Dry eye disease (DED) is a multifactorial disease of the ocular surface characterised by the loss of homeostasis of the tear film and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface (cornea and conjunctiva) inflammation and damage occurs.1 The DED is also called keratoconjunctivitis sicca or dysfunctional tear syndrome.2
DED is a common pathology triggering referrals to ophthalmologists worldwide and has been found to impose a great burden on societies.3 Based on epidemiology studies, the prevalence of dry eye worldwide varies depending on the study population, classification and definition of dry eye, and the various diagnostic tests used. In adults, the prevalence of dry eye, with the presence or absence of symptoms, has been reported to range from approximately 5% to 50% but can be as high as 75% among adults over age 40, with women most affected.4
The most common symptoms of DED are redness, gritty sensation, burning, pain, dryness, ocular fatigue, blurry vision, and headaches. These produce significant impairments in quality of life, daily activity, and work productivity.
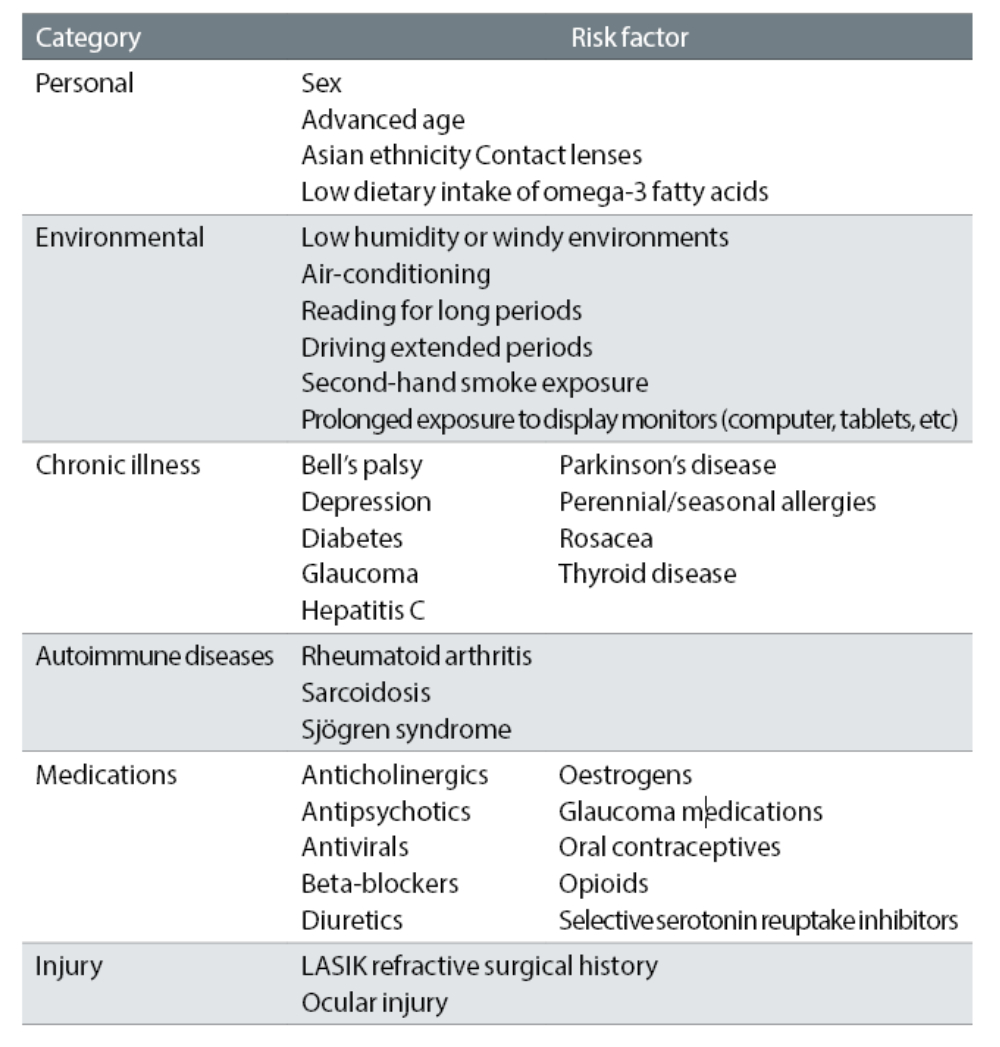
Risk factors for DED (table 1) include advancing age, female, race (commonly seen in Asian ethnicity), certain systemic conditions (such as Sjögren’s syndrome, diabetes, androgen deficiency and connective tissues diseases). The factors that aggravate symptoms are contact lens wear, increased evaporation of tears (central heating/air conditioning), digital device use (reduced blink interval), medications (antidepressants, antihistamines, diuretics), noxious agents (tobacco smoke and cooking fumes), and refractive surgery.4
Table 1: Risk factors for dry eye disease
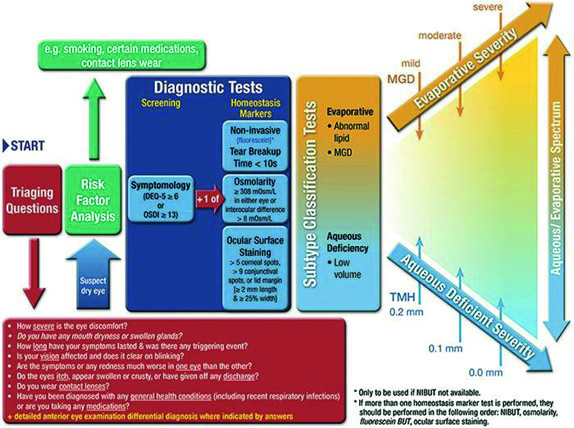
The DED diagnostic test battery (figure 1), which was recommended by the second Tear Film and Ocular Surface Society Dry Eye Workshop (TFOS DEWS2) suggests that if a patient has dry eye symptoms, DED is diagnosed when at least one homeostasis test result is positive. This can still occur even if the practitioner does not have access to the full battery of recommended tests. These tests include non-invasive tear break-up time, invasive tear break up time of <10 seconds, osmolarity ≥308mOsm/L (measured prior to invasive tear break-up time), fluorescein ocular surface staining (observe cornea, conjunctiva, and eyelid margin) and lid wiper epitheliopathy can be observed with fluorescein staining and meibomian glands blockage.5
The Schirmer test is not part of the diagnostic test battery of DED due to the lack of high-level evidence data on repeatability, sensitivity, and specificity of this test. However, the Schirmer test without anaesthetic remains as a diagnostic test for confirming severe aqueous deficiency (such as in Sjögren’s syndrome).5
Figure 1: DED diagnostic test battery by TFOS DEWS25
Classification and Management of DED
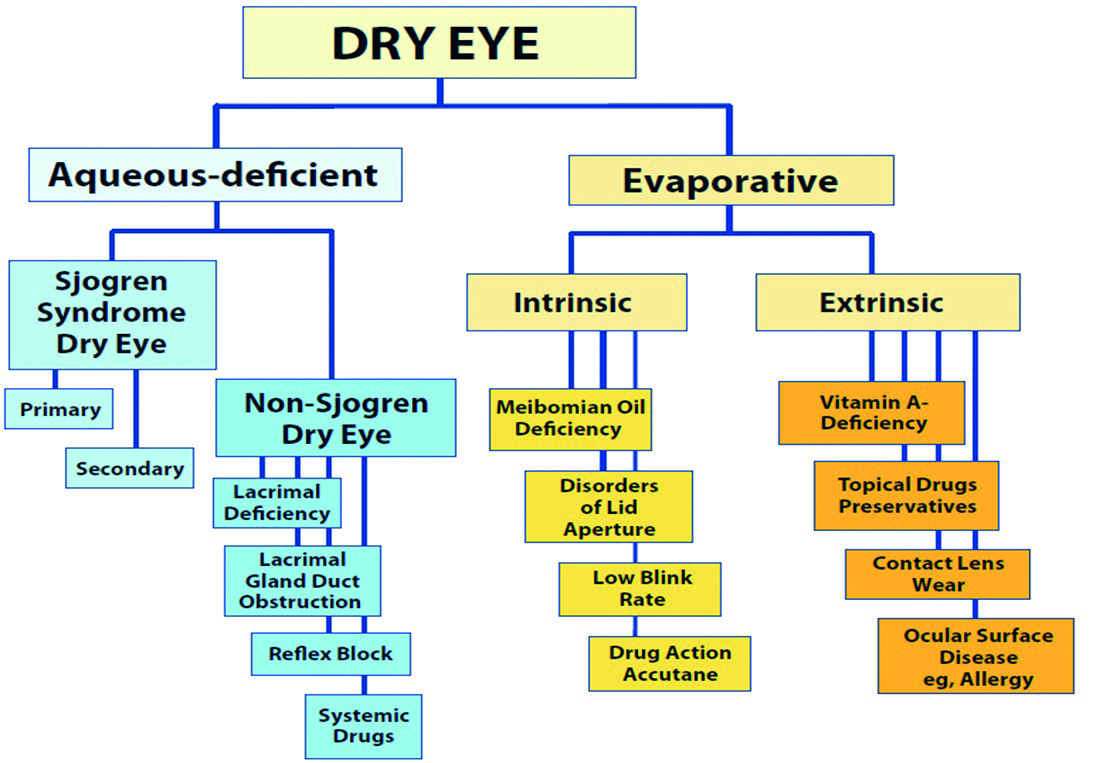
There are two types of DED (figure 2):
- Aqueous deficiency; is when the aqueous secretion from the lacrimal gland is reduced
- Evaporative; caused by abnormalities in the lipid-secreting meibomian glands
