This BCLA CLEAR report1 examined published findings covering changes to the ocular anatomy and physiology with contact lens wear. While there was some small overlap with the BCLA CLEAR complications report, this report mainly considered changes to the eye that may warrant clinical intervention. The report systematically goes through the various structures of the eye in an order that is similar to the order in which an eye care practitioner (ECP) might examine the eye. This article summarises key findings from the report and identifies clinical pearls that are of use to the ECP.
The eyelids and adnexa
The report considers what evidence there is to support the idea that contact lens wear has an effect on blinking; either in terms of spontaneous blink rate or in terms of blink completeness. Spontaneous blinking is an unconscious blink that occurs in the absence of any particular stimulus. The spontaneous blink rate for eyes in the primary position has been found to be between eight and 21 blinks per minute.2 The wide range found in blink rate occurs due to difficulties in accurately measuring the rate, differences in the rate depending on the task being carried out and cognitive and emotional factors.3
Several studies have supported that rigid corneal lens and soft lens wearers are susceptible to increased blink rate. Incomplete blinking is thought to be an unconscious device used to prevent the breaking of an unstable tear film, which might otherwise occur with a complete blink. Incomplete blinking doubles the risk of dry eye disease, meibomian gland atrophy and poor tear film stability. Patients using digital devices and patients of Asian ethnicity have been found to be more likely to be incomplete blinkers.
Figure 1: Example of software capable of blink analysis
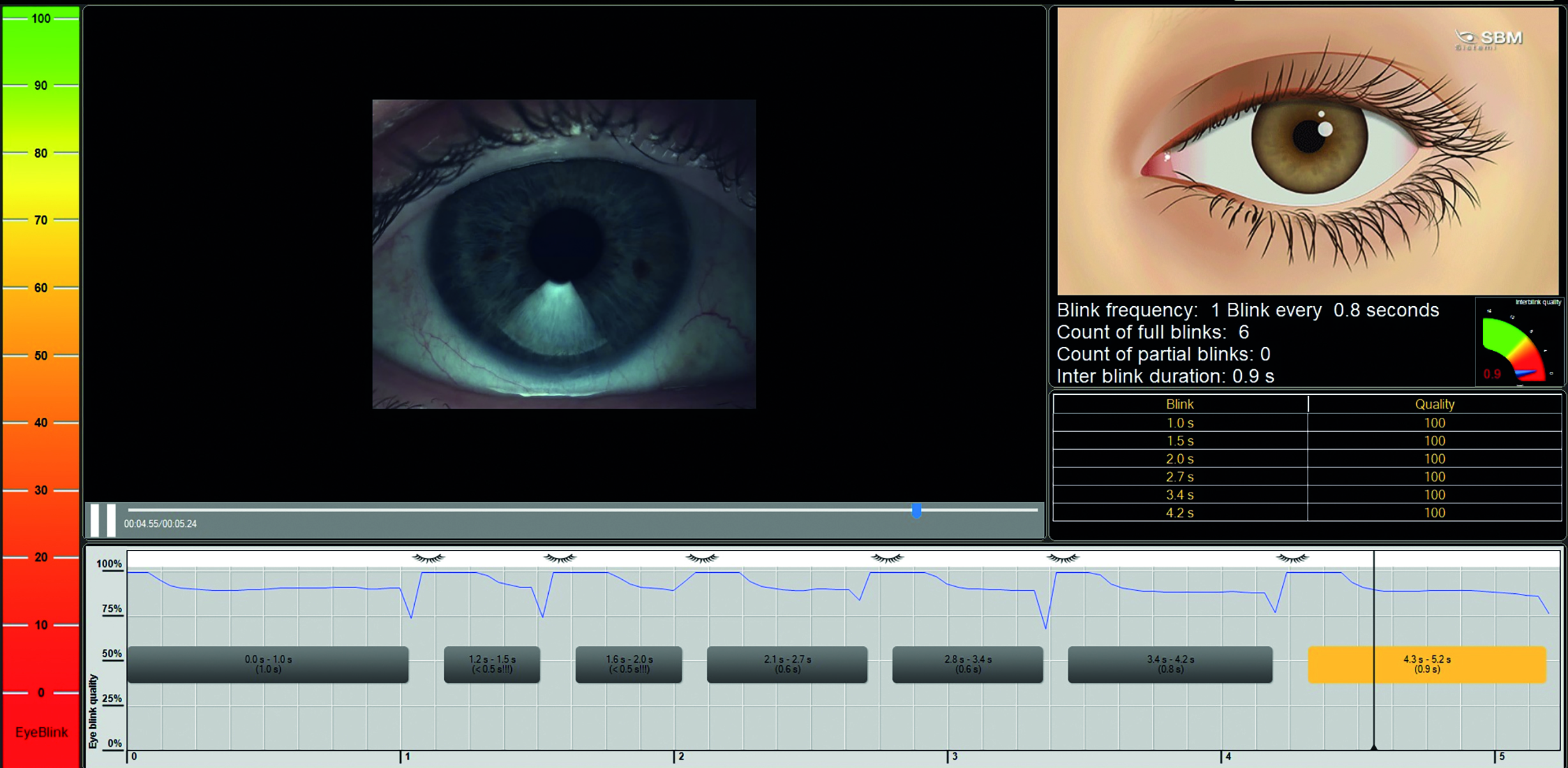
The clinical relevance of increased blink rate is that it could be a sign of dry eye. A baseline measurement of blink rate should be taken on neophytes prior to commencing contact lens wear and this should be re-assessed periodically. The same method of measuring blink rate must be used each time and ideally, the methodology should be noted. It is important to note that blink rate also increases with age, possibly due to age-related dry eye disease.
