’s and Descemet’s layers respectively. For detailed terminology please refer to the BCLA CLEAR report.1
Cornea and limbus
The cornea is a thin, smooth, transparent tissue covering part of the outer surface of the eye. Given the important protective and refractive functions, this structure has been of considerable interest to researchers and clinicians. The cornea has five layers (figure 1), with a sixth layer being postulated more recently, the Dua layer between the stroma and posterior limiting lamina.11
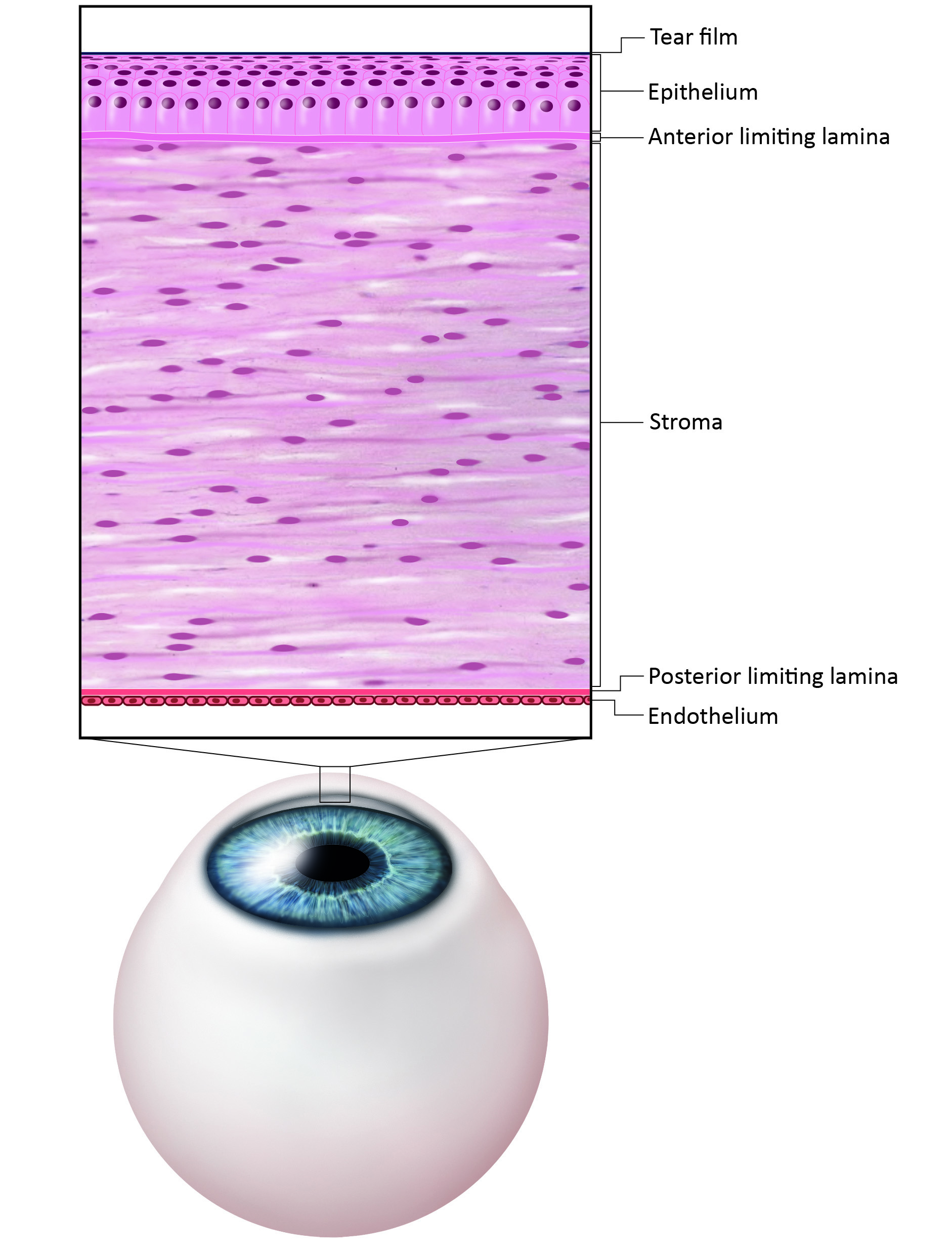 Figure 1: Traditional structure of the cornea (credit: BCLA)
Figure 1: Traditional structure of the cornea (credit: BCLA)
The negative meniscus shape of the cornea, viewed as slightly oval with a slit lamp, is carefully considered in contact lens design, to ensure the best match possible. Table 1 shows the typical characteristics and dimensions of the cornea.
