Many of us are, these days, advocates for dry eye disease management in practice. But how many of us actively consider the ocular microbiome (bacteria, viruses and fungi) in our ocular surface evaluation?
What Is the Microbiome?
A healthy body acts as host to a vast variety of microbes colonising it both externally and internally. These microorganisms, and their genetic material, are collectively known as the human microbiome. The organisms themselves are known as microbiota.1 The microbes gain a habitat and nourishment from the host and, in turn, recent research is establishing that these microbes help the host by regulating various host physiological functions. These include dietary digestion and protective immunity against pathogens.2 In short, humans require commensal microorganisms for vital functions.3
The microbiome of healthy individual consists of the following categories of microbiota:
- Bacteria
- Viruses
- Fungi
These all play a symbiotic role in maintaining homeostasis.3
Bacteria represent the most studied, abundant and diverse group, accounting for 1,014 microbes,3, 4 outnumbering human cells by a ratio of ten to one and contributing more essential genes for survival than human cells. Whereas the human genome carries only approximately 20,000 genes, the microbiome contributes approximately eight million genes.1
The various mucosal regions (including the gastrointestinal tract) within the human body also have characteristic microbial profiles and the make-up of these is influenced by a variety of factors, ranging from pH and oxygen exposure to diet and antibiotic exposure.3
The number of bacteria at each mucosal site varies significantly. Some mucosal sites, like the gastrointestinal (GI) tract, have a bacterial genome that is more than 100 times the size of the human genome, while other surfaces are comparatively lacking in bacteria and described as paucibacterial. The majority of microbiota reside in the GI tract, which also accounts for almost 70% of the immune system in total. For a review of the impact of the gut microbiome upon eye diseases such as AMD, see Optician 25.09.20.
An example of a paucibacterial surface is the ocular surface.
The Ocular Microbiome
Studies of the ocular microbiome show that there are approximately 0.06 bacteria per human cell in the conjunctiva, compared with approximately 12 bacteria per human cell in the buccal mucosa (in the mouth) and approximately 16 bacteria per human cell in the skin adjacent to the mouth, each with markedly different varieties of constituent microbiota.5, 6 Despite the overall low number of bacteria on the ocular surface, studies have demonstrated the existence of an ocular surface microbiome in healthy individuals, which is composed of a specific community of bacteria.3
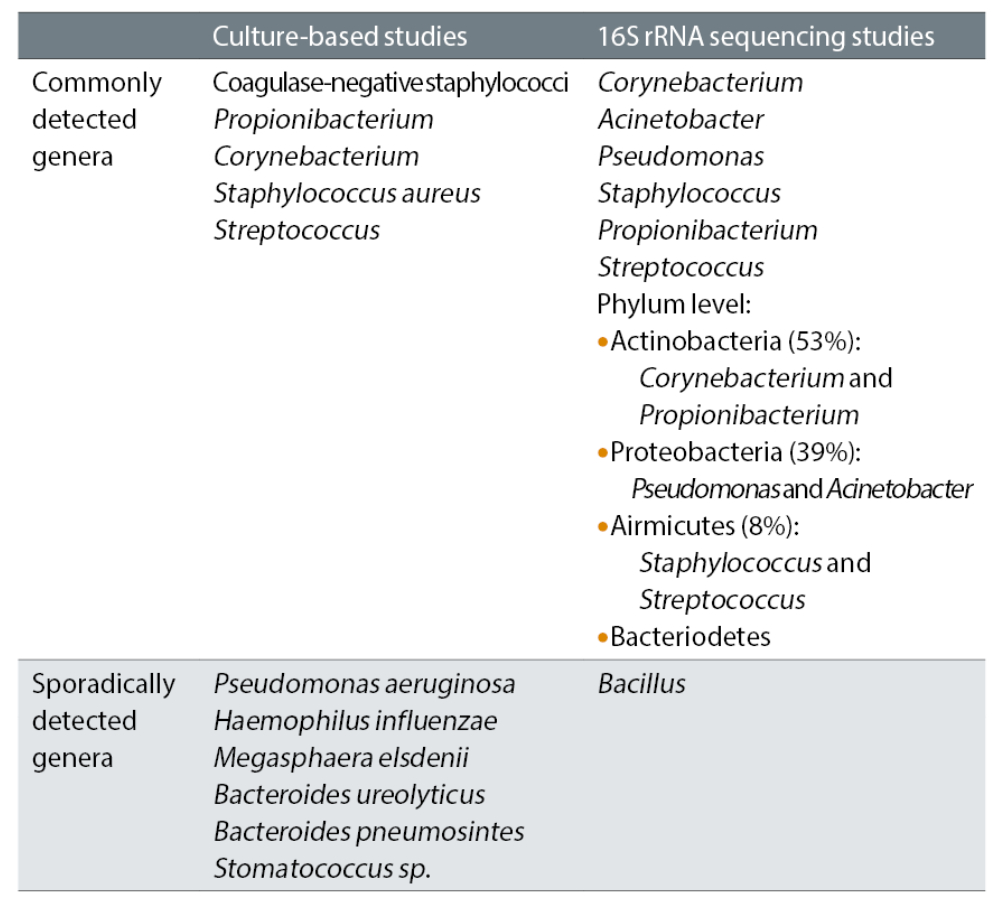
Our current knowledge of the human microbiome has arisen through complex analyses carried out with genome technology, such as to culture-based and 16S ribosomal ribonucleic acid (rRNA) sequencing studies.7 A summary of the typical ocular microbiome as found by such techniques is shown in table 1.