The Tear Film and Ocular Surface Society (TFOS) report on contact lens discomfort found that using contact lens care solutions containing wetting agents could restore normal blinking frequency for longer periods of time than the use of wetting drops could. There are now instruments that can measure blink rate automatically (figure 1). Studies have shown how smartphone applications can be used to characterise patients’ blinks and these applications will soon allow patients to track changes in their own blink rates as well as providing them with exercises to improve their blinking. The simplest way to check for incomplete blinking is to look at the blink during a routine slit lamp exam. If the patient’s blinks are incomplete the ECP should pay close attention when looking for other signs and symptoms of dry eye disease. In particular, a thorough examination of the meibomian glands is warranted.

Ptosis
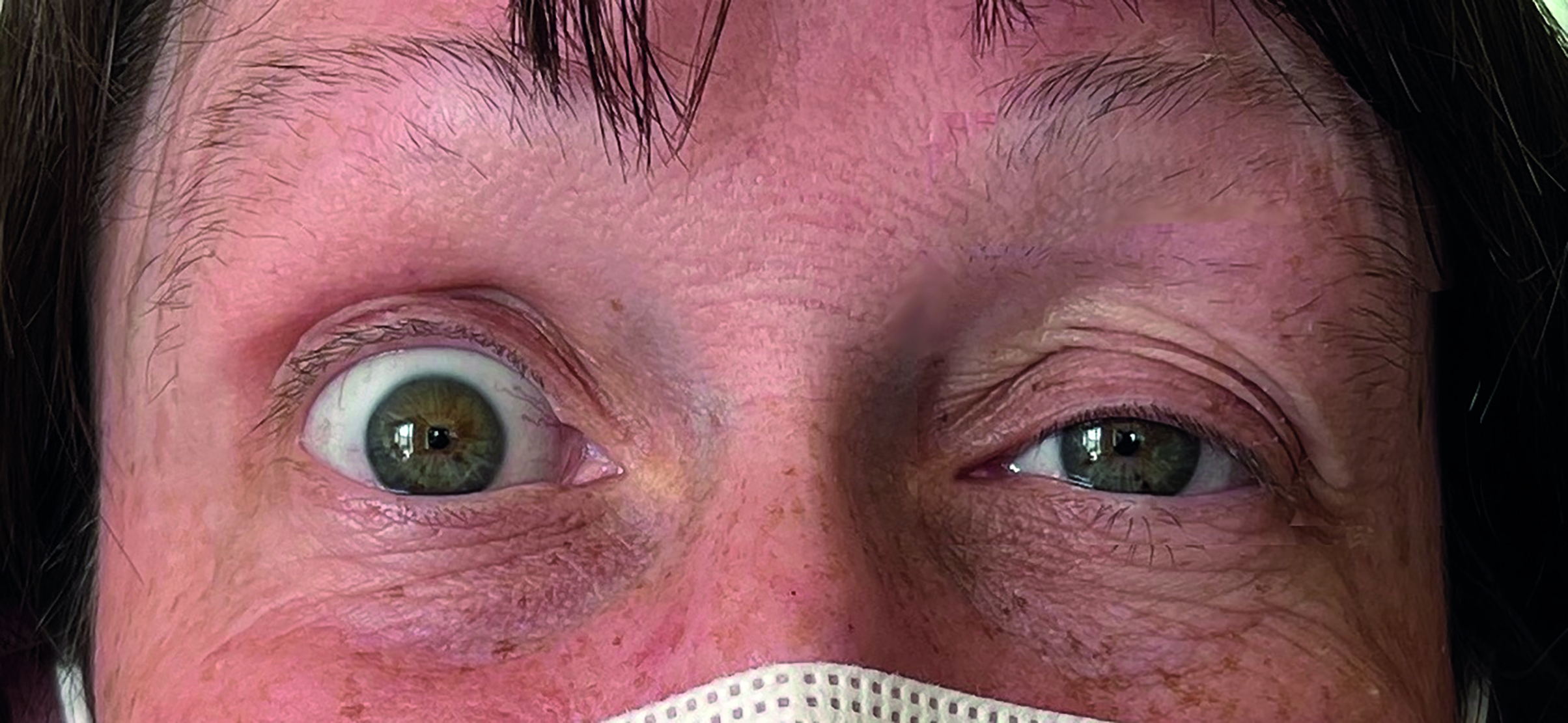
Rigid corneal lens wear is associated with a higher risk of development of ptosis compared to those wearing soft contact lenses (figure 2). In a rare scenario, the ptosis can resolve with discontinuation of wear, but some cases will not resolve spontaneously and may require surgery.