Both of these result in an unstable tear film and lead to chronic inflammation of the ocular tissues. Both of the two types can occur together. Meibomian gland dysfunction (MGD) accounts for the principal cause of evaporative dry eye. These glands in the upper and lower eyelids secrete oils into the surface of the eye and help keep tears from evaporating rapidly.6
Figure 2: Dry eye classification and its causes1
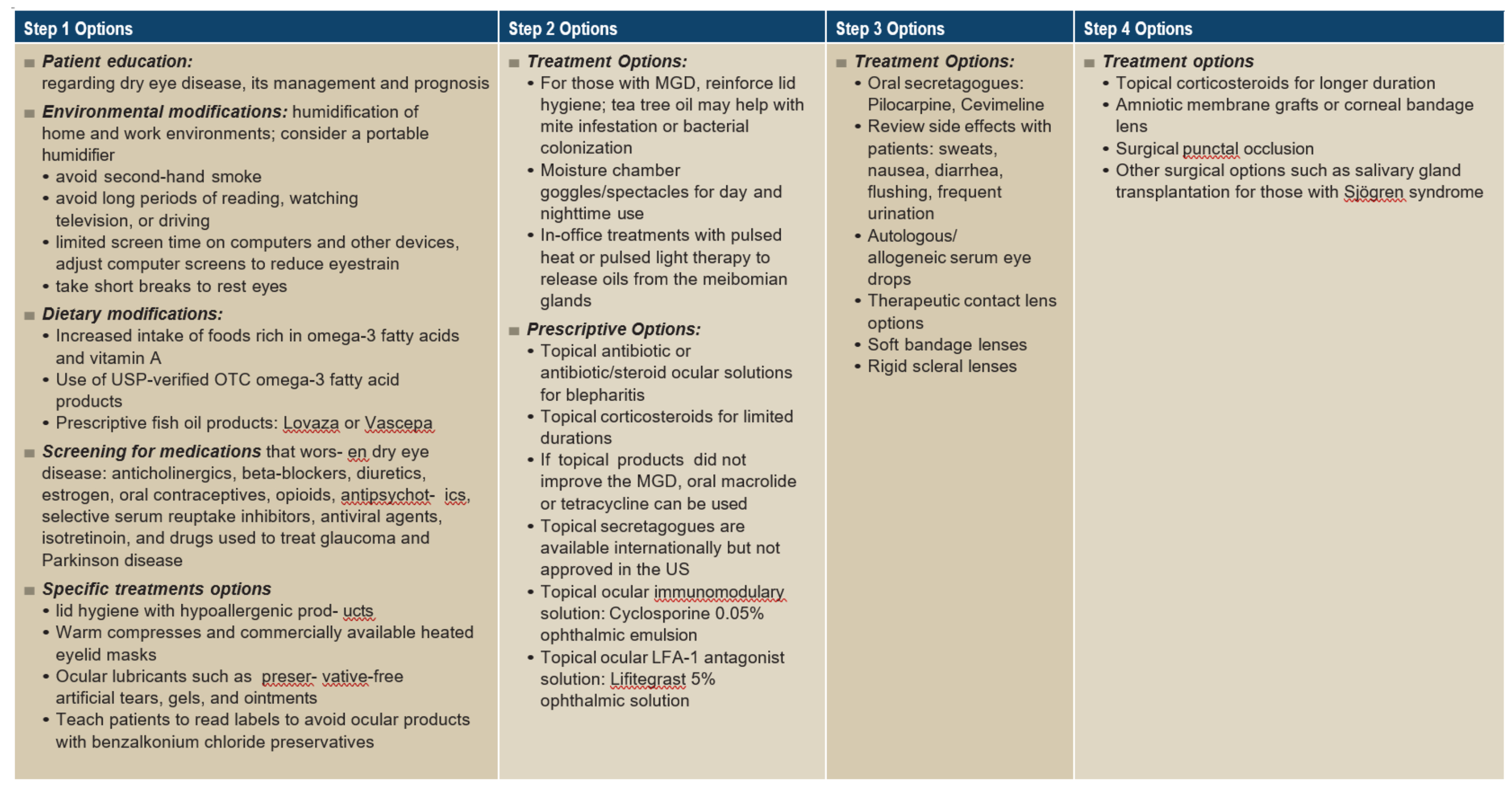
The Tear Film Ocular Society’s Dry Eye Workshop treatment recommendations use a step-therapy approach to guide care (figure 3).6 However, in the current management of DED, artificial tear supplements are the most prescribed, though they do not treat the cause but provide short-term, symptomatic relief and do not reverse metaplastic changes.7 Other pharmacological therapies include anti-inflammatory drops, autologous serum eye drop therapy, preservative-free gels and ointments that are available to lubricate the ocular surface and are often used at night.
Non-pharmacological therapies include a punctal plug, and modification of local environments (desiccating conditions and digital devices use).8 Lid hygiene and hot compresses are effective strategies for MGD. Daily practices with warmed compresses, hypoallergic cleansing products and gentle messages to express the lipid are recommended for evaporative dry eye related to MGD.6
There are disadvantages to each type of intervention. For example, a topical corticosteroid may induce ocular side effects, the autologous serum is taken from the blood. This therapy can lead to blood-borne infections and increase the risk of anaemia.7 Punctal plugs raise the risk of secondary infections by obstructing the canaliculi.9
Unmanaged DED diminishes the quality of life related to vision-focused activities such as reading, driving and computer use, and can adversely impact outcomes in those undergoing cataract removal or refractive procedures.10
Figure 3: Step therapy management for dry eye diseases recommended by TFOS DEWS25
Nutritional Supplements
This systematic review aims to critically appraise scientific evidence regarding the efficacy of oral nutritional supplementation with omega-3 fatty acids (FAs) for the treatment of DED. In recent years, an increasing number of nutritional supplements aiming to improve DED symptoms have been developed. The supplements include vitamin-A, omega-3, omega-6 and omega-9.11 However, this review will specifically investigate the effectiveness of omega-3 in reducing DED signs and symptoms.
There have been reports noting that nutrients from diet were involved in the pathogenesis of dry eye. The prevalence rate of dry eye was significantly lower in people with high omega-3 polyunsaturated fatty acids intake compared to those with low omega-3 intake in the analysis of significantly lower in population with a high intake of tuna containing omega-3.12 As essential fatty acids are necessary to maintain good health because it helps in regulating the inflammatory and immune response in the body.
