The response of the iris to light levels and with accommodation is a result of a neural reflex pathway that involves the iris, retina, visual pathway, midbrain, and parasympathetic and sympathetic innervation of the eye. As such, clinical assessment of the pupil response to light elicits important information about the health of all these structures.
Key Point 1:
- Pupil assessment is an objective test of neurological function.
Until there is more widespread access to practice-friendly electrophysiological testing, assessing the way a pupil responds to light is the best way practitioners have of objectively assessing the functioning of the visual pathway. Visual fields is heavily dependent upon patient response, which, as we all know only too well, can be variable to say the least. Visual acuity is, likewise, subjective by its very nature. So, consider the example in the next column.
Eye care practice primers
To complement our regular CPD features focusing on the latest advanced techniques in optometry, Optician will be publishing a regular series of primers covering the key techniques which are integral to the eye examination.
- Pupil assessment
- Ocular muscle status assessment
- Visual fields assessment
- Tonometry and pachymetry
- Refraction
- Gonioscopy
- Slit-lamp assessment
- Direct and indirect ophthalmoscopy
- Optical coherence tomography
- Photography and imaging
- Paediatric assessment and management
- Low vision assessment and management
Case Example:
A 23-year-old previously healthy female first noticed something wrong with her vision in the left eye when having a hot bath before going to work. By lunchtime, the vision had become cloudy enough for her to be concerned and to seek the opinion of a local optometrist. The acuity in the affected eye had fallen to 6/36, and she reported the vision as ‘like looking through a fog.’ Colours seemed washed out, though colour vision testing and visual fields proved unreliable, if not impossible.
Slit-lamp and fundoscopy showed no anomalies, binocular assessment likewise, and there was no other symptoms reported, other than the drop in acuity. So, at the risk of flippancy, was this actually an eye problem?
The progressive onset and her young age suggests a vascular problem is unlikely. Likewise, unilteral vision loss would suggest a problem either in the left eye (ruled out by examination) or the left optic nerve. So how to find if the left optic nerve is not working as well as the right? Check pupils.
Confirmation of a left relative afferent pupil defect (see later) would confirm an issue with the optic nerve and the onset (age, hours onset, trigger by a hot bath – possible Uhthoff’s phenomenon) would lead to the reasonable conclusion of a retrobulbar neuritis. In this case the pupils are diagnostic, objective and confirm other findings that are reliant upon the patient’s subjective input.
This case illustrates why an assessment of the pupils is an essential part of every routine eye examination.
Anatomy and Physiology
The pupil describes the aperture defined by the iris, which regulates the amount of light entering the posterior chamber of the eye and therefore influences the image at the retina.
The word is from the Latin ‘pupillae’ meaning ‘little female doll’ and refers to the tiny image one sees of oneself reflected in the eye of another seen against the dark aperture background. The Greek word for similar was ‘kore,’ hence the suffix -coria in medical terms relating to the pupil.
In a healthy human, the pupil is circular and the diameter is controlled by the interaction of two iris muscles:
- Sphincter pupillae; a circular muscle, around 1mm in width and 6µm thick and comprised of smooth muscle fibres. Contraction narrows the pupil.
- Dilator pupillae; radial myoepithelial muscle tissue of 2µm thickness
The muscles act antagonistically to maintain pupil size. Importantly for clinicians, the two muscles have different innervation. The sphincter muscle is stimulated by parasympathetic fibres via the neurotransmitter acetylcholine. The dilator muscle has sympathetic innervation, mediated by noradrenaline. This explains the pupil dilation that occurs during moments of heightened emotional (sympathetic) response. It also allows for selective control of the pupil by topical agents that can interfere with normal neurotransmitter activity. There is also reciprocal innervation between the eyes so the activity of one will influence the other and, in theory, maintain constancy between the two eyes (a consensual response).
Key Point 2:
- Sympathetic innervation causes mydriasis, parasympathetic innervation.
Though there is a large variability both between the average pupil diameters of one individual’s eyes and also between the diameters of different individuals. A typical resting diameter of a pupil in a healthy young adult in daylight conditions is 4mm. Arterioscleriosis of the vessels within the iris results in an age-related miosis and pupils get smaller with age. Indeed, muscle control declines with iris atrophy and makes clinical dilation more difficult in elderly patients. Other influences upon averaged pupil size have been suggested, including eye colour (blue eyes have bigger pupils than brown) and ametropia (myopic pupils are bigger than emmetropic and then hyperopic).
Key Point 3:
- Pupil responses become sluggish and asymmetric with age ¬ beware the record of PERRLA in the 90-year-old.
Drugs for Dilation
A drug which prevents the action of acetylcholine (anti-cholinergic), by binding to its receptor sites and blocking it, will prevent sphincter activity and cause dilation (mydriasis). These are:
- Tropicamide
- Cyclopentolate
- Atropine
A drug which mimics acetylcholine (a parasympathomimetic) will enhance sphincter activity and cause constriction or miosis. Pilocarpine is the best known such drug, though its use has declined.
A drug that mimics noradrenaline (a sympathomimetic), such as phenylephrine, will enhance dilation, though not impact upon the sphincter so an antagonistic constriction may still occur in response to light stimulus. Phenylephrine is often used as a synergistic dilating drug, along with an anti-cholinergic, to enhance the dilation of a stubborn pupil, as with an elderly patient or where as large a dilation as possible is required for viewing peripheral retina.
Action of the Pupils
As would be expected from the autonomic nervous innervation, the pupils are not under voluntary control but, instead, react to external or pharmacological influences. There are a number of reflexes dictating pupil activity.
- Light reflex; the best known reflex is the contraction of the pupil in response to external light stimulus. This response is a combination of a immediate miotic response to a brief light stimulus (phasic reflex) and a maintained response to sustained light stimulus (tonic reflex). The two reflexes explain the sometimes staggered nature of response to incident light. Miosis may result from a direct stimulus (direct reflex) or from stimulation of the other eye (consensual reflex).
- Near reflex; this is the contraction of the pupil on viewing a near target and is associated with, but distinct from, the concurrent accommodation and convergence that also takes place.
- Other; there is an array of other pupil responses related to a wide range of systemic influences. These include;
- Sleep response; pupils are miosed during sleep, and are known to contract prior to rapid eye movement (REM) sleep phases, though diameters vary due to other varying emotional input such as from dreams
- Psychosensory; dilation due to heightened emotional arousal as already described
- Vestibular; dilation linked with balance responses
- Orbicularis phenomenon; miosis linked with nearby muscle activity
- Trigeminal reflex; usually a brief initial mydriasis followed by miosis
The first two are of most interest to the eye care practitioner. The third reminds us that the control of the pupils has influences from many higher centres and is not just a response for light adaptation and vision.