Figure 2: Unilateral ptosis related to rigid corneal lens wear
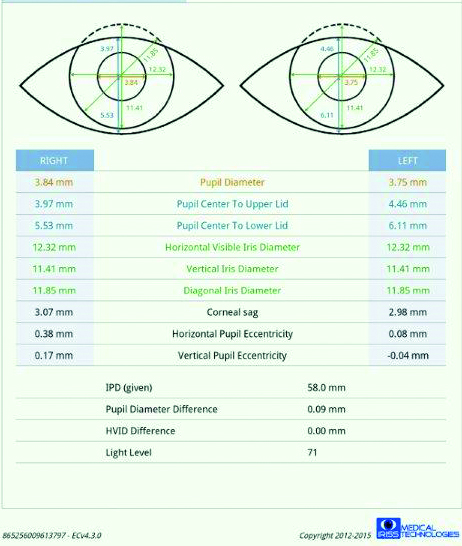
The progression of ptosis can be charted by using vertical palpebral aperture (VPA) size over time. However, it should be noted that the changes in VPA may sometimes be less than 1mm and therefore measurement with a ruler may not be accurate enough to detect subtle, early changes. To identify these small changes, it is necessary to measure VPA using instrumentation that has image acquisition with an in-built measurement system such as a topographer, anterior segment OCT or an automated photo-ophthalmic device (figure 3).
Figure 3: Data sheet from an automated photo-ophthalmic device
Meibomian Glands
Meibomian glands have attracted significant attention recently due to their relevance to lipid production and ocular surface disease. The research into possible negative effects of contact lens wear on the meibomian glands is very much equivocal. Some studies have shown that contact lens wear can affect meibomian gland dropout and function in the early stages of contact lens wear but not thereafter. Other studies show problems worsening over time and sometimes no association has been reported.
Clinically these glands operate maximally with good blinking. If contact lens wear changes the way a patient blinks, then this is likely to affect the secretion of meibum. Addressing poor blinking may go some way to ameliorating problems associated with the meibomian glands. The simplest way to assess the meibomian glands (in the absence of specialist equipment) is to press on the glands to determine if the glands can be expressed and to examine the nature of the glands’ secretions. This procedure should be carried out after all other tear quality and quantity tests have been completed, as the secreted meibum will affect the results of other tests.4 In-office treatment of meibomian gland dysfunction with a hot compress and lid massage can be useful both to kick-start the treatment of meibomian gland dysfunction and to demonstrate the technique to patients in order that they may carry out the treatment correctly themselves.
Figure 4: Meibomian gland dysfunction with minimal expression (left) and grade 1 expressability (right)

Conjunctiva
Bulbar and limbal hyperaemia
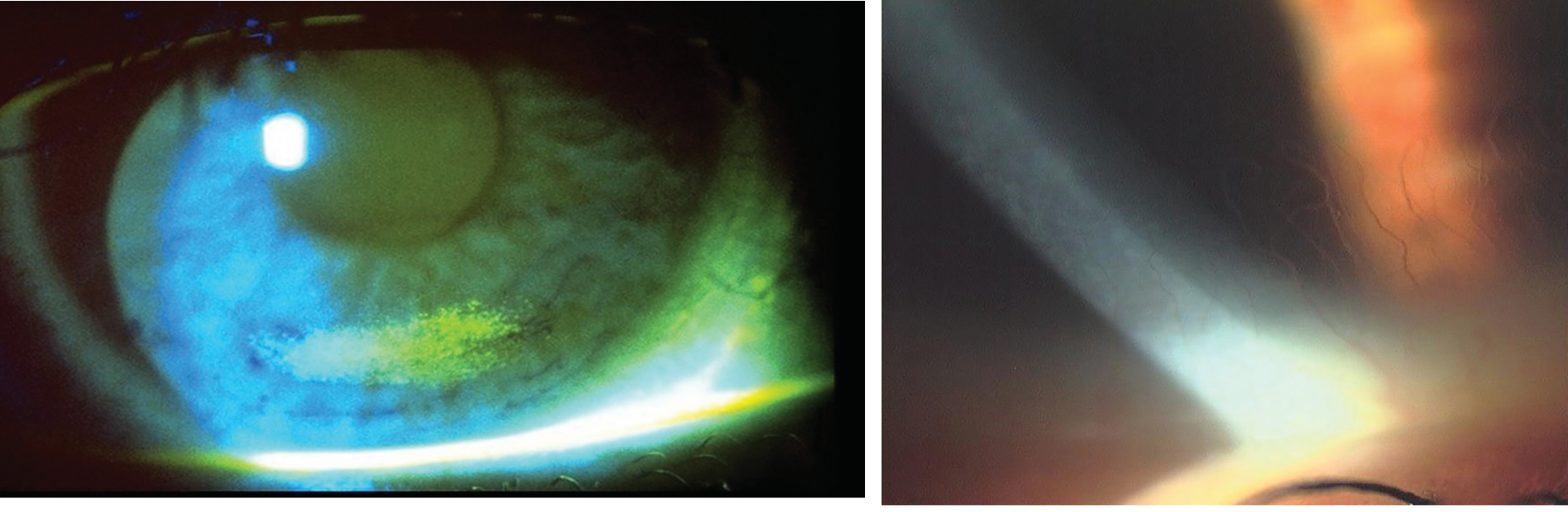
Hyperaemia can be so commonplace in contact lens wearers that it can be forgotten that hyperaemia is actually a sign that the ocular surface is under stress (figure 5). When recording observed hyperaemia, it is important to note the location, the extent and the grade of hyperaemia. Grading should be undertaken using a recognised scale and a note should be made of the scale used. When taking history and symptoms at a contact lens aftercare, it is important to ask patients about any redness observed since their previous check-up. The ECP should try to establish the cause of the hyperaemia, in order to take steps to prevent future hyperaemia.