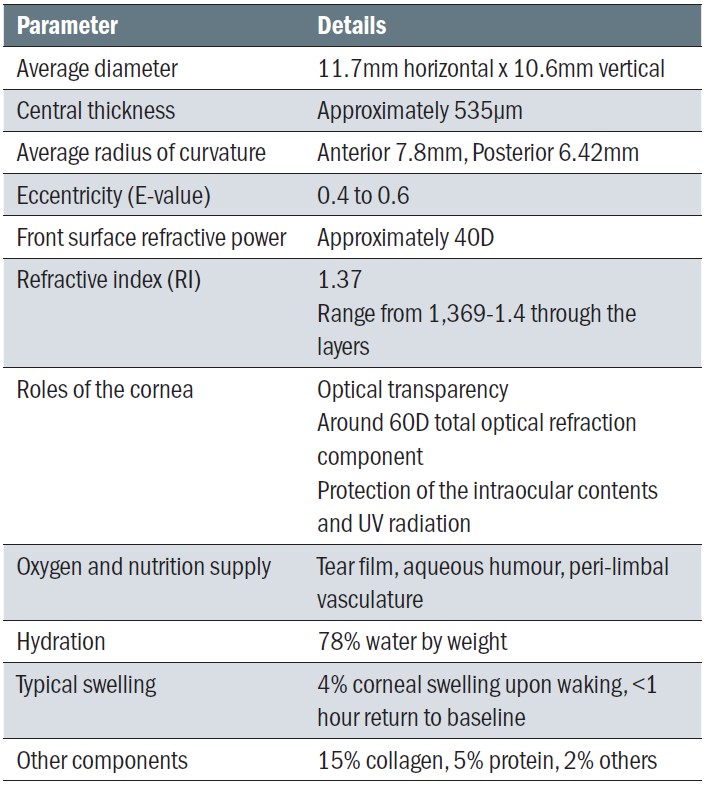 Table 1: Features of the cornea
Table 1: Features of the cornea
The cornea is avascular with no lymphatic drainage, although it can boast about being one of the most innervated tissues in the body, with an estimated 700 nociceptors per square mile, derived primarily from the trigeminal nerve. There may also be a small level of sympathetic corneal innervation. This high sensitivity explains why even slight compromise can result in symptoms. When corneal nerves are stimulated so is tear production by the trigeminal parasympathetic reflex and a foreign body can lead to an impressive 100-fold increase in tear flow.
The epithelial goes through constant renewal by a process known as mitosis and the stroma can heal too, primarily by scar tissue, which could negatively impact vision. Both the anterior and posterior limiting lamina (Bowman’s membrane and Descemet’s membrane) do not show regenerative responses if damaged. The single cell layer comprising the corneal endothelium, which is important for maintaining hydration and transparency, also does not regenerate, declining at an average of 0.6% per year.
The limbus, also known as the corneoscleral junction marks the border where the optically clear cornea meets the opaque sclera and is around 1.5-2mm in width. Anteriorly, this transitions the corneal epithelium and the bulbar conjunctiva and contains stem cells defined by radially orientated fibrovascular ridges known as the ‘Palisade of Vogt’. The limbus is important for wound healing and corneal epithelial cell replacement.
Conjunctiva and sclera
The conjunctiva (table 2) is considered to have three main regions: bulbar, forniceal and palpebral. It is a loose vascular connective tissue that has an overlying epithelium layer. The main functions of the conjunctiva are: forming a protective barrier and secreting mucin.