The Light reflex
The light reflex pathway is shown in figure 1.
Afferent signals triggered by light on the retina travel the first part of the visual pathway as follows:
- Optic nerve of stimulated eye
- Decussation at the chiasma to enter both optic tracts
- Leave the pathway prior to the lateral geniculate nucleus (LGN) and synapse at the pretectal nuclei (input from each eye goes to both nuclei) in the region of the superior colliculus
- Pretectal nuclei send signals to each Edinger-Westphal nucleus
- Efferent fibres pass to each eye along the oculomotor (3rd
cranial) nerve parasympathetic pathway via the ciliary ganglion and synapse at the sphincte.
Figure 1: The light reflex pathway
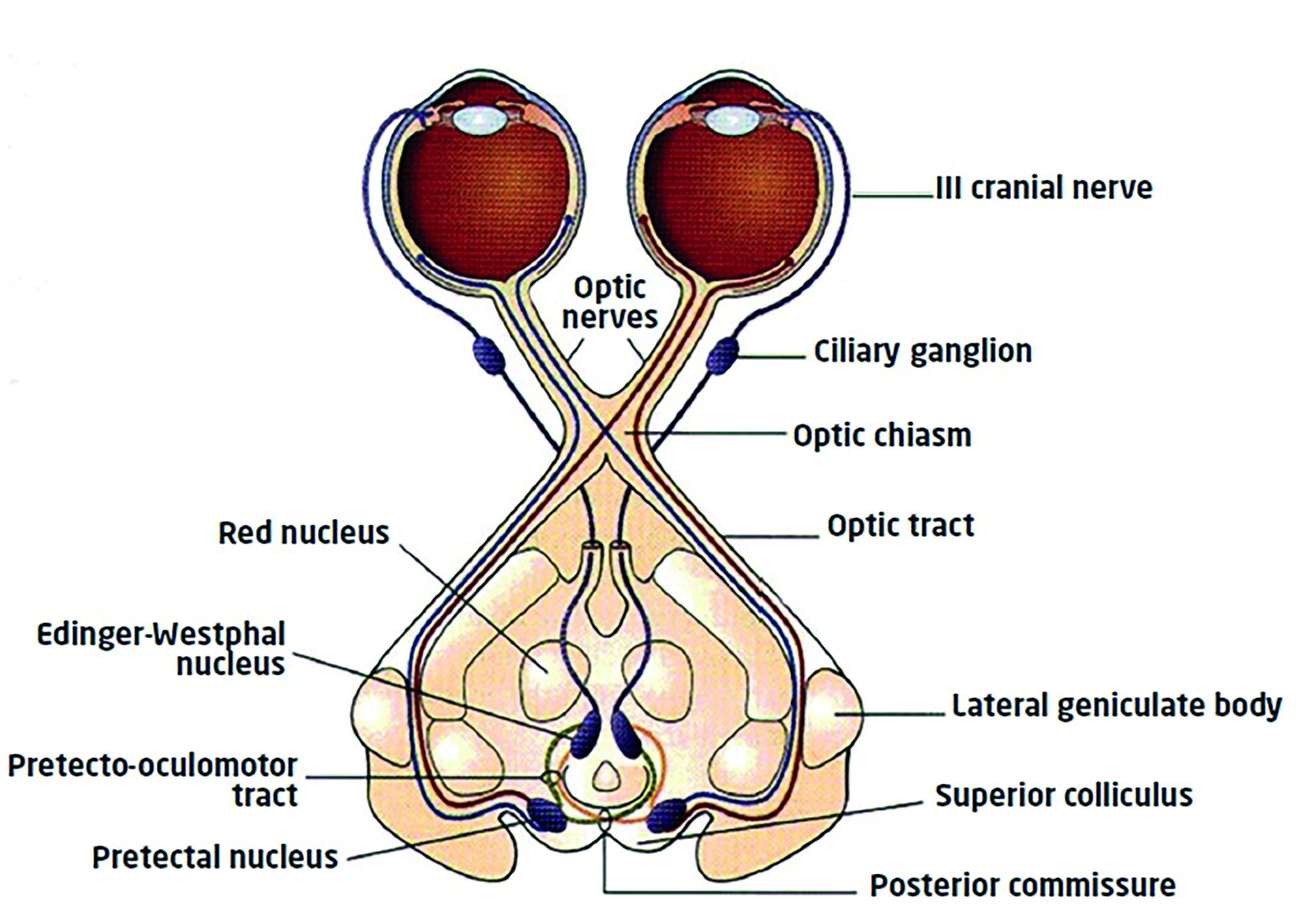
So, decussation to both sets of supranuclear control areas allows the consensual response. Constant supranuclear input maintains constriction while inhibition of supranuclear activity will allow redilation. Dilation will also be complemented by sympathetic activity.
Also, the afferent fibres leaving the pathway just prior to the LGN means that lesions to the pathway before this point (affecting vision and causing field loss) would also impact on pupil response. Lesions to the pathway beyond this point, for example a cerebrovascular accident (stroke) in the radiations would not affect the pupil light response.
The Near Reflex
The constriction of the pupil as a response to viewing a near target (figure 2) can be split into two components:
- Convergence pathway; afferent signals from the medial rectus muscle pass along the oculomotor nerve to the mesencephalic nucleus of the trigeminal (5th cranial) nerve, and then to so-called convergence centre in the tectal/pretectal region and then to Edinger-Westphal and beyond as with the light reflex.
- Accommodation pathway; signals from the retina pass along the visual pathway to the LGN and then to the striate cortex, the parastriate cortex and on to the Edinger Westphal nuclei via the occipiomesencephalic tract and pontine centres. Back to the eye again via the oculomotor nerve.