Figure 5: Limbal hyperaemia, low grade (left) and high grade (b)
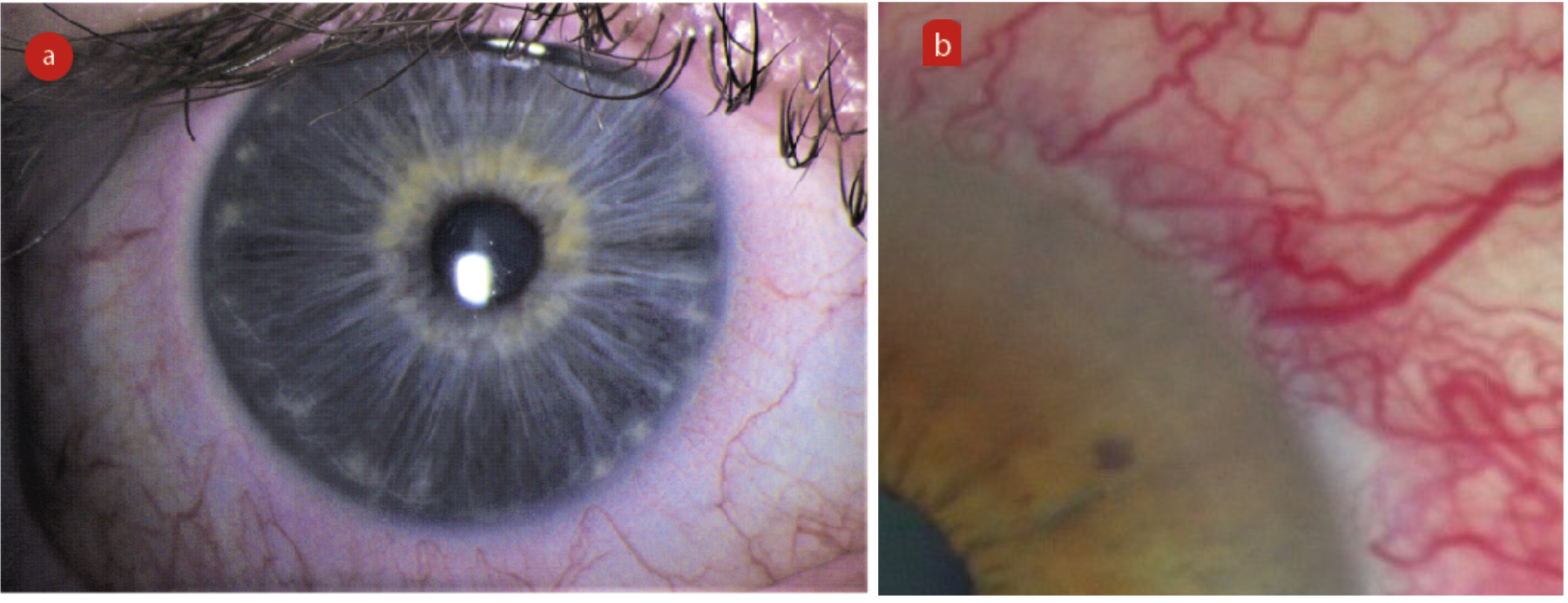
Limbal hyperaemia can be caused by both a tight-fitting and a loose-fitting lens. In cases where it is suspected that the lens fit is the cause of the hyperaemia, the fit should be modified for optimisation. In the case of soft lenses, this will often require a change of lens product.
If deposits underneath the lens are thought to be the cause of ocular surface irritation, and hence hyperaemia, then it may be appropriate to change the lens modality, for example from monthly disposable to daily disposable. Reinforcing the rub and rinse step, the importance of case hygiene and reminding patients to change their lenses at the correct time can also help here.
In cases where it is suspected that the hyperaemia is a result of hypoxia, then a change in lens material to increase oxygen transmission may be required. Papas estimated that a Dk/t of 125 was needed to avoid limbal hyperaemia in contact lens wearers but the 95% confidence limits for this estimate were 56 to 274.5 This means the critical oxygen requirement to maintain a healthy ocular surface varies considerably between patients. When manufacturers quote values for Dk/t, they do so for a -3.00DS lens. Clearly, lenses in higher prescriptions will be thicker and will therefore have lower Dk/t values. There are also variations in the methodology used across the literature.
Poor tears in contact lens wearers can cause some dehydration of the contact lens. This can in turn cause hyperaemia, either from a lens that has tightened or from friction between the ocular surface and the lens surface. Possible solutions would be to change the lens material or lens modality. Lower water content lenses will tighten less than higher water lenses as a result of dehydration. Lenses that become partially dehydrated lose their ability to fully rehydrate over time6 so a daily disposable lens may be a better option than a reusable lens for patients with poor tears.
Lens care systems can produce limbal and bulbar hyperaemia, but this is easily remedied by changing systems. Unpreserved solutions will invariably produce less ocular irritation than preserved solutions. Hyperaemia may be secondary to lid problems such as meibomian gland dysfunction or blepharitis.
Treatment or management of these conditions should alleviate hyperaemia.