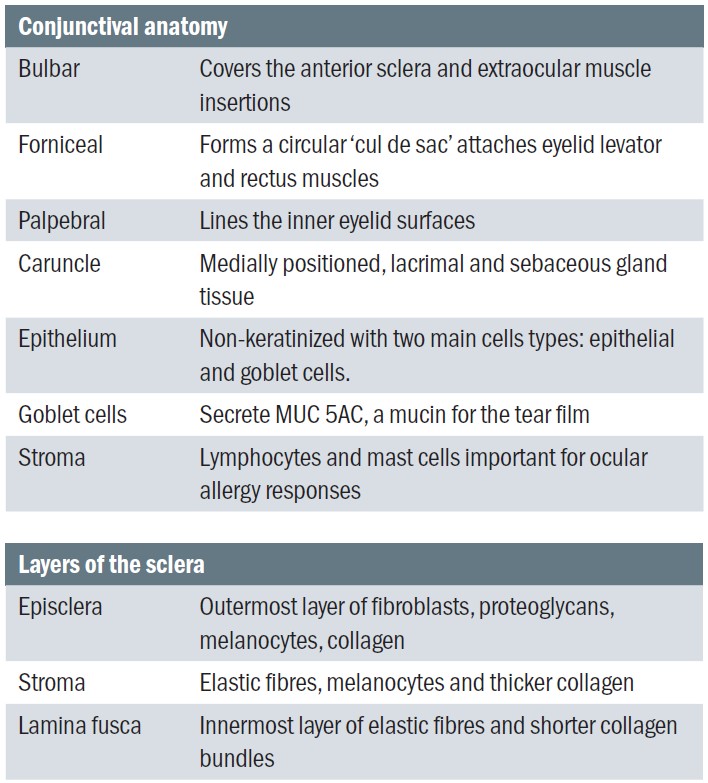 Table 2: The regions of the conjunctiva and sclera
Table 2: The regions of the conjunctiva and sclera
The sclera is opaque and impressively covers most of the globe’s surface, connecting to Tenon’s capsule. The main function is protection over the intraocular contents and provides a firm insertion point for the extraocular muscles. There are two openings, anteriorly surrounding the cornea and posteriorly the optic nerve.
Eyelids and eyelashes
The eyelids protect the anterior ocular surface, distribute the tear film helping to prevent it from spilling out of the eye; and assist in the control of light entering the eye. Anatomically, the eyelids can be divided into two lamellae sections separated by the grey line (figure 3).
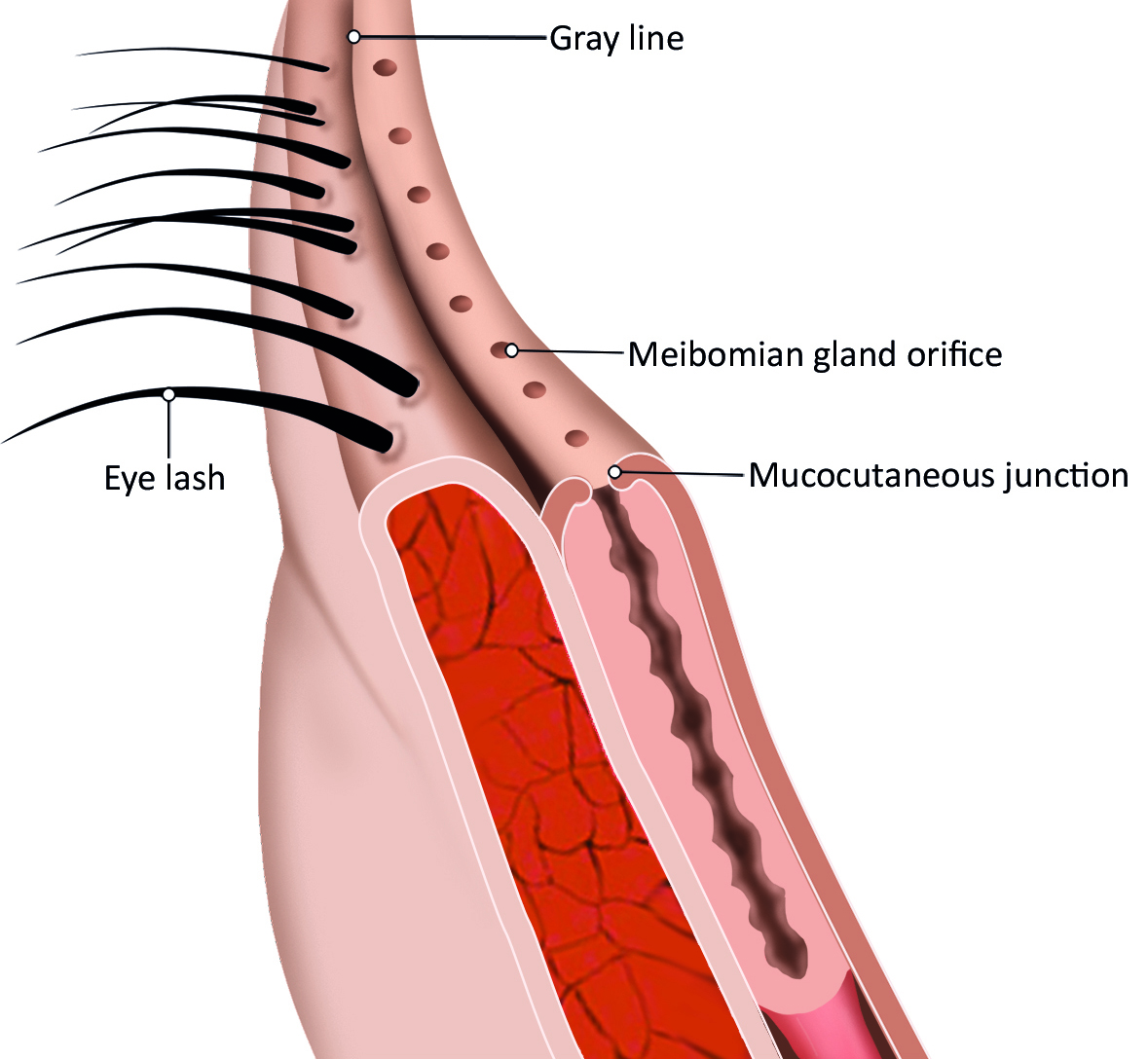 Figure 3: Anatomy of eyelid (credit: BCLA)
Figure 3: Anatomy of eyelid (credit: BCLA)
The orbicularis oculi muscle and the levator palpebrae superioris mediate eyelid movements. The eyelid margins are important for maintaining lubrication of the anterior structures by continuous re-formation and distribution of the tear film with blinking. The entire mucocutaneous junction is kept moist by the presence of the tear film meniscus.
The lid wiper is the portion of the marginal conjunctiva of the upper eyelid that wipes the ocular surface during blinking. The blood vessels underneath the superficial layers of the lid wiper region are more superficial than those at the mucocutaneous junction and immune cells have also been observed in this area. The eyelids are innervated by both sensory nerves from the trigeminal nerve, and have motor innervation from the facial nerve.
There are various glands in the eyelids, which include:
- Meibomian glands are responsible for producing meibum (lipid-based secretions) that enters the tear film and contributes to creating an optically smooth surface over the cornea and to helps retard tear evaporation. There are approximately 25-40 glands in the superior eyelid, and 20-30 in the inferior.
- Ciliary glands include the sebaceous gland (of Zeis) and ciliary gland (of Moll). These glands produce oily sebum whose exact function is not well defined. The sebum may condition the eyelashes or maintain the hair follicles; it is unlikely to contribute significantly to tear composition. Its secretions contain antimicrobial peptides that are important for defence against microorganisms at the eyelid margin.