Figure 2: The convergence pathway (left) and accommodation pathway (right)
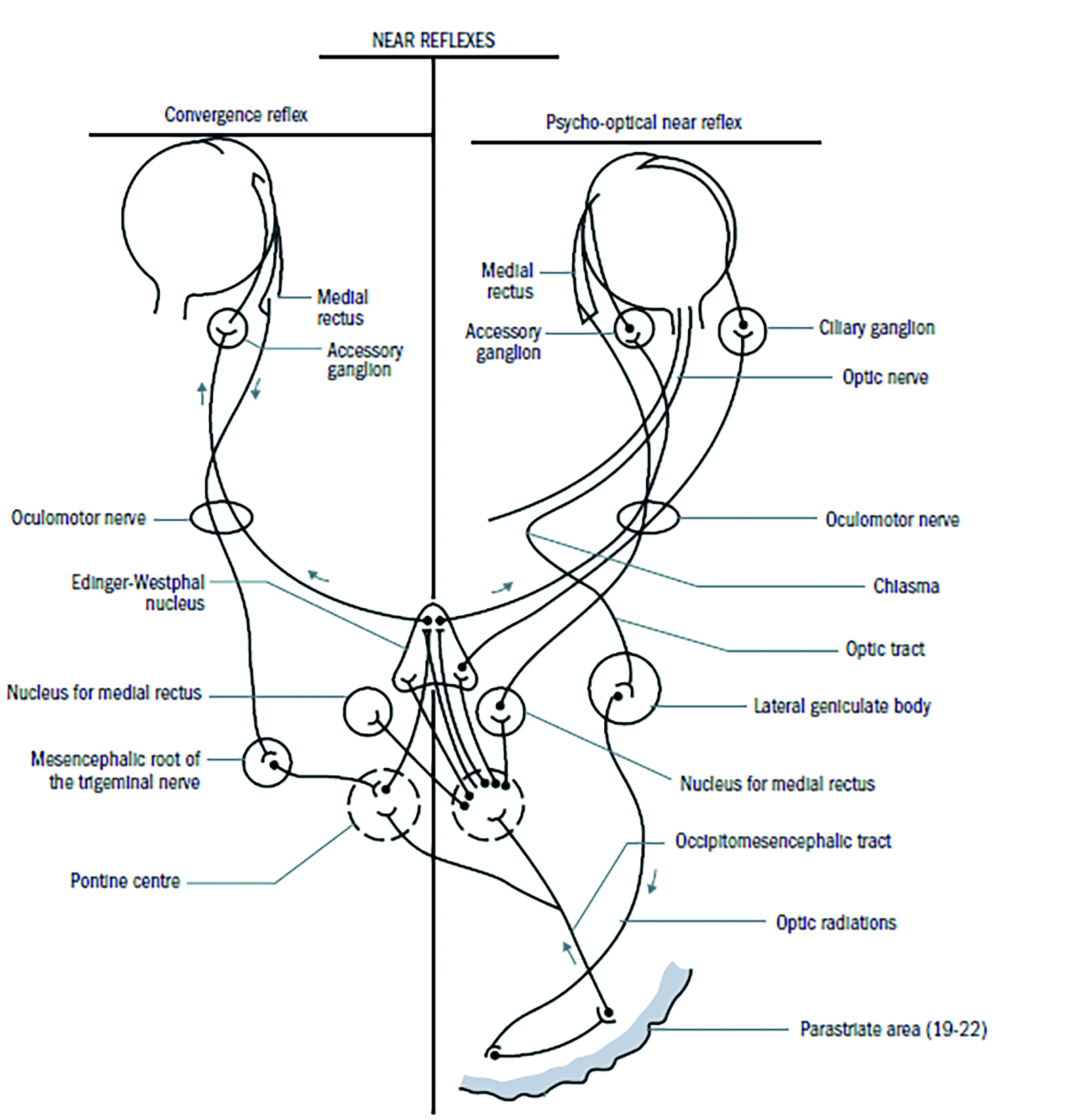
Role of the Pupil
The pupil, bearing in mind it is simply a hole, has a remarkably wide influence on humans. Debate may continue about whether such influences are pre-designed and favoured by evolution or serendipitous artefacts (such as age-related miosis improving depth of focus, and therefore near vision, in elderly presbyopes). That said, it is possible to categorise pupil function as having four key roles.
Social signalling
Pupil dilation is linked to sympathetic innervation as already mentioned. It follows then that the pupils will dilate, and in a way enhance by younger age due to the state of health of the iris, in responses of fear, anger, sexual attraction, and so on. That this serves as a signal to others of emotional state has been suggested by a whole host of evidence, not all of which might always satisfy a Cochrane report analysis.
It is well known, for example, that women in the Middle Ages deliberately dilated their pupils to make them more attractive to men. The theory might be that men might find them more attractive, perhaps by actively or subliminally assuming such a women to be in a state of sexual or emotional arousal. This is how the deadly nightshade plant came to be known as belladonna (‘beautiful woman’), as the atropine-containing (and therefore highly toxic) berry juice of the plant was squeezed into the eyes to cause pupil dilation.
Some wackier psychological experiments of the sixties suggested that people were more likely to be aroused or attracted to images of otherwise similar characteristics when the pupils were airbrushed to appear larger. More recently, functional MRI testing has supported this, showing how viewing dilated pupils in others triggers activity in the amygdala and supports attraction to another. Is it coincidental that this activity is most enhanced during the early years of fertility?
Light regulation
The iris aperture limits the amount of light entering the eye and so can optimise visual resolution at different ambient light levels and viewing distances. It can reduce the risk of retinal bleaching and therefore response times at high light intensity levels, and similarly facilitate photostress recovery and dark adaptation. These would be favoured functions in evolutionary survival terms.
Optical aperture stop
A pencil of non-collimated light entering the eye will suffer from increasing amounts of spherical aberration the further from the initial axis of light direction one moves. Use of aperture stops, for example in astronomical telescopes and OCT instruments, reduces aberration and therefore improves the quality of the image formed by any directional light focusing upon the retina. The aperture diameter, combined with the photoreceptor alignment and distribution at the retina, serve to minimise the increased spherical (Stiles-Crawford effect1) and chromatic (Stiles-Crawford2) aberrations away from the axis and so maintain as good an image as possible.
As everyone who regularly refracts the very elderly will know, sensitivity to spherical power change in people with very small pupils is poor. This may make refraction frustrating (for example, limited response to a +1.00D blur test), but has the advantage of improving focusing ability without correction in the patient. Older readers may remember pilocarpine users who had thrown away their spectacles since using the miotic drug. As shown in figure 3, a smaller pupil increases both the depth of field within which an object may lie and the depth of focus of the image it forms at the retina.