Staining
Two main types of conjunctival staining have been noted in contact lens wearers:
- Interpalpebral bulbar staining related to dryness
- Circumlimbal staining from the edge of the contact lens
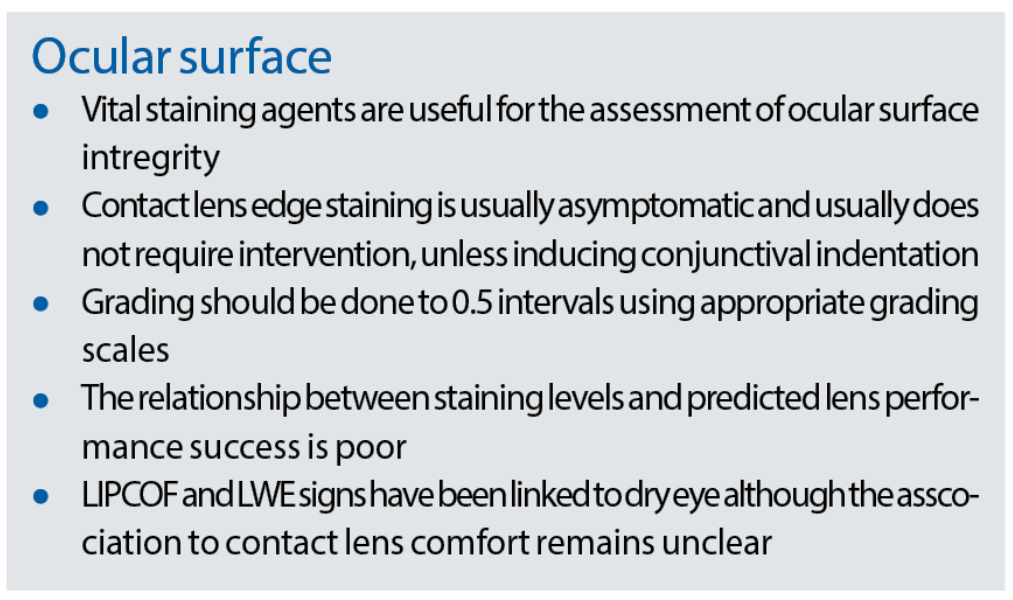
Non-contact lens wearers will also show bulbar staining but usually of lower grades than that of contact lens wearers. Lissamine green staining of the bulbar conjunctiva is less commonly seen than fluorescein staining but is also more likely to be associated with symptoms of dry eye (figure 6).
Contact lens edges fall broadly into three categories:
- Round edge
- Chisel edge
- Knife edge
Round edges are associated with the least comfort but also the least staining. Knife edges tend to be more comfortable but produce more staining. Silicone hydrogel lenses with their higher modulus, also tend to produce more staining than hydrogel lenses. Circumlimbal lens edge staining is usually asymptomatic and does not produce hyperaemia, so intervention is not always necessary unless there is evidence that it is producing conjunctival indentation. This can be seen as pooling of fluorescein in a circular pattern corresponding to the lens edge. In this case the patient should be changed to a lens with a rounder edge and/or lower modulus material.
Figure 6: Staining of the conjunctiva; (a) lissamine green, (b) fluorescein
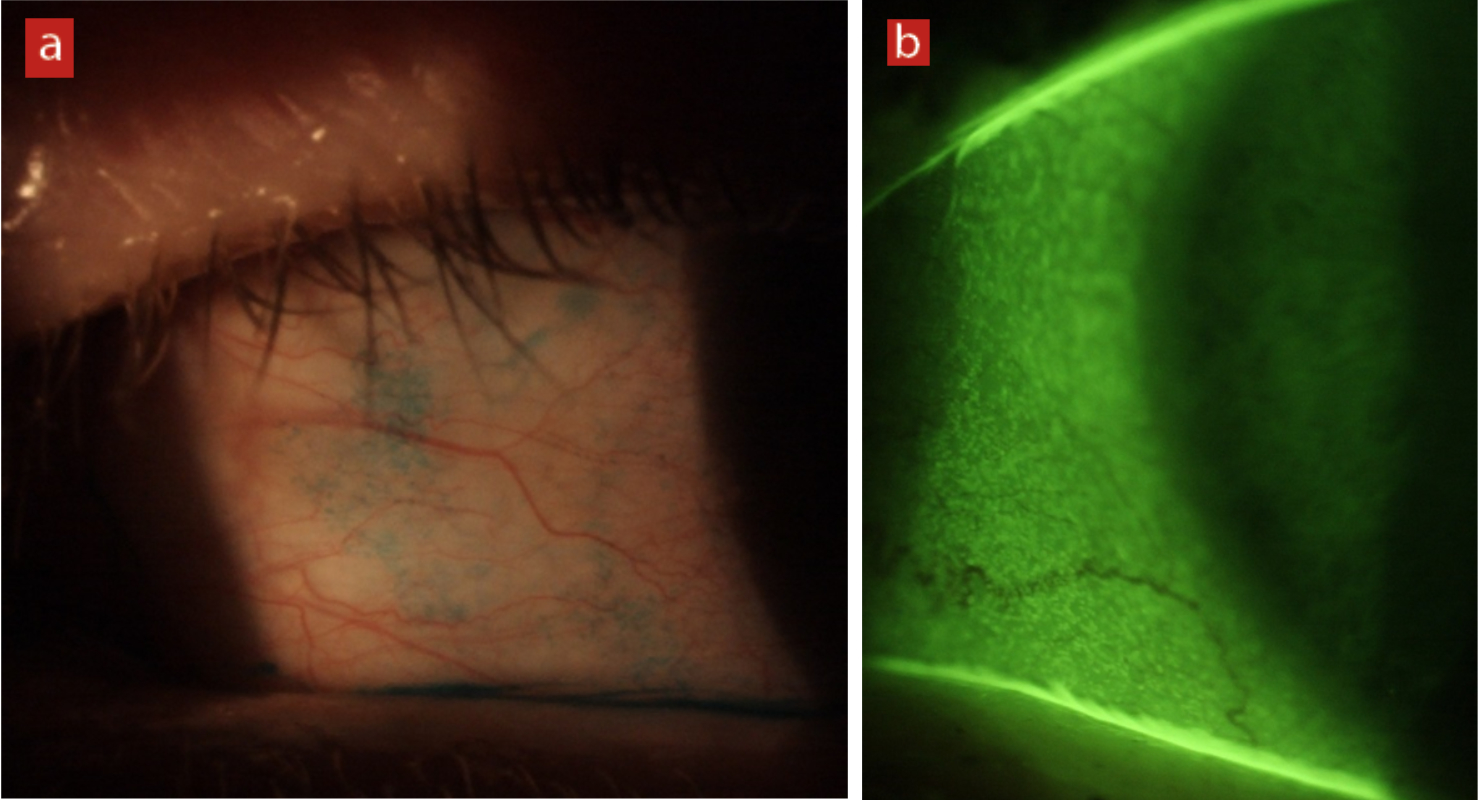
Lid parallel conjunctival folds (LIPCOF)