In a randomised double-blind clinical trials, it was found that the treatment group received a daily intake of omega-3 FAs (450mg EPA, 300mg DHA, 1000mg flaxseed oil) for 90 days. This group showed a significant improvement in DED symptoms. The changes in the tear volume also were significant, whereas the changes in meibum lipid composition and aqueous tear evaporation rate were not significant.13 From recent evidence of two systemic reviews and one meta- analysis demonstrates increased intake of omega-3 FAs improves tear production and DED symptoms.14-16
Furthermore, DED is referred as chronic inflammation in the ocular surface by disturbing the tear osmolarity leading to ocular changes increasing dry eye symptoms and severity. According to these findings, oral supplementation of omega-3 FAs could improve the inflammation of the ocular surface and give relief to symptoms of DED, given this new therapeutic alternative, which will be reviewed in this paper.
Omega-3 Supplementation for the Management Of DED
Omega- 3 fatty acids are a family of essential fats that cannot be synthesised in the body and must be supplemented in the diet. There are three compounds: eicosatetraenoic acid (EPA), docosahexaenoic acid (DHA); being the long chain FAs and α-linolenic acid (ALA) being the short chain FAs.
EPA and DHA modulate prostaglandin metabolism towards anti-inflammatory prostaglandin synthesis due to competitive inhibition of arachidonic acid pathway. In DED, to keep inflammation under control is very important, it is shown that increased concentration of cytokines such as interlukin-1, interlukin-6
and tumour necrosis factor-alpha have been found in the tear film of dry eye patient.17 Hence, taking EPA and DHA through supplement, will help the body overcome inflammation and enhance immune response.
Omega-3 FAs are preferred over the omega-6 or omega-9 as the omega-3 FAs contain eicosatetraenoic acid (EPA) and docosahexaenoic acid (DHA), which have anti-inflammatory properties that improve tear function and DED symptoms. DHA blocks oxidative reactions and prevents the releases of arachidonic acid, a potent inflammatory compound.18 In contrast, increased intake of omega-6 FAs may incur risk for DED as when the FA is activated it releases mediators that increase inflammation.19
The long chain omega-3 is derived from fatty fishes such as salmon, light tuna, sardines and lake trout, which are low mercury, and the larger fatty fishes, which are a rich source of omega-3 but they are advised not to be taken in excess due to concern regarding the heavy mercury metal contamination.20, 21
ALA is found in some plants and vegetables including flaxseeds, walnuts, kale, spinach, broccoli, squash, whole grains, wheat germ, kidney and mung beans.22 Within the body, the short chain ALA can be converted to the long chain omega-3 (EPA and DHA), although the efficiency of this conversion may be age-dependent and may not be as efficient.22
The extraction and purification of omega-3 FAs is an important factor to determine the quality of omega-3; otherwise, low-quality omega-3 can have an unpleasant smell, which can be a barrier to consuming them. There are three stages to obtaining the finest quality of omega-3 FAs: extraction, refining and concentration.