Figure 3: Depth of field and depth of focus depend on the pupil diameter
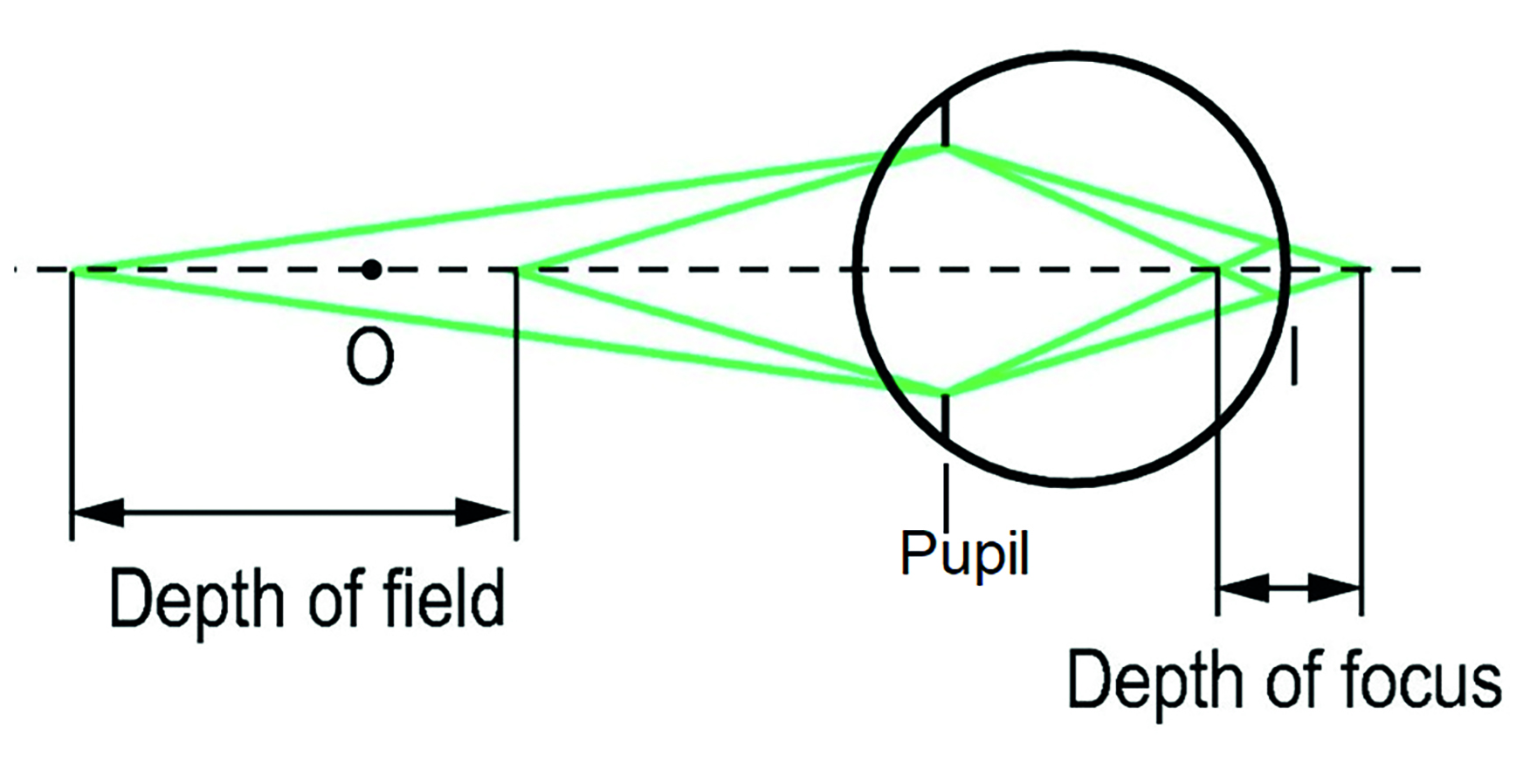
These effects are, of course, clinically useful. A pinhole stop (figure 4) is able to reduce off-axis aberrations and light scatter in patients with reduced acuity and help to indicate whether there is any residual ametropia that might be corrected. Also, by restricting the width of incident beam, increased macular dependency for interpretation may be a useful indicator of early maculopathy, were the pinhole acuity to be reduced.
Health marker
Knowing what the normal pupil appearance and response should be allows clinicians to interpret abnormality and so the pupils can support diagnosis of ocular disease. It is this last section that will form the bulk of the rest of this article.
Irregular Pupils
A pupil is essentially round, though never perfectly so due it its reliance upon a large number of fibres producing different forces to dictate its shape. A common finding clearly seen with paler irises, is a rim of pigment around the aperture, a so-called pupillary ruff (figure 5).
Figure 4 & 5 (left to right): A pinhole stop; and a round pupil with a narrow pupillary ruff
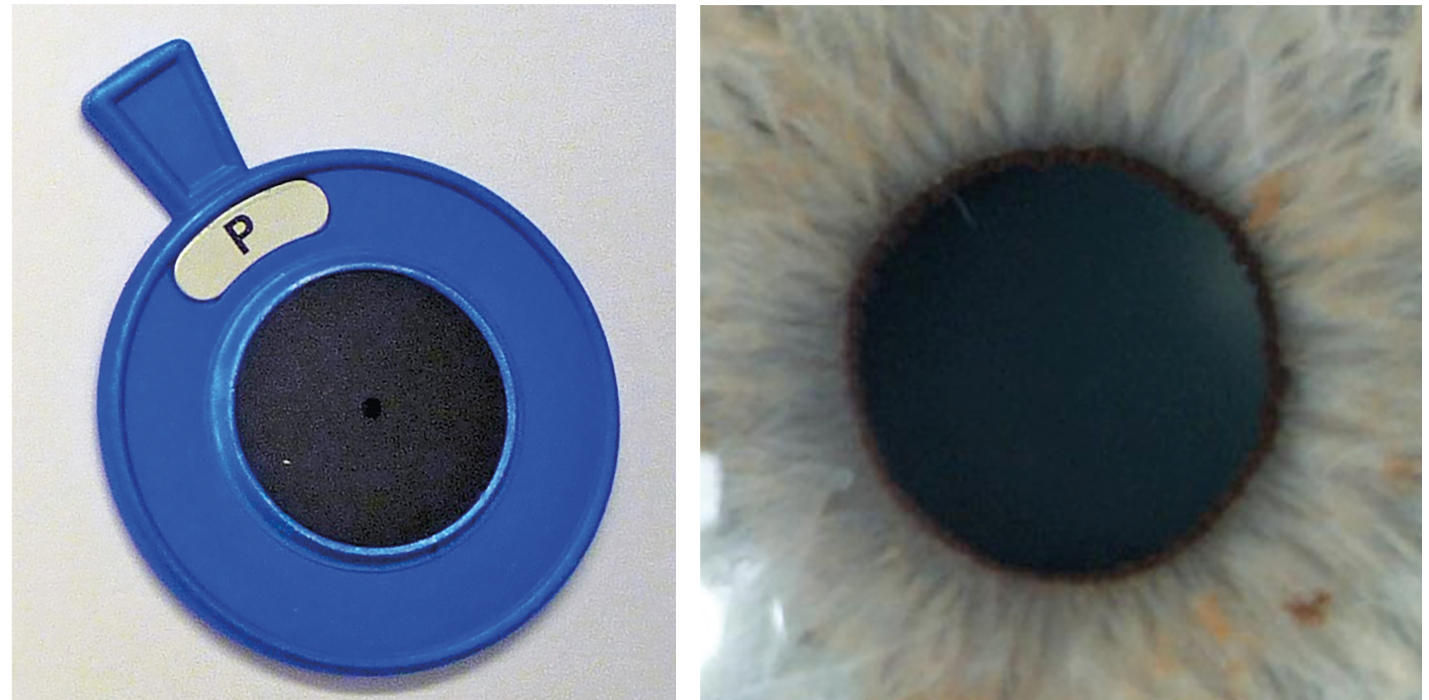
Because the pupil forms later in gestation, there are sometimes strands or remnants of the original tissue in the centre of the iris seen on slit lamp, waving into the pupil from iris attachments. In a very few cases, these residual strands may span the pupil and have attachment at opposite sides on the iris. This can cause some pupil distortion when the pupil dilates as they may not stretch adequately. It has been known for these persistent iris strands to be broken by argon-YAG laser if thought beneficial to the patient.