- Lacrimal accessory glands are divided into two groups: fornix (Krause) and palpebral (Wolfring). These are ‘mini’ lacrimal glands that secrete a similar water component containing electrolytes, immunoglobulins, and antimicrobial proteins.
Three different types of blink are recognised during eye movements: spontaneous, reflex and voluntary. During blinking, the globe is displaced posteriorly by approximately 1.0mm and rotates upwards (Bell’s phenomenon). A spontaneous blink is an unconscious blink in the absence of an evident stimulus, while a reflex blink is an involuntary protective mechanism that occurs in response to a stimulus. The 200-300ms rhythmic opening and closing of the eyelids during spontaneous blinking distributes the tear film over the ocular surface and provides a pumping mechanism to facilitate aqueous tear flow through the lacrimal drainage apparatus. While movement of the superior eyelid is predominantly vertical, the inferior eyelid is smaller and has a horizontal and inward movement. The major forces involved in tear drainage are the blink and gravity.
Lacrimal system
The lacrimal apparatus is the physiological system containing the orbital structures for tear production and drainage. The lacrimal system has three major divisions (figure 4). The first is the secretory component, consisting of the exocrine tubuloalveolar serous lacrimal gland and two types of accessory lacrimal glands (Krause and Wolfring), which contribute to the aqueous components of the tear film. Distribution is the second component, consisting of the tear meniscus and blinking. The third component is the system that mediates the drainage of these secretions, which includes the puncta, lacrimal canaliculi, lacrimal sac, and nasolacrimal duct. The almond-shaped lacrimal gland is approximately 20×12×5mm and is in the lateral portion of the roof of the orbit, at the lacrimal gland fossa of the frontal bone. Tear fluid secreted by the lacrimal and accessory glands drains via the lacrimal drainage apparatus, comprising the lacrimal canaliculi, lacrimal sac and nasolacrimal duct.