LIPCOF are very small folds of the bulbar conjunctiva, which run parallel to and close to the lower lid centrally. They are thought to be caused by friction between the eyelid and the bulbar conjunctiva in eyes with a poor tear film, but this has yet to be proven. LIPCOF has been shown to be associated with contact lens discomfort. The management of LIPCOF in contact lens wearers is to recommend the use of wetting drops or to change the wearer to a lens with better surface wettability.
The BCLA CLEAR biochemistry report7 has found that once a contact lens is removed from its blister pack and is then worn for a few days, its surface wetting decreases, therefore changing a patient from other modalities to daily disposable may help with surface wettability and thus prevent further increases in LIPCOF.
Figure 7: Lid parallel conjunctival folds (LIPCOF)
Palpebral Conjunctiva
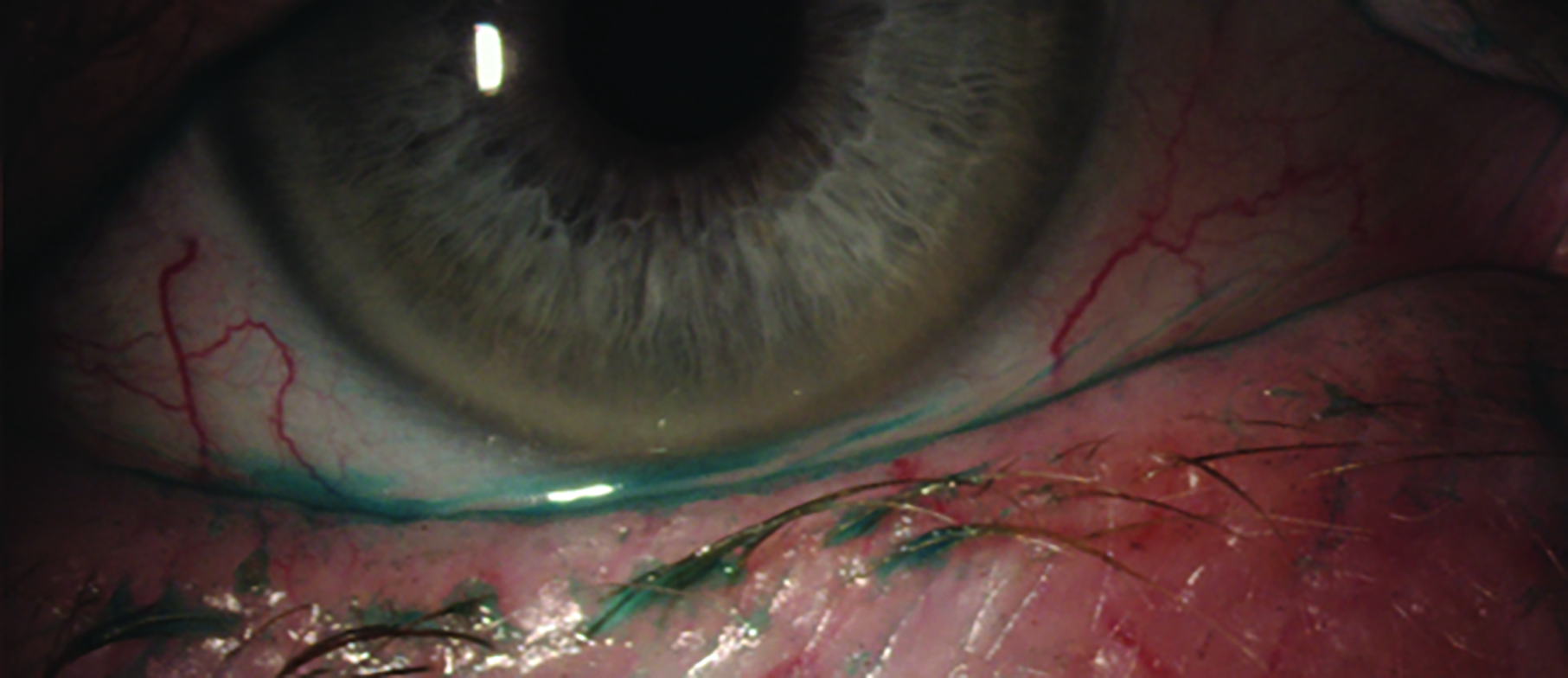
Only about 14% of the population have smooth palpebral conjunctiva. The majority of patients (85%) have small uniform micropapillae (figure 8). It is important therefore to establish the baseline appearance of a patient’s palpebral conjunctiva prior to commencing contact lens wear. In determining the baseline with lid eversion, only the central palpebral conjunctiva should be considered as other areas of the conjunctiva can be affected by the eversion process. Contact lens induced papillary conjunctivitis occurs in around 6% to 18% of soft contact lens wearers. Changing patients to daily disposable lenses can be beneficial in avoiding papillary conjunctivitis as only 2% of patients in this modality seem to develop these changes.