Also during development, sometimes the pupil forms off-centre and eccentric to the visual axis. This is known as corectopia and, while it can result from a number of rare genetic and development disorders, is most likely seen in practice as a result of surgical or traumatic events.
Pupil irregularity may also occur due to the following:
- Iris disruption; neoplastic iris diseases, such as melanoma or metastatic growth may distort the pupil
- Iris atrophy; this may be more noticeable when atrophy is due to disease and non-concentric in nature
- Synaechiae; attachment of the iris to the lens (posterior synaechiae) or the cornea (anterior) is likely to result in a deformed pupil
- Iris coloboma; failure of the anterior inferior fissure to close during gestation may result in a keyhole-like pupil appearance
- Vermiform constriction; where only parts of the sphincter are operating, the resultant pupil may be irregular. In extreme cases, this may look almost shamrock-like, but is usually described as ‘worm-like’ or vermiform
- Irregular when fixed; a fixed pupil does not respond to stimuli. Irregularity may be present depending on the nature of the cause. For example, very high intraocular pressure, as with angle closure glaucoma, will prevent the iris from working. The resultant pupil is commonly vertically oval due to the distribution of the damage to the iris
Anisocoria
As already explained, most people are asymmetric and so it is likely that their two pupils are of different diameters. Differences in pupil diameters is termed anisocoria. In most cases, therefore, this is a physiological anomaly and usually of no concern. Most authorities suggest that clinicians need only be concerned when presented with anisocoria of greater than 2mm difference, in the absence of other determining factors of course.
Key Point 4:
- Most people have a degree of anisocoria, but rarely is it a concern. Diameter differences of greater than 2mm should be investigated. Where the difference remains constant under differing light levels, the anisocoria may be considered physiological.
Anisocoria may have a number of aetiologies. These can be categorised into three types;
- Physiological; this describes pupils that are unequal simply due to variation in anatomy. As such they are not assumed to be related to any disease process or tissue damage and the patient may simply need reassurance
- Efferent defects; these are due to damage to the efferent innervation of the pupils resulting in anisocoria
- Secondary; this blanket term is here used to describe unequal pupils due to previous damage, either pathological (for example after recurrent anterior uveitis), iatrogenic (for example as a complication of intraocular surgery), pharmacological (for example as a result of asymmetric influence of a systemic drug), or age-related (as due to asymmetric atrophy of the iris muscle fibres with age).
Note that afferent defects, where there is disruption of the reflex pathways, may present with apparent isocoria (equal pupil size) and will only be elicited by testing of the reflexes.
Key Point 5:
- Efferent defects present as anisocoria while afferent defects may not.
Leukocoria
Leukocoria literally means ‘white pupil’. When light is shone onto a pair of healthy eyes and viewed on axis from a short distance, the reflection from the retina of each eye will make the pupils appear red. This is the basis of the so-called red reflex test (though not a neural reflex in this case). Where the reflex appears dull in one eye, or the pupil is pale or white then this indicates something preventing retinal reflection. Intervention is essential to prevent significant amblyopia or disease progression. The test is most commonly performed on neonates as a screening tool for significant eye diseases. A white pupil in infancy can be caused by conditions such as the following:
- Congenital cataract
- Persistent hyperplastic primary vitreous; the previously vascularised foetal vitreous has failed to clear during gestation
- Retrolental fibroplasia; a neovascular scarring response found with retinopathy of prematurity
- Retinoblastoma; large neoplasms may cause major retinal detachment, seen as whiteness in the pupil
It is worth noting that a significant difference in the retinal reflex, such as one bright and one dull, can indicate unilateral high ametropia.
Leukocoria, anisocoria and pupil eccentricity can be sensitively screened for using these days by automated photo-ophthalmic device, included hand-held adapted smartphone devices (figure 6).
Figure 6: Use of an automated photo-ophthalmic device (courtesy of Professor Simon Barnard)

Routine Assessment
Initial observation
Initial observation should immediately help to identify mis-shapen pupils (as already discussed) or anisocoria. As stated, anisocoria may be physiological, due to efferent pathway damage, or secondary. Let us consider how to confirm each in turn:
- Physiological anisocoria; to confirm that any pupil diameter difference is physiological, it is simply a matter of confirming that each pupil responds in a normal manner and both behave symmetrically. This is best done by simply dimming the room lights and bringing them back up again. As this is done, the pupils should first dilate with decreasing light levels and then constrict as brightness is restored. Physiological anisocoria can be confirmed (and a patient reassured) if the difference between the two diameters remains constant throughout.
- Pathological anisocoria; the pupils respond differently as ambient light levels are changed. One may remain large or small and fixed or each might respond at differing rates.