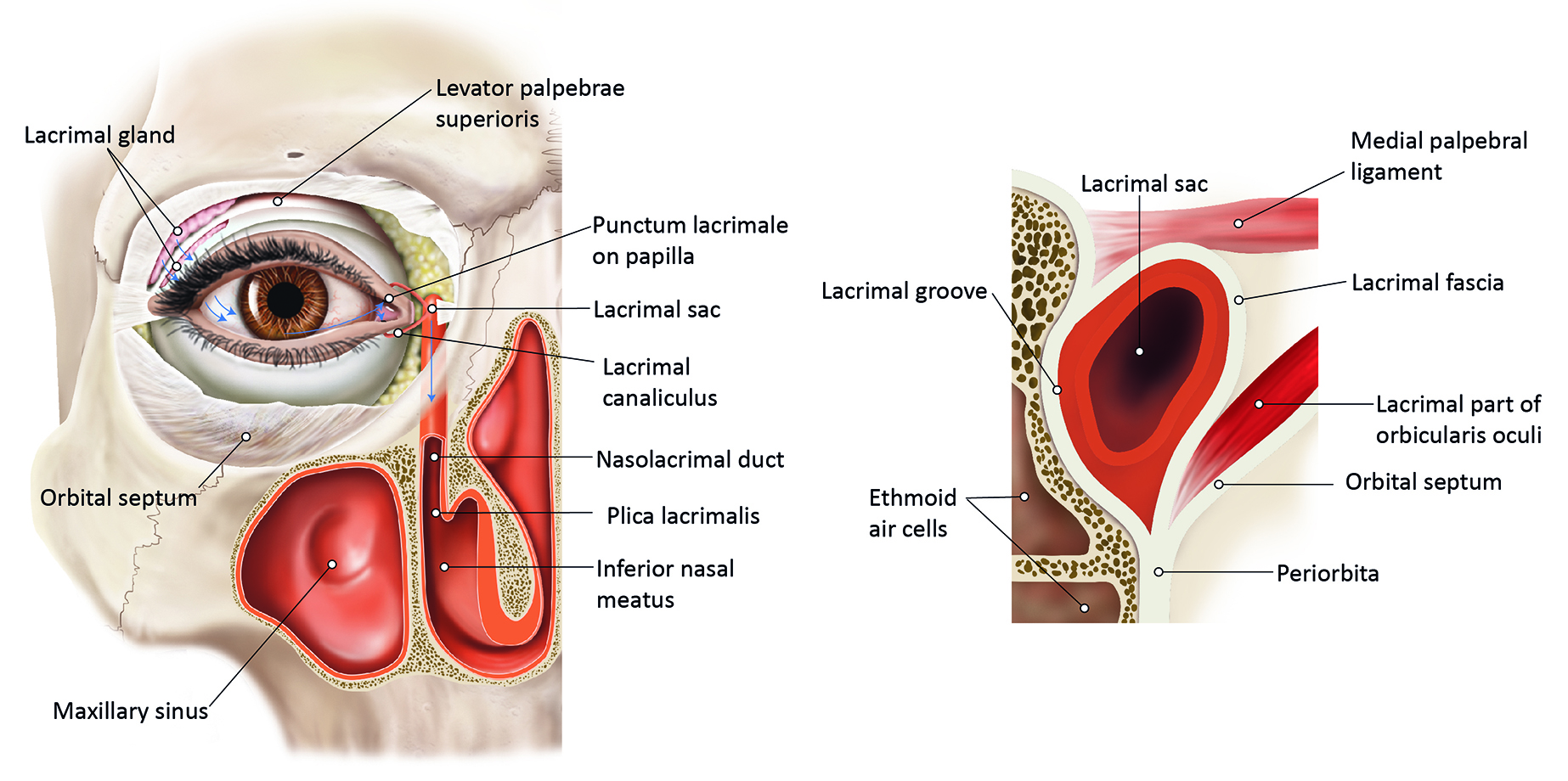 Figure 4: (a) Anatomy of lacrimal system.(b) Cross-section of the lacrimal sac and associated drainage structures (credit: BCLA)
Figure 4: (a) Anatomy of lacrimal system.(b) Cross-section of the lacrimal sac and associated drainage structures (credit: BCLA)
Tear Film
This protective fluid layer, which overlies the eye’s surface, has been a major focus of contact lens research.