Extraction
The methods used to extract omega-3 FAs include the use of solvents, supercritical fluids, wet pressing, and fish silage by employing enzymes in fish and other chemicals.23
The most used method for the industrial-scale production of omega-3 FAs is wet pressing, which consists of four stages: fish cooking, pressing, decanting and centrifugation. Another conventional method of extraction is using solvent, based on the solubility of lipids in organic solvents and their insolubility in water. There is a limitation to this method as it requires a dry sample that is destroyed and it is very time-consuming while generating large amounts of residual solvent which produces low-quality omega-3.23
Refinement
During the refining process, the impurities in the oil reduce its quality and must be eliminated while minimising oil loss and maximising the availability of beneficial constituents.24 The refining process includes four stages: degumming, neutralisation, bleaching and deodorisation. Each stage is important to remove the different impurities’ compounds.
Concentration
Lastly, several techniques have been proposed to maintain the high concentration of omega-3, including winterisation, concentration by enzymatic methods, fractionation by supercritical fluids and by chromatographic methods, formation of complexes with urea, and concentration by membranes.
It has been reported that high attainable purity for EPA >95% and DHA >99% from a single isocratic separation of fish oil within five to 10 minutes was achieved using silver-thiolate argentation chromatography.25 Some studies have shown that combination of different techniques seems to offer a good alternative to increase the purity omega-3 efficiently.26
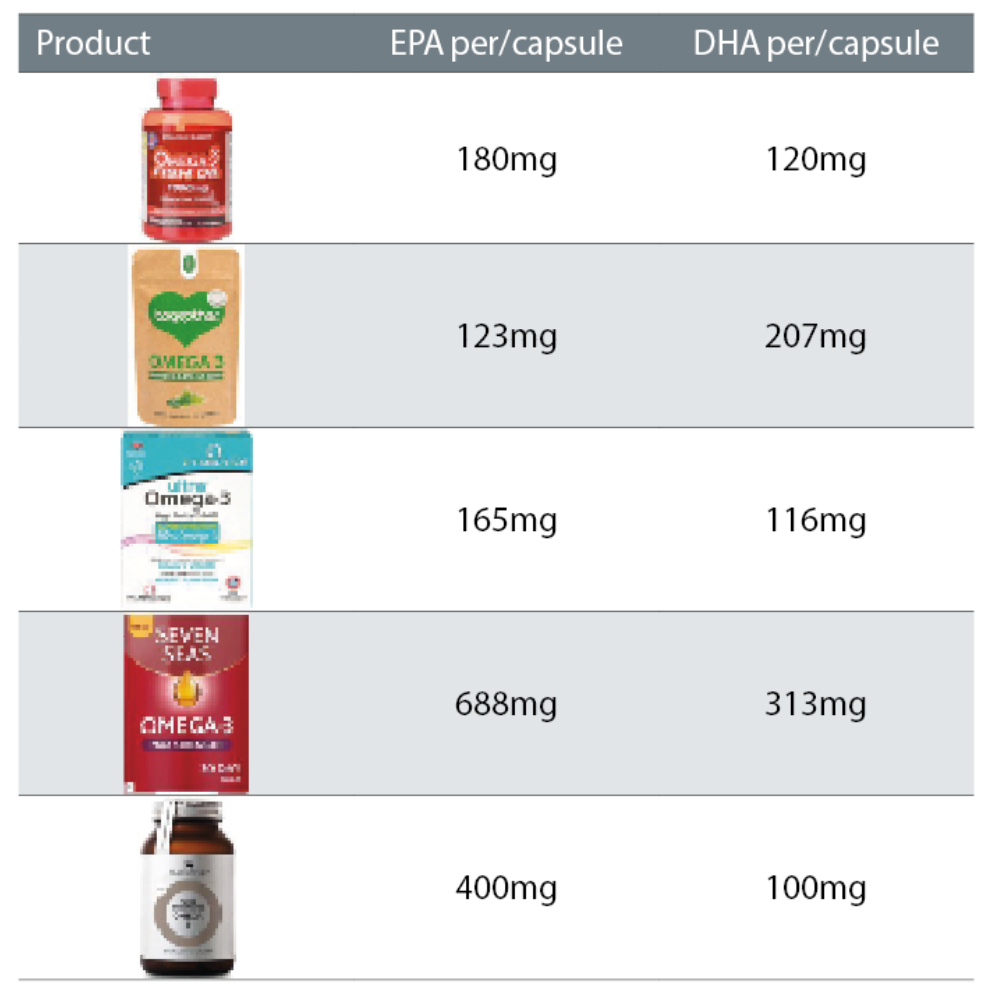
As table 2 suggests, the availability of omega-3 supplements in the UK market, is overwhelming. Hence, it is a challenging task for a patient to choose a suitable omega-3 with the correct unit of EPA and DHA that will benefit them and help to reduce their DED symptoms.