Testing reflexes
Typically, the light reflex is tested in practice using a pen-torch. It is important to establish the best background lighting to best assess pupils and this will vary from patient to patient. Ambient lighting should be reduced to exaggerate the resting pupil diameter, but be of sufficient levels to allow easy viewing of the pupil, particularly in patients with very dark irises. A dimmer switch for the consulting room main lights is useful here. The lights should be dim enough to enhance pupil dilation prior to introducing the pen-torch light while bright enough to still allow the consensual reflex to be seen without the pen-torch. So darker irises tend to need more background lighting than paler irises.
It is also worth remembering that the torch should be held close enough to the eye to minimise light spread to the other, which would impact on the sensitivity of any direct reflex by introducing consensual influence. A focusable beam (or torch with a directional illuminator extension) is ideal for this. Some US practitioners advocate use of an occluder along the nose to prevent contralateral light stimulation.
A typical sequence for the overall testing of pupils would be to assess the direct and consensual light reflexes for each eye first, then to look for an RAPD, and finally to assess the near reflex as follows:
- Direct and consensual reflexes; the light should be shone directly onto the pupil. The pupil will constrict (the direct reflex) as will that of the other eye (the consensual reflex). This is seen on the left side of figure 7a. This should then be repeated for the other eye and again both the direct and consensual responses should be noted and recorded. This should be repeated at least three times to look for any gradual reduction in reflex, which may indicate an abnormality. Each time the light is removed, a direct and a consensual dilation should also be noticed. The same should be repeated for the opposite eye. The absence of a direct reflex necessitates the investigation of a near reflex.
- Near reflex; though there is some debate as to the usefulness of this as a routine test, most practitioners still undertake an observation of the pupil response when the patient is asked to change their focus from a distant to a near (non-light stimulating) target. The pupils should be seen to constrict equally, though this response becomes difficult to gauge in older patients. This is most useful when a direct reflex is absent as a maintained near reflex without direct reflex is indicative of some neurological disease (Argyll-Robertson pupil), but its usefulness in general eye care practice might be questioned.
- Relative afferent pupillary defect (RAPD) test; this checks the strength of the direct afferent response against the consensual response of the other eye. In cases where the afferent signal is compromised, then consensual dilation may over-ride any direct constriction response. This is usually tested using a swinging pen-torch test. When the light is held in front of one eye for one to two seconds and then moved across to the opposite eye for a further one to two seconds, then back to the first and so on, a sequence of bilateral equal constrictions should be observed. In the case of damage to the afferent pathway (as with, for example, the earlier case example of retrobulbar neuritis), the affected eye will show a dilation when the light is moved in front of it from the opposite healthy eye.
Key Point 6:
- Checking for the presence of an RAPD is the most important part of a pupil assessment in everyday eye care practice.
Key Point 7:
- To confirm a positive RAPD, move the light from the healthy eye to the affected eye and look for an initial dilation (figure 7).
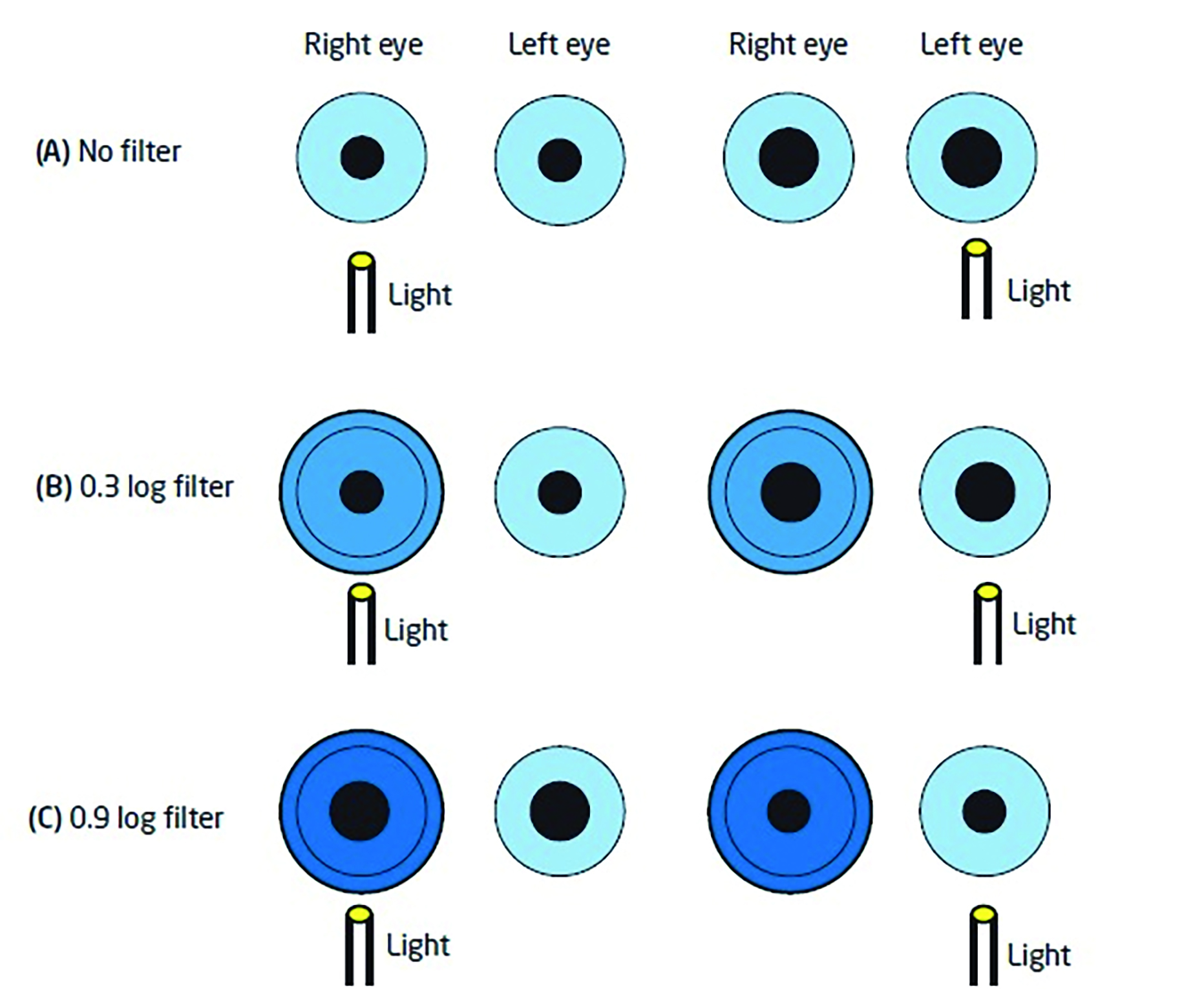
The dilation occurs because the consensual reflex from the healthy eye over-rides the compromised direct response in the affected eye. It is possible to use neutral density filters, therefore, to establish how big a defect is – and indeed grade it if required. By placing filters before the healthy eye of increasing density, there will come a point at which the consensual response from the healthy eye will be reduced to a point where the initial direct response in the affected eye will be restored. The greater the relative afferent defect, the denser the filter needs to be. This can then be recorded (figure 7). This should also remind us that media opacification (cataract, corneal haze and so on) may affect pupil response, but does this is not an RAPD.