The recently accepted tear film model comprises a superficial thin 40nm lipid layer overlying a thicker aqueous-mucin phase, and a glycocalyx structure (membrane-bound mucins: MUC 1, MUC 4, MUC 16) that binds the film to the ocular surface epithelium (figure 5). The outer lipid layer prevents evaporation and reduces surface tension, arising predominantly from the meibomian gland secretions. The aqueous-mucin phase makes up most of the tear volume with the lacrimal gland being the primary source of the aqueous fluid. The tear film impressively has over 2,000 proteins, electrolytes and has tightly regulated inflammatory mediators, which contribute to the ocular surface defence.
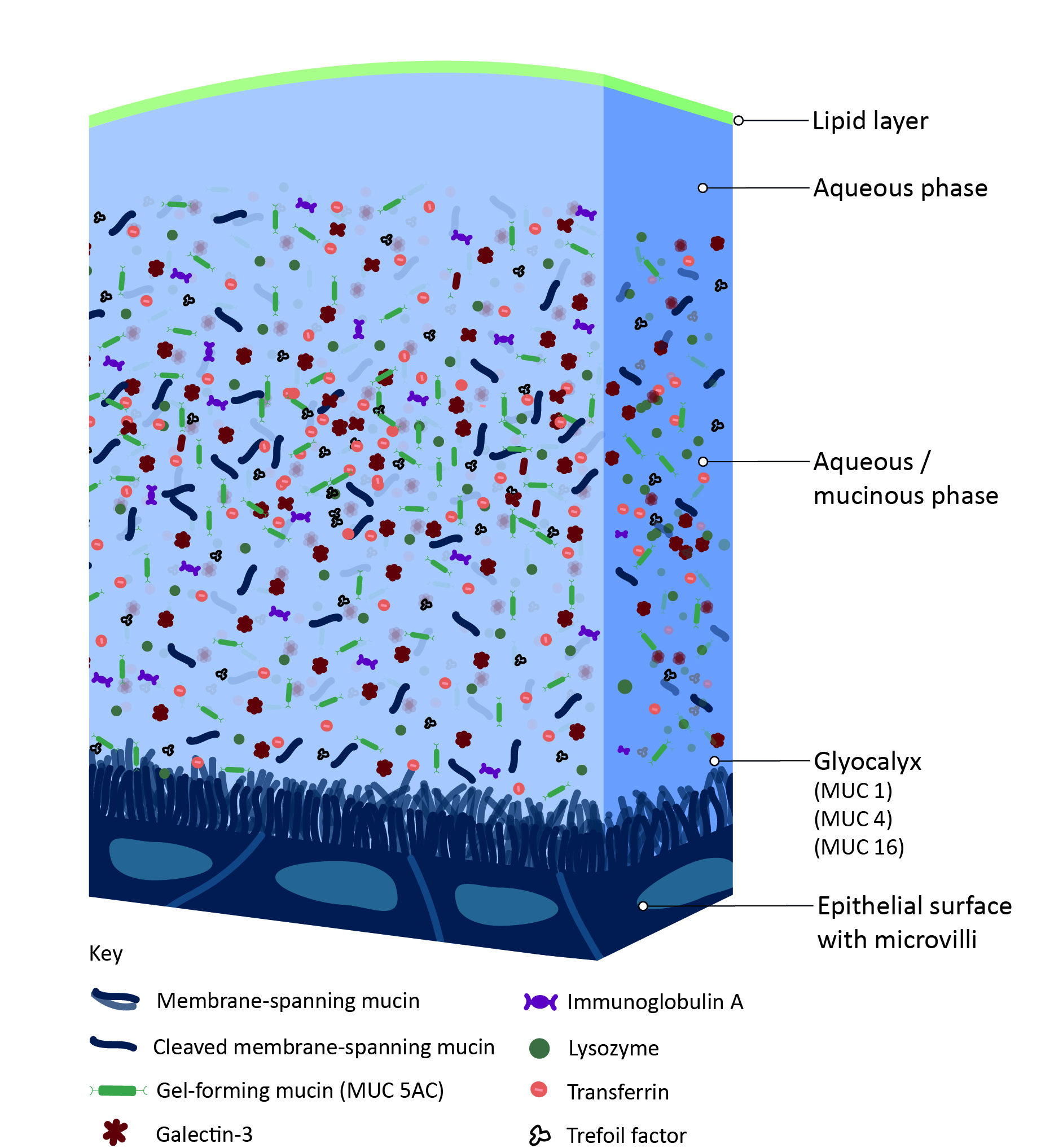 Figure 5: The structure of the tear film (credit: BCLA)
Figure 5: The structure of the tear film (credit: BCLA)
Tear osmolarity is an important parameter that can be used to quantify losses in tear homeostasis due to hyperosmolarity; it is an overall indicator of the balance between tear production,
tear electrolytes, evaporation, drainage, and absorption. Tear instability results in excessive evaporation of the aqueous tear component, resulting in tear hyperosmolarity. Interesting facts about the tear film can be found in table 3.