Table 2: Brands of omega-3 supplements available in the UK and the amount of EPA and DHA present per capsule (in moderate dosage)
Systematic Review
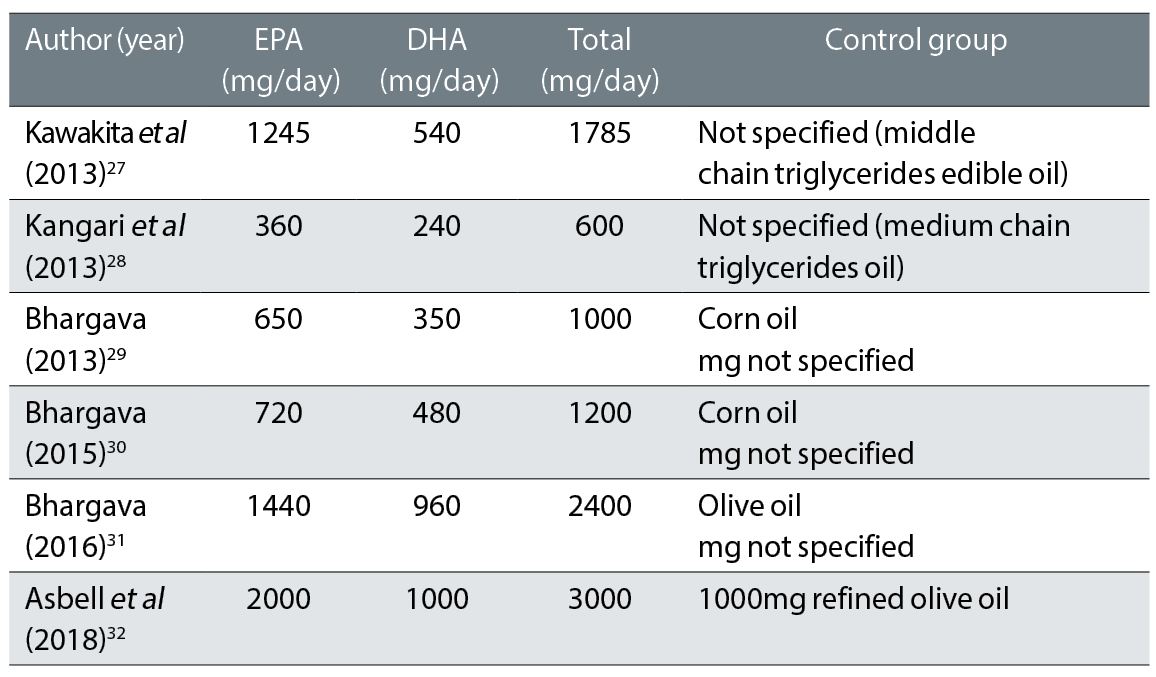
The composition of the supplements used in this review are outlined in table 3.
A targeted, systematic review and critical analysis were conducted by reviewing quantitative data published in the scientific literature.
The keywords that were used to search the scientific literature systematically were:
- ‘Dry eye disease’ OR ‘keratoconjunctivitis sicca’ ‘Keratitis sicca’ combined with ‘diet’ OR ‘supplement’ OR ‘therapeutic’ treatment of dry eye disease
- ‘Omega’ ‘Omega-3’
- ‘Fatty acids’ OR ‘Omega-3’ ‘eicosatetraenoic acid (EPA)’ ‘docosahexaenoic acid (DHA)’ ‘(n-3)’ ‘(PUFAs)’ ‘(n-6)’ ‘(DHA)’ ‘(EPA)’
Table 3: Oral supplement composition from six studies that are included in the systematic review
The electronic platforms EBSCOhost were used to search the scientific literature. The databases included were Embase, Medline and Cochrane library. Google Scholar and the City University library were visited to attain the full text if not available through the platforms.
Inclusion criteria were randomised controlled trials (RCTs), publications from the year 2000 onwards, human studies only, and participants with dry eye disease (DED) irrespective of aetiology, gender, or age. To address the primary aim of this systematic review, included studies that investigate the effect of omega-3 supplementation in reducing the DED symptoms with any placebo drug, and conducted at least one of these tests: non-invasive TBUT, invasive TBUT, and ocular staining.
Exclusion criteria involved animal studies, any other languages except English, and any other intervention except the omega-3 oral supplementation.
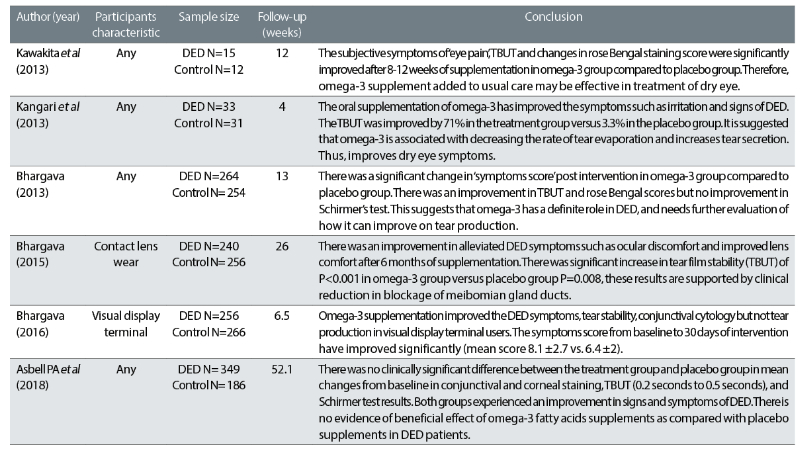
The key characteristics of the six papers included in the review are summarised in table 4.
Table 4: The characteristics of six literature papers that were included in the review27-32
Each of these studies were then assessed for risk of bias and quality of data.
Five studies (83%) have a low risk of bias in selection bias, as they utilised adequate methods of sequence generation to allocate the patients in the study. However, Kangari et al did not provide a clear method of allocation, despite being a ‘randomised controlled’ study.
In allocation concealment, five studies have a low risk of bias whereby investigators and patients were prevented from the knowledge of intervention allocation before patients were assigned. One study29 used a sequentially numbered sealed envelope, and the other two studies30,31 used secured randomisation codes in a sealed obscure envelope. In another, two studies8, 32 had a third party coded and labelled the supplements. The remaining study27 did not provide sufficient detail to make a judgement of this bias and performance bias while the other five studies had a low risk of bias for performance bias as they described methods of masking patients and the investigator.
The highest risk of bias for three out of six studies (50%) was assessed to be due to attrition bias, patients were randomised but were lost in follow up and some dropped out due to various issues. Then, high selective bias was assessed in the longitudinal study32 and it did not report all their pre-specified outcomes measure in the final published paper. The remaining studies (except Kangari et al, 2013) have unknown risk of bias because of unclear and insufficient detail in the paper.
Lastly, two studies27, 32 were assessed to have high risk of other bias, as the studies were funded, materials were supplied by industry and the author had a vested interests with industry that was relevant to intervention. One study29 was assessed as having an unknown risk of bias due to insufficient details in the paper.
Discussion and Conclusion
A systematic review of six RCTs from four different countries, involving 2162 patients with specific and non-specific DED characteristics, was conducted. The number of participants ranged from 27 to 535 in individual trials. Study follow-up periods ranged from one month to 12 months.