Figure 7: Assessment of a left relative afferent pupillary defect and grading its depth.
A. The light before the healthy right eye causes a direct constriction and a consensual constriction in the left. Swinging the light from the healthy to the affected eye, a dilation is seen in the affected eye as the direct reflex is weakened allowing the consensual from the good eye to override.
B. Placing filters before the healthy eye allows the depth of defect to be assessed. A 0.3 filter does not change to pupil activity.
C. A 0.9 filter before the healthy eye reduces the consensual signal from the healthy eye to a level that allows the weaker direct reflex in the affected eye to dominate. This is the filter that first shows this reversal so may be recorded for this RAPD
Recording of Pupil Responses
Most practitioners use some form of acronym when recording the responses, such as a tick next to DCN and no RAPD. Others write PERRLA representing;
- Pupils
- Equal
- Round
- Respond to
- Light and
- Accommodation
With defective pupil responses, a description of the exact defect needs to be recorded.
Key Point 8:
- Remember that, for elderly patients, PERRLA is as unlikely as the absence of artieriosclerosis, lenticular changes and a wide open anterior chamber angle, despite what many records suggest.
Causes of Pupil Anomaly
As mentioned, anisocoria of physiological origin is easily confirmed. Where a disease is suspected, there are some further pharmacological tests that may help with a final diagnosis. Figure 8 summarises a diagnostic systematic approach.
The efferent defects worth remembering are:
- Adie’s tonic pupil; part of what is termed Holmes-Adie syndrome. This presents as a dilated tonic round or vermiform pupil (but can be bilateral), typically in 30 to 50-year-old women. Like Bell’s palsy, it is thought to result from an autoimmune response to residual infective material from an old viral infection. It is usually transient and recovery is hoped for after a few months. Mydriasis and cycloplegia present as blurring and photophobia and may be managed as required.