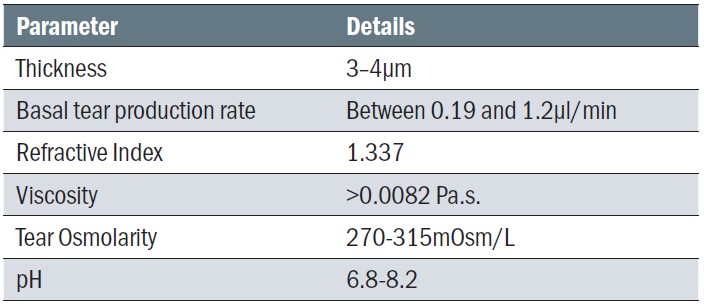 Table 3: Normal tear film characteristics
Table 3: Normal tear film characteristics
Advanced testing techniques
A range of additional clinical instruments are available to assess and measure the tear film and ocular surface, which will be covered in the review of the BCLA CLEAR-Evidence based practice report. Some specialist techniques beyond those typically conducted in practice include:
- Pachymetry, using optical or ultrasound methods, which can be used to measure corneal thickness.
- Corneal sensitivity, which can be quantified using a variety of approaches. Cochet-Bonnet aesthesiometry measures ocular surface sensitivity to a mechanical stimulus using nylon filaments. Other methods to measure ocular surface sensitivity include aesthesiometers with saline delivery, carbon dioxide laser and/or air pulses.
- Conjunctival impression cytology, which involves applying a specialised filter paper to the conjunctiva to collect goblet cell samples. It is generally performed under topical anaesthesia. The samples are subsequently used to evaluate the goblet cells and their capacity to secrete mucin.
Conclusion
This report has reviewed the anatomy and physiology of the anterior eye relevant to contact lens practice. Biological changes to structures naturally occur as we age and in response to the environment, which means clinical decision making should be linked to the individual’s needs. Peer-reviewed scientific understanding of these complex structure-function relationships provides a basis for ongoing advancements in contact lenses and provides opportunities to further optimise on-eye contact lens comfort, biocompatibility and safety.
Neil Retallic, above left, is the president of the BCLA (Credit: BCLA) and Dr Debarun Dutta, above right, is a lecturer in optometry, Optometry & Vision Science Research Group, Aston University, and BCLA Council member.
- The full report and supplementary information can be accessed at doi:https://doi.org/10.1016/j.clae.2021.02.009
- The BCLA CLEAR Summary report is a short bite-size evidenced based practical guide for clinicians, bringing together the key findings from the report. Accessed via CLEAR (bcla.org.uk).
Acknowledgements
Acknowledgement and recognition to Laura Downie, Stefan Bandlitz, Jan Bergmanson, Jennifer Craig, Debarun Dutta, Carole Maldonado-Codina, William Ngo, Jaya Sowjanya Siddireddy, James Wolffsohn who were the paper’s authors, Joanna Culley for the illustrations and the educational grants from Alcon and CooperVision.
The authors would like to thank Associate Professor Laura Downie for her review of this article.
Original article: Downie, LE; Bandlitz, S; Bergmanson, JPG; Craig, JP; Dutta, D; Maldonado-Codina, C; Ngo, W; Siddireddy, JS; Wolffsohn, JS. CLEAR – Anatomy and physiology of the anterior eye. Contact Lens & Anterior Eye; 2021, 44, 132-156.
References
- Downie, LE; Bandlitz, S; Bergmanson, JPG; Craig, JP; Dutta, D; Maldonado-Codina, C; Ngo, W; Siddireddy, JS; Wolffsohn, JS. CLEAR - Anatomy and physiology of the anterior eye. Contact lens & anterior eye: 2021, 44, 132-156, doi:10.1016/j.clae.2021.02.009.
- Willcox, M; Keir, N; Maseedupally, V; Masoudi, S; McDermott, A; Mobeen, R; Purslow, C; Santodomingo-Rubido, J; Tavazzi, S; Zeri, F; et al. CLEAR – Contact lens wettability, cleaning, disinfection and interactions with tears. Contact lens & anterior eye: 2021, 44, 157-191, doi:10.1016/j.clae.2021.02.004.