Table 1: Distribution of the typical microbiome of the ocular surface, according to culture-based and 16s ribosomal ribonucleic acid (rRNA) sequencing studies7
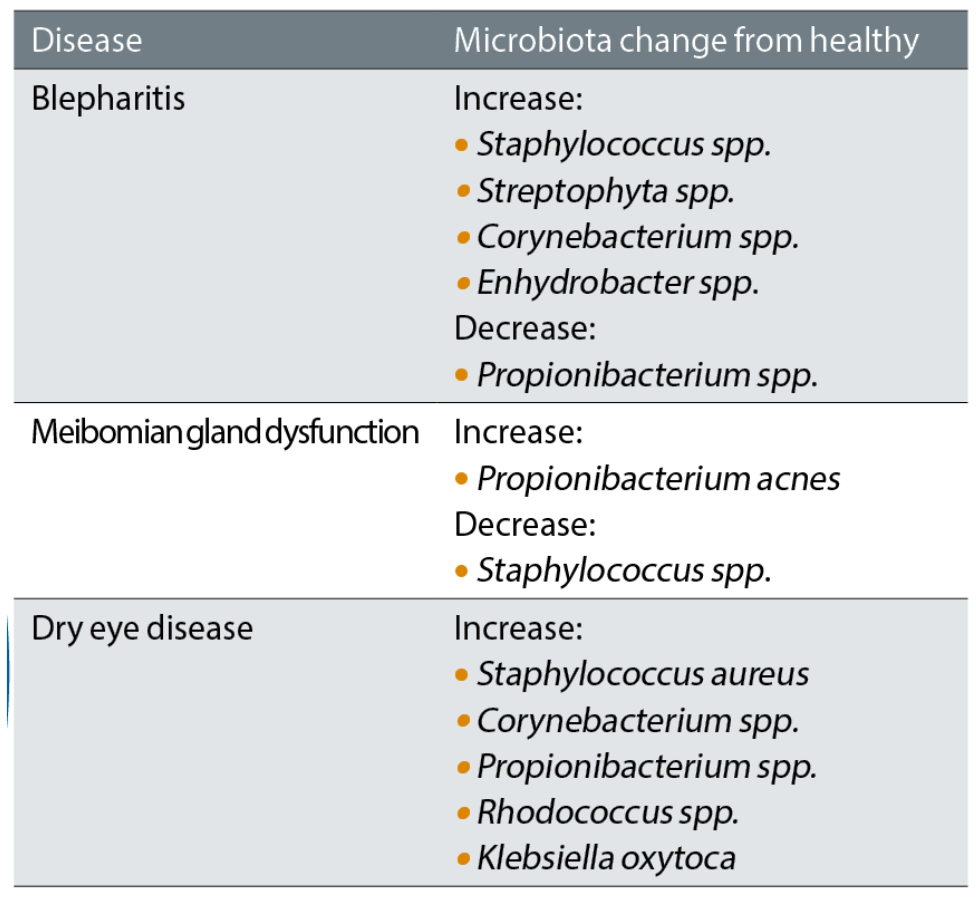
There is an increasing evidence base indicating the relevance and importance of the role a healthy ocular microbiome has on maintaining a healthy, homeostatic balance for the ocular surface and adnexa. The immune system has a role in maintaining the balance of resident microbial communities while, at the same time, these bacteria also shape the immune system. With certain diseases of the ocular surface and adnexa, studies have shown alterations in the make-up of the microbiome.7 Some of these are summarised in table 2.