Most of the studies found that omega-3 fatty acid supplementation improved the symptoms of DED, although results were variable across studies. RCTs and double-blinding studies were reviewed because they are the best way to evaluate any intervention in which the subject’s or investigator’s expectations might affect data collection.
The evidence from the reviewed studies confirms a correlation between omega-3 and a reduction of DED symptoms and signs. This was observed in both, overall population and distinct sub- population of environment and diseases-induced patients. The strength of the correlation is difficult to measure considering the wide range of dry eye conditions and discrepancy of clinical endpoints. However, all six studies reviewed show a statistical and clinically significant health gain to be derived from omega-3 supplementation.
“The evidence from the reviewed studies confirms a correlation between omega-3 and a reduction of DED symptoms and signs.”
One study reported that subjective symptoms were improved such as ‘eye pain’ using a Visual Analog scale and objective measurements: TBUT and rose Bengal staining scores in the omega-3 group were significantly improved after eight to 12 weeks of supplementation compared to those of the placebo group. Hence, this suggests that fish oil supplementation may be effective in treating DED by enhancing tear stability and improving mucin disorder. A similar subjective result of a reduction in symptoms such as comfort and eye pain were reported in the other three studies, but a different scoring scale (Dry Eye Severity Score) was used in these studies.
Although the Schirmer test shows no significant difference between the two groups, quantitative data was not provided in the study’s findings. However, it reported that TBUT was improved (two seconds to four seconds pre- and post-intervention, respectively). This indicates that tear stability was improved but tear volume was not. Such findings were also reported by the other three studies.
Furthermore, the improvement of TBUT was observed in the omega-3 group after the washout period of four weeks, suggesting the omega-3 treatment continues to affect the tear film stability and maintains its effect. Moreover, side effects of omega-3 and patient drop-outs due to intolerance to omega-3, such as gastrointestinal issues were not observed in this study, implying that omega-3 is safe and easy to consume. This was in contrast with other studies, where dropouts of patients due to intolerance to omega-3 were as high as 19%.
One study27 also reported how symptoms of the placebo group were also improved but the omega-3 supplementation accelerated the recovery time of DED symptoms compared to the placebo group.
While the TBUT and rose Bengal staining showed statistically significant improvement, they are not clinically significant as the TBUT is <5 seconds, which indicates the presence of dry eye symptoms that correspond to the subjective measurement of dry sensation score between both groups; so no significant difference was found. There is also a high risk of ‘attrition bias’, as one patient dropped out and final data was calculated with 26 patients, but it is not clear which intervention participants were assigned to. In addition, the patients were using artificial tear drops alongside the intervention, which may have impacted the tear film stability and osmolarity, which could have improved on the results of TBUT and rose Bengal staining. Therefore, the findings from the study may not be reliable and not clinically significant to suggest definitive improvement in DED symptoms by using omega-3 supplementation as a standalone treatment.
On the other hand, another study,28 which investigated the efficacy of omega-3 as a standalone treatment, has found an improvement in the reduction of DED symptoms and signs. The subjective measurement of the ocular surface disease index symptoms questionnaire (OSDI) reported a 26% reduction in DED symptoms from baseline to fourth week, compared to the placebo group with a 4% increase (P<0.001). While we were expecting to see a placebo effect in the control group, the DED symptoms deteriorated by 4%. This denotes that failure to receive the proper treatment surpasses the placebo effect.
In the same study,28 the mean TBUT in the placebo group from baseline to intervention was 4.7 (±2.6 seconds), which was not significantly different from the baseline value (P=0.353). The increase in mean TBUT was observed in the treatment group by 1.7 seconds, this change was statically significant.
The overall outcome suggests a 71% increase in TBUT in the treatment group compared to 3.3% in the placebo group. The intergroup difference was statically significant (P=0.004). The study28 found a positive correlation between the use of omega-3 supplementation for one month in reducing the DED signs and symptoms. The limitation of this study is that the diet of the patients was not controlled and it was a short duration study to establish a correlation between DED and omega-3. Also, a high risk of ‘attrition bias’ was found as a total of nine patients failed to complete the study; three had digestive problems, two were advised by the doctor to discontinue the supplementation and four were lost in follow-up. It is not clear how the data were calculated after they dropped out.
Similarly, one study32 reported 5% to 19% (66 out of 349) patients in the treatment group had gastrointestinal disorders and, in another study,31 22 out of 256 patients had gastric, digestive problems, were not compliant and not taking omega-3 regularly due to bad breath and an unpleasant fishy smell.
Poor quality omega-3 may reduce patient compliance with taking a supplement. Therefore, this marks the importance of high-quality extraction and purification methods to be adapted by pharmaceutical manufacturers. It also highlights the fact that eye care practitioners should have knowledge of the quality of omega-3 taken to make a beneficial recommendation to dry eye patients.
Three of the studies30-33 found an improvement in DED signs and symptoms. They assessed dry eye symptoms using a subjective Dry Eye Questionnaire and Scoring System (DESS). The level of heterogeneity was high and, in each of these studies, participants assigned to the omega-3 treatment had significantly lower symptom scores at the study endpoint than participants assigned to placebo. In these trials, the daily dose of combined EPA and DHA ranged from 1,200mg to 2,400mg. One study29 found 5% of symptomatic patients were asymptomatic and 35% had moderate improvement in the omega-3 group by three months and in the placebo group 33% of symptomatic patients were asymptomatic and 65% had moderate improvement. Thus, the placebo effect is apparent in this study, which was not seen in a previous study.27
One study28 reported the mean change in Schirmer score (mm/5 minutes) at three months of follow-up, indicating that omega-3 supplementation was found to improve tear production (mean ±SD: 0.62 ±1.06, P<0.001). While another30 reported a higher Schirmer test score, diagnosing the omega-3 individuals with contact lens-associated dry eye at six months of follow-up (2.21 to 2.99 mm/5minutes). Therefore, both studies have found the positive impact of omega-3 supplements in improving the volume of tears, hence, aiding27-31 in lubrication of the ocular surface.
Though, all five studies have used Schirmer’s test as one of the diagnostic tests to investigate their hypothesis. In accordance with DED diagnostic test battery recommended by TFOS DEWS2, the Schirmer’s test is no longer used as a diagnostic test battery of DED, which was used as a routine diagnostic test for tear volume formerly, due to a lack of high-level evidence data on repeatability, sensitivity and specificity of this test.