Figure 8 & 9 (left to right): Micropapillae of the upper eyelid palpebral conjunctiva; Lid wiper epitheliopathy (LWE),
graded 1
Lid wiper epitheliopathy (LWE)
The lid wiper is the portion of the eyelid marginal conjunctiva that wipes the ocular or contact lens surface during blinking. The epitheliopathy of the region is characterised by increased staining by dyes such as fluorescein, lissamine green or rose Bengal (figure 9). While LWE is an established diagnostic biomarker of dry eye disease, its relationship with contact lens wear is unclear. The results of the various reports are largely influenced by the varying lissamine green methodology used, staining protocol, grading technique and dye concentration. Nevertheless, patients showing severe LWE could benefit from increasing lubrication, improved blinking behaviour, and altering the type and modalities of lens wear.
Cornea
Staining
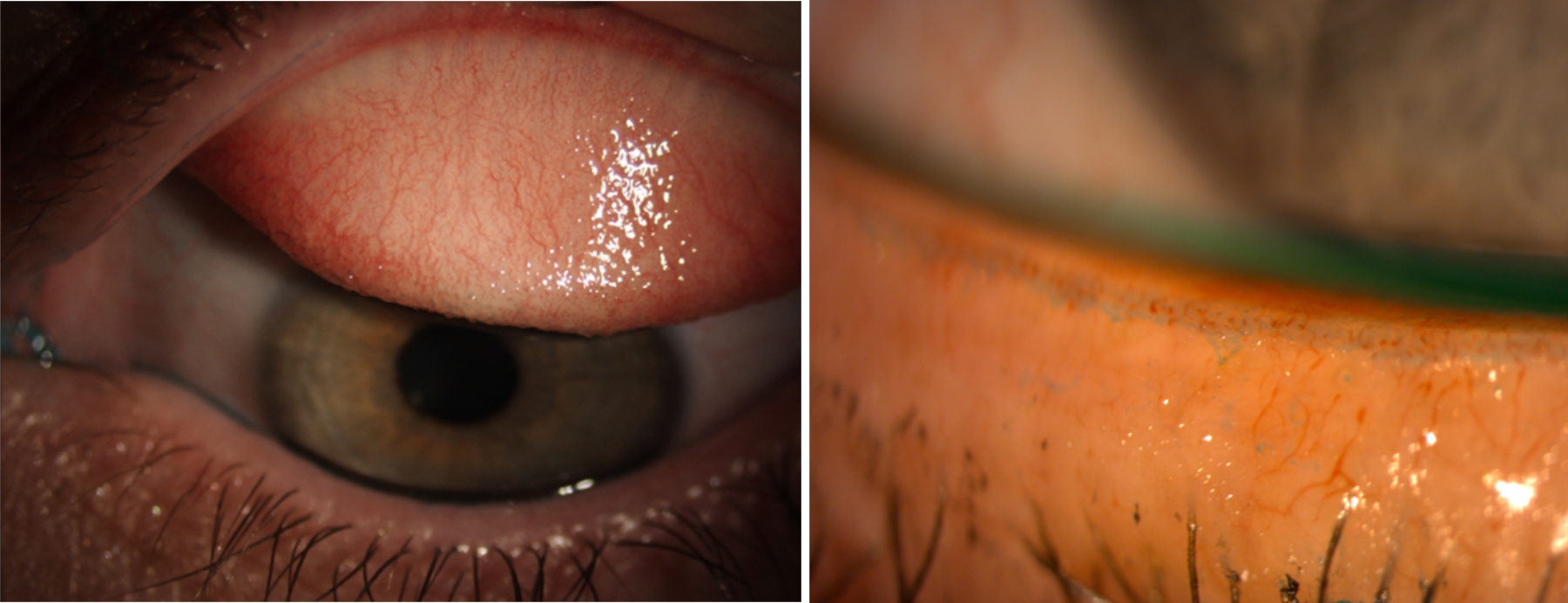
Low levels of corneal smile or desiccation staining can be seen even in non-contact lens wearers (figure 10). More moderate levels of staining can be improved by improved blinking, the use of artificial tears, reducing wearing time and using lenses that are less likely to dehydrate (such as low water content lenses). A study of 413 contact lens wearers showed that the wearing of silicone hydrogel lenses appeared to produce less corneal staining than the wearing of hydrogel lenses, so a change of lens material may be beneficial for some patients.