- Oculomotor (3rd cranial) nerve palsy; the expected triad of signs would be a full ptosis (loss of levator palpebrae innervation), hypo and exotropia, and a dilated pupil. Where the nerve damage is vascular in origin, as with for example diabetes, the pupil dilation might not be present (so-called pupil sparing).
- Horner’s syndrome; the triad of signs is a miosed pupil (figure 9), a partial ptosis (the paralysis of Muller’s muscle may be subtle and hard to see), and dryness (anhidrosis) on the cheek of the affected side. The sympathetic innervation of the iris follows a long and tortuous path from the spinal ganglion in the lower cervical region (where there is input from the hypothalamus), over the apex of the lung (where a Pancoast neoplasm may damage the nerve, and adjacent to many important vascular structures. The trauma of birth can damage the nerve pathway, and congenital Horner’s is often differentiated by a heterochromia, with the affected eye being pale and depigmented. Recently acquired Horner’s may be due to an aneurysm so ophthalmological advice should be immediately sought.
Figure 8: Differential diagnosis of anisocoria
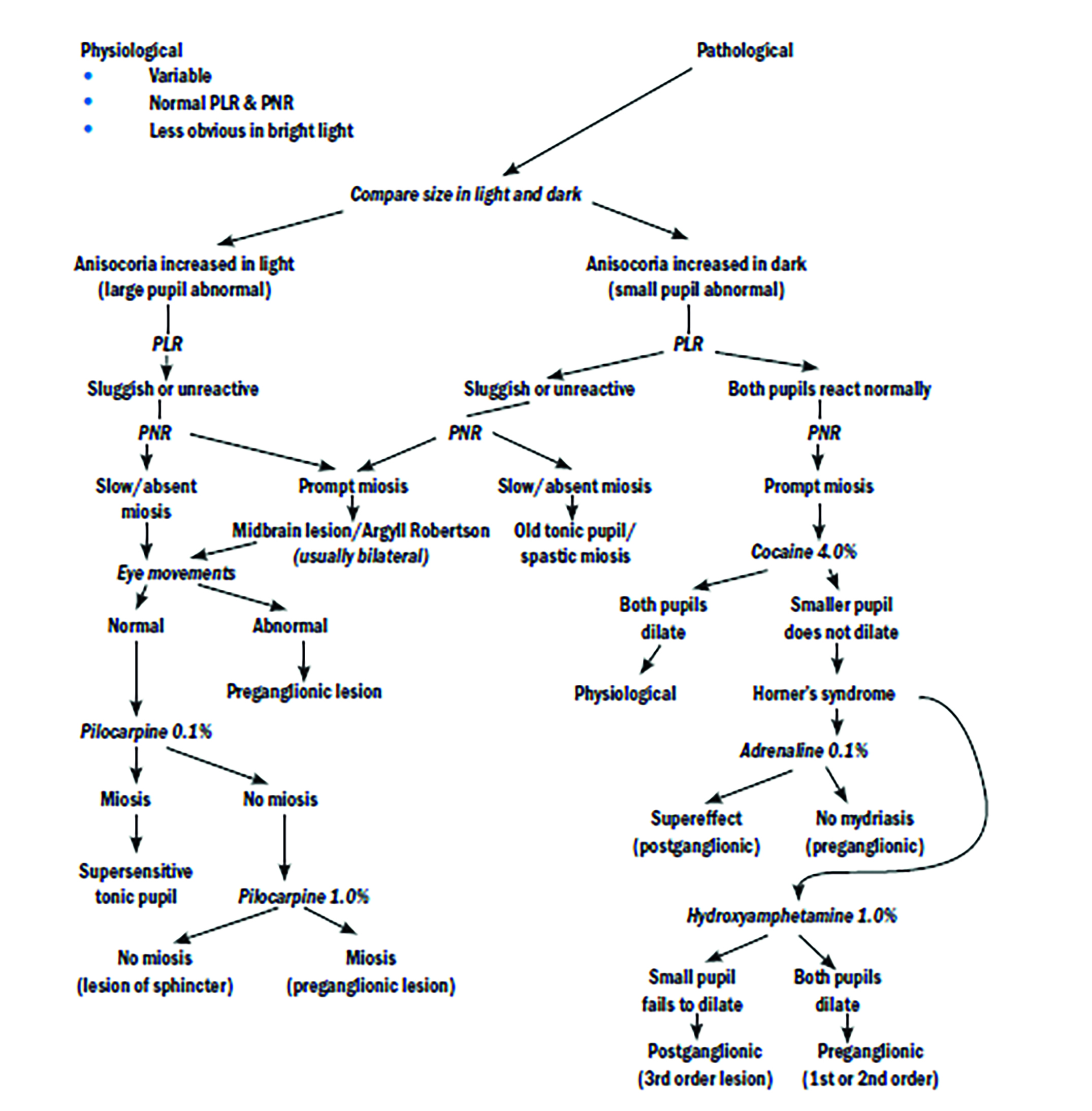
Figure 9: On dimming the lights, the left pupil dilates further while the right pupil remains small and fixed throughout. This suggests a right Horner’s syndrome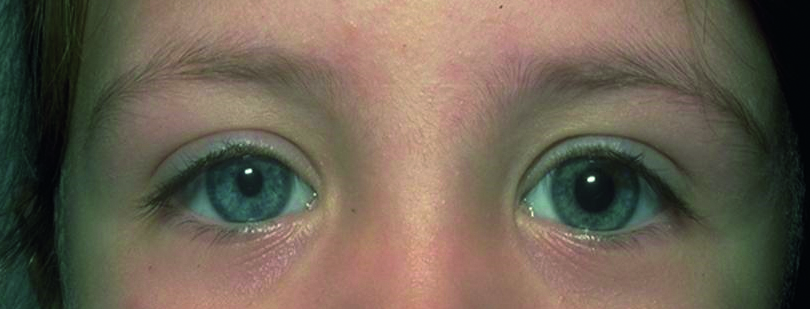
Anisocoria due to secondary influences includes a wide range of possible causes and careful history is necessary to elicit the cause. This may be:
- Iris atrophy; it is common for the irises to atrophy in asymmetric fashion
- Previous history of eye disease; for example, unilateral chronic anterior uveitis, iris neoplasm and so on
- Iatrogenic; iris damage subsequent to surgery
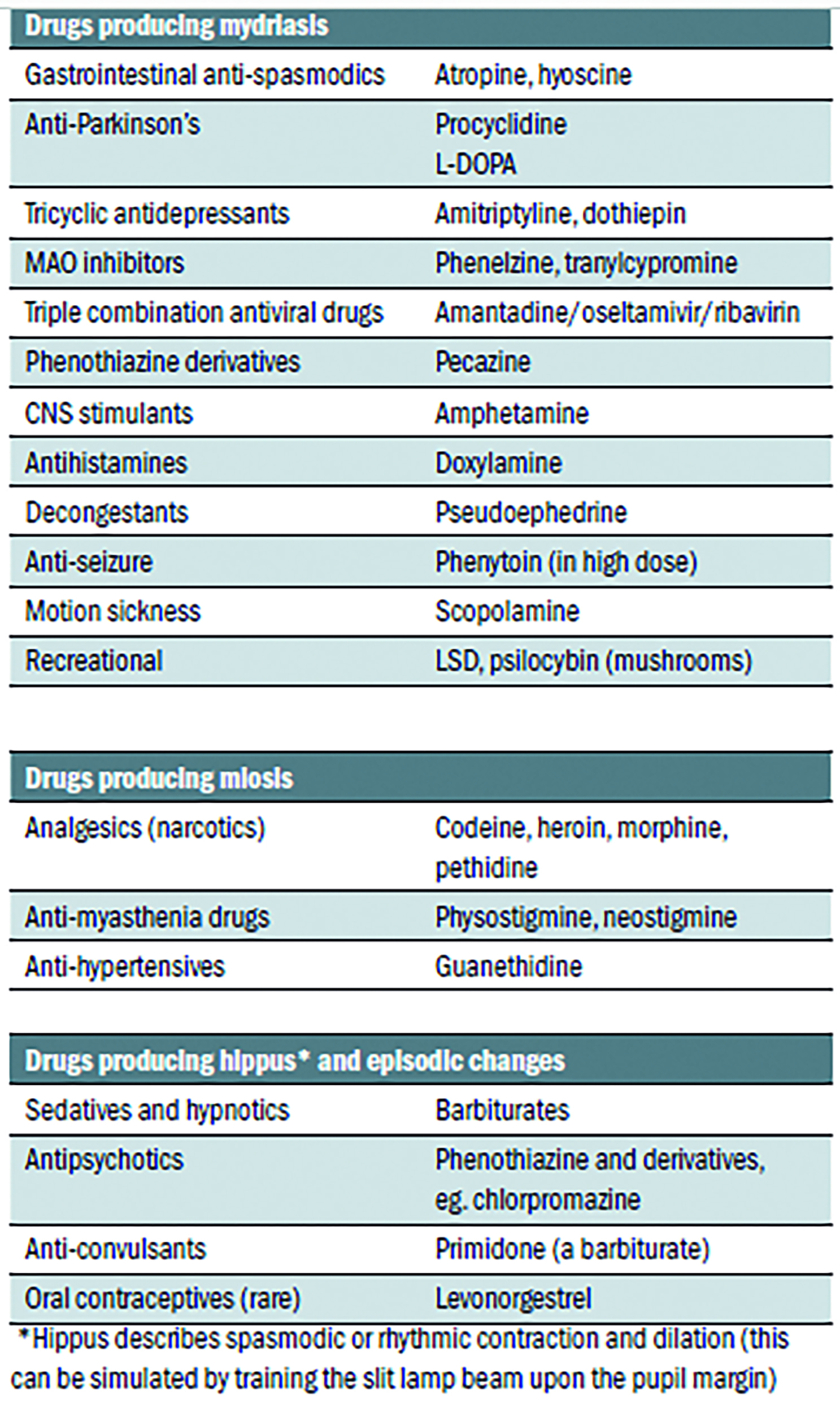
- Pharmacological; there are many legal and licensed drugs that can influence pupil state. Important examples are summarised in table 1.
Table 1: Some drugs known to affect the pupil
Try this:
- RAPDs are common, though often minimal and so missed. Next time you see a patient with asymmetrical acuities, trying looking for a subtle initial dilation in the weaker eye on swinging the light.