- Morgan, PB; Murphy, PJ; Gifford, KL; Gifford, P; Golebiowski, B; Johnson, L; Makrynioti, D; Moezzi, AM; Moody, K; Navascues-Cornago, M; et al. CLEAR - Effect of contact lens materials and designs on the anatomy and physiology of the eye. Contact lens & anterior eye : 2021, 44, 192-219, doi:10.1016/j.clae.2021.02.006.
- Richdale, K; Cox, I; Kollbaum, P; Bullimore, MA; Bakaraju, RC; Gifford, P; Plainis, S; McKenney, C; Newman, S; Tomiyama, ES; et al. CLEAR – Contact lens optics. Contact lens & anterior eye : 2021, 44, 220-239, doi:10.1016/j.clae.2021.02.005.
- Vincent, SJ; Cho, P; Chan, KY; Fadel, D; Ghorbani-Mojarrad, N; González-Méijome, JM; Johnson, L; Kang, P; Michaud, L; Simard, P; et al. CLEAR - Orthokeratology. Contact lens & anterior eye : 2021, 44, 240-269, doi:10.1016/j.clae.2021.02.003.
- Barnett, M; Courey, C; Fadel, D; Lee, K; Michaud, L; Montani, G; van der Worp, E; Vincent, SJ; Walker, M; Bilkhu, P; et al. CLEAR - Scleral lenses. Contact lens & anterior eye: 2021, 44, 270-288, doi:10.1016/j.clae.2021.02.001.
- Jacobs, DS; Carrasquillo, KG; Cottrell, PD; Fernández-Velázquez, FJ; Gil-Cazorla, R; Jalbert, I; Pucker, AD; Riccobono, K; Robertson, DM; Szczotka-Flynn, L.; et al. CLEAR - Medical use of contact lenses. Contact lens & anterior eye : 2021, 44, 289-329, doi:10.1016/j.clae.2021.02.002.
- Stapleton, F; Bakkar, M; Carnt, N; Chalmers, R; Vijay, AK; Marasini, S; Ng, A; Tan, J; Wagner, H; Woods, C; et al. CLEAR - Contact lens complications. Contact lens & anterior eye : 2021, 44, 330-367, doi:10.1016/j.clae.2021.02.010.
- Wolffsohn, JS; Dumbleton, K; Huntjens, B; Kandel, H; Koh, S; Kunnen, CME; Nagra, M.; Pult, H; Sulley, AL; Vianya-Estopa, M; et al. CLEAR - Evidence-based contact lens practice. Contact lens & anterior eye : 2021, 44, 368-397, doi:10.1016/j.clae.2021.02.008.
- Jones, L; Hui, A; Phan, CM; Read, ML; Azar, D; Buch, J; Ciolino, JB; Naroo, SA; Pall, B; Romond, K; et al. CLEAR - Contact lens technologies of the future. Contact lens & anterior eye : 2021, 44, 398-430, doi:10.1016/j.clae.2021.02.007.
- Dua, HS; Faraj, LA; Said, DG; Gray, T; Lowe, J Human corneal anatomy redefined: a novel pre-Descemet’s layer (Dua’s layer). Ophthalmology 2013, 120, 1778-1785, doi:10.1016/j.ophtha.2013.01.018.
- Bandamwar, KL; Papas, EB; Garrett, Q. Fluorescein staining and physiological state of corneal epithelial cells. Contact lens & anterior eye : 2014, 37, 213-223, doi:10.1016/j.clae.2013.11.003.
- Morgan, PB; Maldonado-Codina, C. Corneal staining: do we really understand what we are seeing? Contact lens & anterior eye : 2009, 32, 48-54, doi:10.1016/j.clae.2008.09.004.
- Zola, E; van der Meulen, IJ; Lapid-Gortzak, R; van Vliet, JM; Nieuwendaal, CP. A conjunctival mass in the deep superior fornix after a long retained hard contact lens in a patient with keloids.Cornea: 2008, 27, 1204-1206, doi:10.1097/ICO.0b013e318180e5b9.
- Holicki, C; Sims, J; Holicki, J. Two Soft Contact Lenses Retained in the Superior Fornix for 15 Years in a Patient With Unique Orbital Anatomy. Eye Contact Lens : 2020, 46, e13-e16, doi:10.1097/icl.0000000000000609.