Sensitivity
The cornea is highly innervated and corneal sensitivity adaptation to soft lenses does not occur to the same level as seen with rigid lenses. Increased limbal sensitivity from mechanical interaction by any contact lens material can contribute to symptoms of lens discomfort, although more research is needed to explore this relationship further. Lid margin sensitivity is the highest of all conjunctival regions although is generally reduced in contact lens wearers.
Neovascularisation
Contact lens related neovascularisation (figure 11) historically resulting from chronic corneal hypoxia is now rare with modern lens materials. Risk factors are; compromised unhealthy eyes (for example in corneal pathology or post-operative patients), non-compliant patients or those classified as ‘high-swellers’ (individuals with a higher swelling response to hypoxic conditions). All of these patient types should be reviewed with caution.
Signs of hypoxia can be found in all layers of the cornea and include epithelial microcysts, stromal swelling, striae, folds, haze and endothelial blebs.
Sub-clinical inflammatory responses
Increases in dendritic cell levels suggests that sub-clinical inflammatory responses do occur with contact lens wear, with higher levels reported in reusable compared to daily disposable lenses wearers. Further research is needed to understand what impact this may have.
Myopic progression and ocular growth
The term ‘myopic creep’ had been coined to describe small myopic shifts that had previously been identified with daily wear and overnight wear of hydrogel contact lenses and which appeared to be greater than myopic shifts in spectacle wearers. However, a large study of 484 children divided into two groups of single vision spectacle wearers and single vision contact lens wearers showed no significant difference in myopia progression (axial or refractive) between the two groups. Thus ‘myopic creep’ is no longer considered a possible side effect of contact lens wear.
Figure 10 & 11 (left to right): Inferior desiccation staining; Corneal neovascularisation
Conclusion
This article focused on summarising the most clinically relevant areas of the BCLA CLEAR report. The full report covers far more side-effects of contact lens wear, but with modern lens designs and materials, many of these consequences of contact lens wear are no longer of concern to contemporary ECPs in most countries. Key findings from the report include the importance of blinking, which is discussed under several headings, and the use of daily disposable lenses as a solution to problems that may be more prevalent with other lens modalities.
Our ability to study sub-clinical responses to contact lens wear, through the use of instrumentation such as confocal microscopes, should continue to provide new insights into the mechanisms of the physiological side-effects of contact lenses. With these new insights, further improvements in designing truly biocompatible contact lenses will surely be forthcoming.
- The full report and supplementary information can be accessed at https://www.contactlensjournal.com/article/S1367-0484(21)00020-5/fulltext
- The BCLA CLEAR Summary report is a short bite-size evidenced-based practical guide for clinicians, bringing together the key findings from the report. Accessed via CLEAR (bcla.org.uk)
- The editors for this series are Neil Retallic and Dr Debarun Dutta
Acknowledgements
Acknowledgement and recognition to Philip Morgan, Paul Murphy, Kate Gifford, Paul Gifford, Blanka Golebiowski, Leah Johnson, Dimitra Makrynioti, Amir Moezzi, Kurt Moody, Maria Navascues-Cornago, Helmer Schweizer, Kasandra Swiderska, Graeme Young, Mark Willcox who were the paper’s authors and the educational grants from Alcon and CooperVision.
Original paper: Morgan, PB; Murphy, PJ; Gifford, KL; Gifford, P; Golebiowski, B; Johnson, L; Makrynioti, D; Moezzi, AM; Moody, K; Navascues-Cornago, M; Schweizer, H; Swiderska, K; Young, G; Willcox, M. BCLA CLEAR – Effect of contact lens materials and designs on the anatomy and physiology of the eye. Contact lens and anterior 2021, 44, 192–219.
References
- Morgan PB, Murphy PJ, Gifford KL, Gifford P, Goblebiowski B, Johnson L, Makrynioti D, Moezzi AM, Moody K, Navascues-Cornago M, Schweizer H, Swiderska K, Young G, Willcox M. BCLA CLEAR - Effect of contact lens materials and designs on the anatomy and physiology of the eye. Contact lens & Anterior Eye, 2021, 44, 192–219.
- Doughty MJ. Consideration of three types of spontaneous eyeblink activity in normal humans: during reading and video display terminal use, in primary gaze, and while in conversation. Optometry & Vision Science, 2001;78:712–25.
- McMonnies CW. The clinical and experimental significance of blinking behavior. Journal of Optometry, 2020;13:74–80.
- Wolffsohn JS, Arita R, Chalmers R, Djalilian A, Dogru M, Dumbleton K, Gupta PK, Karpecki P, Lazreg S, Pult H, Sullivan BD, Tomlinson A, Tong L, Villani E, Yoon KC, Jones L, Craig JP. TFOS DEWS II Diagnostic Methodology report. Ocular Surface, 2017 Jul;15(3):539-574.
- Papas E. On the relationship between soft contact lens oxygen transmissibility and induced limbal hyperaemia. Experimental Eye Research, 1998 Aug;67(2):125-31.
- Morgan PB, Efron N. Hydrogel lens ageing. Contact Lens Association of Ophthalmologists Journal (CLAO J), 2000; 26: 85-90
- Willcox M, Keir N, Maseedupally V, Masoudi S, Mc Dermott A, Mobeen R, Purslow C, Santodomingo-Rubido J, Tavazzi S, Zeri F, Jones L. BCLA CLEAR – Contact lens wettability, cleaning, disinfection and interactions with tears. Contact lens & Anterior Eye, 2021, 44, 157–191.