Therefore, this may suggest that Schirmer’s results from all these studies may not be as reliable for this investigation. Furthermore, each study has proved the significant difference in invasive TBUT between the treatment and placebo groups by comparing pre- and post-intervention.
However, most of the differences are by 1.5 to 2.5 seconds and TBUT remains <10 seconds, which is not a clinically significant value. This measurement is against the normal values of TBUT which is >10 seconds in accordance with TFOS DEWS2.5
One study32 contradicts the other five RCTs study and reported no evidence of a difference among patients with DED, instead finding that those who were randomly assigned to 3,000mg of omega-3 for 12 months did not have significantly better improvement than the placebo group. The evidence shows no change in corneal fluorescein staining score from baseline (0.1point; 95% Cl, -0.2 to 0.4), TBUT (0.2 seconds; 95% Cl, -0.1 to 0.5), and result on Schirmer’s test (0.0mm; 95% Cl, -0.8-0.9). However, subjective measurement of mean OSDI scores showed an improvement in symptoms from baseline and 12 months between omega-3 (-13.9±15.6) and placebo group 12.5±18.2 (P<0.001 for change in each group). Yet, no difference was seen between the two groups in four key signs of DED: conjunctival staining score, corneal staining score, TBUT and Schirmer’s test.
This study has used fundamental aspects of the design and conduct of the DREAM (Dry Eye Assessment and Management) trial to contribute to the validity and generalisability of the results. This trial was a ‘real world’ clinical trial, which includes patients with moderate-severe dry eye and used their current treatment of DED. The advantage of a 12-month follow-up is that it reduces the effect of seasonal factors, which may exacerbate the signs and symptoms of DED. The disadvantage of the longitudinal study design is that patient compliance reduces over time, which was evident as 47 patients in total were lost to follow-up.
Although the study, stated that it was performed in accordance standard of the DREAM trial, it has a high risk of ‘attrition bias’ and it is evident in the study as 36 out of 535 patients’ results were not included in the final analysis. The mean OSDI scores from six months to 12 months were missing from the outcomes. Other limitations of this study includes other bias and selective reporting bias, as prespecified clinical outcomes were not included in the published paper such as ocular redness, cytokine level, and frequency of artificial tears used.
The study that used the DREAM trial, which is the largest trial on this matter, has argued that the study32 designs used in other five RCTs studies were not applicable to the real world, as a variety of DED patients are needed to be included in the trial. Also, it argued that the RCTs28 that excluded the use of other eye medications had different results.
However, the results of the DREAM trial can only be construed as there being no benefits in moderate to severe DED from omega-3 treatment. The real effect of omega-3 on DED should be proven objectively by pure data and without other eye medications. Yet, for example, Kawakita et al,27 reported significant improvements in both intervention and placebo groups alongside the use of eye drops. In Asbell et al, about 38% of patients used the cyclosporine drop and 50% used other treatments, suggesting the results may have been affected by other treatments rather than omega-3 intervention.32
Collectively, in all six studies, the total daily dose (table 3) of combined EPA and DHA ranged from 600mg to 3,000mg and a range (240mg to 1,000mg) of different comparators was adopted for both the placebo (corn oil, olive oil and unspecified – middle chain triglycerides edible oil). The recommendations from the American Dietetic Association and the International Society for the study of Fatty Acids reported a daily intake of 500mg of EPA and DHA. While the FDA remains quiet on a minimum dosage of eye and vision health, it has been established by Generally Regarded As Safe values (GRAS) that 3,000mg of EPA and DHA per day is safe for consumption.33 Some eye practitioners have prescribed total dosages of 200mg to 3,000mg per day.33 This may explain the reason for gastric intolerance and the increase in drop-out rate in the RCTs studies.
The subjective improvement was measured using different scoring systems in all the studies: OSDI, DESS, and VAS scores. Hence, making it difficult and unreliable to compare the results among the studies. The diagnostic tests used to test objectively were different in most of the studies, which creates subtle differences among the studies. The only two diagnostic tests that were used in all studies were TBUT and Schirmer’s test (which is not used anymore).
Furthermore, any comparison between studies should be made carefully due to differences in the features of study samples such as age, gender, the characteristic of DED and the epidemiology of studies. Also, each study included in this systematic review had some degree of bias, which confirms that, in the future, a well designed study or meta-analysis is needed to establish strong scientific evidence of this matter.
This systemic review focused solely on omega-3 supplements and could, instead, have reviewed other available supplements (such as omega-6 and 9). Other limitations included limited access to papers, it only viewed papers in English language, only six RCTs studies were reviewed, and was only concerned with RCTs (not many RCTs are available on omega-3 specifically). Therefore, the future implication is that more RCTs should be conducted to investigate and explore the effects of duration of treatment and determine the optimal dosage for omega-3 treatment for different patients with DED. And I recommend the use of thorough and validated diagnostic assessment instruments or tests in future studies, which should recruit a homogenous sample of patients.
The pooled finding on the efficacy of omega-3 for DED treatment is consistent in reducing DED signs and symptoms except for one study.32 The data included in the review appears to be associated with better TBUT, corneal, and conjunctival staining scores (parameters of tear functions) and OSDI (DED symptoms such as comfort were improved). In Schirmer’s test (parameter of tear production) a small improvement was observed. The impact of reducing symptoms will improve the quality of life and improve work capacity. Therefore, the clinical implications are that the eye care practitioner should recommend the use of omega-3 supplementation to their dry eye patients. From a patient perspective, it is very difficult to choose a suitable omega-3 supplement with the correct dosage, due to the overwhelming availability of omega-3 supplements in the UK market.
It is the duty of the eye care practitioner to educate patients on suitable omega-3 with the correct dosage of EPA and DHA per day. Therefore, eye care practitioners should explore and have sufficient knowledge of the effects of duration of treatment and determine the optimal dosage (EPA and DHA per day) of omega-3 treatment and the effects of purification methods on the quality of omega 3 because the poor quality may impact patients’ compliance, tolerance and inconsistency of taking the supplement.
- This is an edited version of the 2022 City University/Optician Best Dissertation prize-winner written by the author as a final year undergraduate.
- The author would like to thank Dr Byki Huntjens for her guidance and help.
References
- Craig, J.P et al. 2017. TFOS DEWS II definition and classification report. The Ocular Surface, 15(3), pp.276-283.
- Behrens, A. et al. 2006. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea, 25(8), pp.900-907
- Waduthantri, S et al. 2012. Cost of dry eye treatment in an Asian clinic setting. PloS one, 7(6), p.e37711
- Stapleton, F. et al. 2017. Tfos dews ii epidemiology report. The Ocular Surface, 15(3), pp.334-365
- Wolffsohn, J.S. et al 2017. TFOS DEWS II diagnostic methodology report. The Ocular Surface, 15(3), pp.539-574
- Jones, L et al. 2017. TFOS DEWS II management and therapy report. The Ocular Surface, 15(3), pp.575-628
- Kumar, P et al. 2014. The correlation of routine tear function tests and conjunctival impression cytology in dry eye syndrome. Korean Journal of Ophthalmology, 28(2), pp.122-129
- Welder, J.D et al 2011. Limbitis secondary to autologous serum eye drops in a patient with atopic keratoconjunctivitis. Case Reports in Ophthalmological Medicine
- Parikh, N.B., Francis, J.H. and Latkany, R.A., 2010. Retention rate of silicone punctal plugs placed by residents in a general clinic setting. Ophthalmic Plastic & Reconstructive Surgery, 26(6), pp.400-402
- Milner, M.S et al. 2017. Dysfunctional tear syndrome: dry eye disease and associated tear film disorders–new strategies for diagnosis and treatment. Current Opinion in Ophthalmology, 28, (Suppl1), p3
- Alanazi, S.A et al. 2019. Effects of short-term oral vitamin A supplementation on the ocular tear film in patients with dry eye. Clinical Ophthalmology (Auckland, NZ), 13, p.599
- Miljanovic, B et al. 2005. Relation between dietary omega− 3 and omega− 6 fatty acids and clinically diagnosed dry eye syndrome in women. The American Journal of Clinical Nutrition, 82(4), pp.887-893
- Wojtowicz, J.C et al. 2011. Pilot, prospective, randomized, double-masked, placebo-controlled clinical trial of an omega-3 supplement for dry eye. Cornea, 30(3), pp.308-314
- Kaya, A. and Aksoy, Y. 2016. Omega-3 fatty acid supplementation improves dry eye symptoms in patients with glaucoma: results of a prospective multicenter study. Clinical Ophthalmology, 10, pp.911-912
- Liu, A. and Ji, J. 2014. Omega-3 essential fatty acids therapy for dry eye syndrome: a meta- analysis of randomized controlled studies. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research, 20, p.1583
- Molina-Leyva, I et al. 2017. Efficacy of nutritional supplementation with omega-3 and omega-6 fatty acids in dry eye syndrome: a systematic review of randomized clinical trials. Acta Ophthalmologica, 95(8), pp.e677-e685
- Amparo, F et al. 2013. Topical interleukin 1 receptor antagonist for treatment of dry eye disease: a randomized clinical trial. JAMA Ophthalmology, 131(6), pp.715-723
- McCusker, MM et al. 2016. An eye on nutrition: The role of vitamins, essential fatty acids, and antioxidants in age-related macular degeneration, dry eye syndrome, and cataract. Clinics In Dermatology, 34(2), pp.276-285
- Funk, C.D. 2001. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science, 94(5548), pp.1871-1875
- Bosch, A.C. et al. 2016. Heavy metals in marine fish meat and consumer health: a review. Journal of the Science of Food and Agriculture, 96(1), pp.32-48
- Hark, L., Ashton, K., Deen, D. (editors). 2012. The Nurse Practitioner’s Guide to Nutrition. John Wiley & Sons
- Brenna, J.T. 2002. Efficiency of conversion of α-linolenic acid to long chain n-3 fatty acids in man. Current Opinion in Clinical Nutrition & Metabolic Care, 5(2), pp.127-132
- Adeoti, I.A. and Hawboldt, K. 2014. A review of lipid extraction from fish processing by-product for use as a biofuel. Biomass and Bioenergy, 63, pp.330-340
- Vaisali, C. et al. 2015. Refining of edible oils: a critical appraisal of current and potential technologies. International Journal of Food Science & Technology, 50(1), pp.13-23
- Dillon, J.T. et al. 2013. Purification of omega-3 polyunsaturated fatty acids from fish oil using silver-thiolate chromatographic material and high-performance liquid chromatography. Journal of Chromatography A, 1312, pp.18-25
- Bonilla-Méndez, J.R. et al. 2018. Methods of extraction refining and concentration of fish oil as a source of omega-3 fatty acids. Ciencia y Tecnología Agropecuaria, 19(3), pp.645-668
- Kawakita, T. et al. 2013. Effects of dietary supplementation with fish oil on dry eye syndrome subjects: randomized controlled trial. Biomedical Research, 34(5), pp.215-220
- Kangari, H. et al. 2013. Short-term consumption of oral omega-3 and dry eye syndrome. Ophthalmology, 120(11), pp.2191-2196
- Bhargava, R. et al. 2013. A randomized controlled trial of omega-3 fatty acids in dry eye syndrome. International Journal of Ophthalmology, 6(6), p.811
- Bhargava, R. and Kumar, P. 2015. Oral omega-3 fatty acid treatment for dry eye in contact lens wearers. Cornea, 34(4), pp.413-420
- Bhargava, R. et al. 2016. Short-term omega 3 fatty acids treatment for dry eye in young and middle-aged visual display terminal users. Eye & Contact Lens: Science & Clinical Practice, 42(4), pp.231-236
- Asbell, P.A. et al. 2018. n− 3 Fatty acid supplementation for the treatment of dry eye disease. New England Journal of Medicine, 378(18), pp.1681-1690
- Mendoza, R.L. 2018. Clinical trials with multiple endpoints can establish a correlation, but not (yet) causality, between dietary supplementation with omega-3 fatty acids and keratoconjunctivitis sicca. Journal of Medical Economics, 21(8), pp.733-744
- Parolini, C. 2019. Effects of fish n-3 PUFAs on intestinal microbiota and immune system. Marine Drugs, 17(6), p.374