A better understanding of the disruption of the microbiome in specific disease processes may hold the key to better diagnosis and also management of some common ocular surface and adnexal disease. It is also important to not overlook the skin surrounding the area, nose and cheeks for other signs of irregularities, dermatitis or telangiectatic vessels, which can give clues to other more holistic issues such as dermatitis or acne rosacea.
Table 2: Distribution of ocular microbiome in selected diseases7
A Holistic View of Dry Eye Disease
Studies have shown in dry eye disease the microbiome is usually very similar in diversity, but the relative abundance of each type differ, so changing the dominant flora and relative abundance of the local microbial environment.8 Many types of bacteria live on our lids, and when you have an imbalance of bacteria, the body may react to the proteins and the toxins that some of these bacteria produce. Research has also found increases in certain isolates in the microbiome, such as Staphylococcus and Corynebacterium spp.9
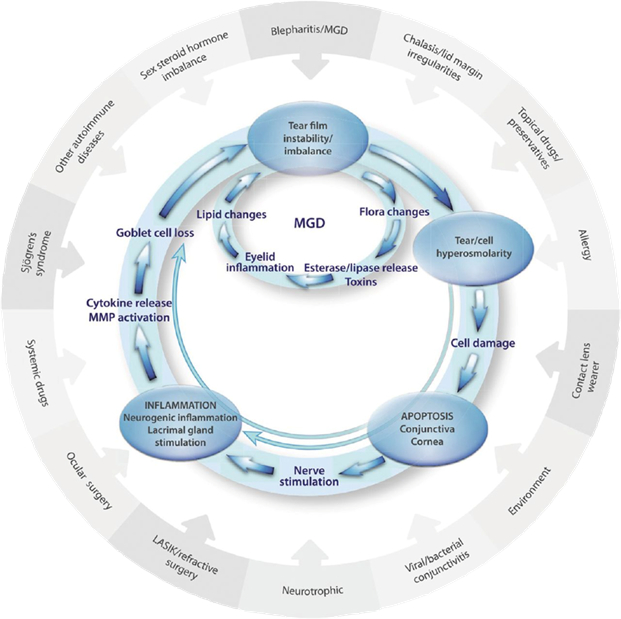
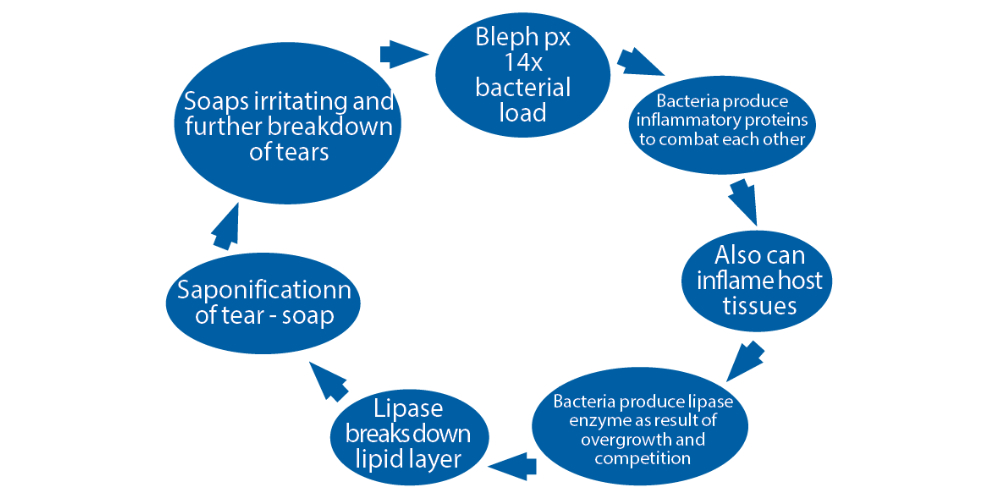
The pathogenesis of dry eye disease was elegantly summarised by Badouin et al10 and is represented in figure 1. In this schematic representation, the outer circle indicates all of the contributory factors that can exacerbate or cause dry eye disease. The inner two circles illustrate the vicious cycle established on the ocular surface in the diseased state. We can see in the innermost cycle, the one associated with meibomian gland dysfunction (MGD), that flora (microbiota) changes lie at the very heart of the MGD cycle.
Figure 1: A proposed vicious circle of the pathology of dry eye disease10
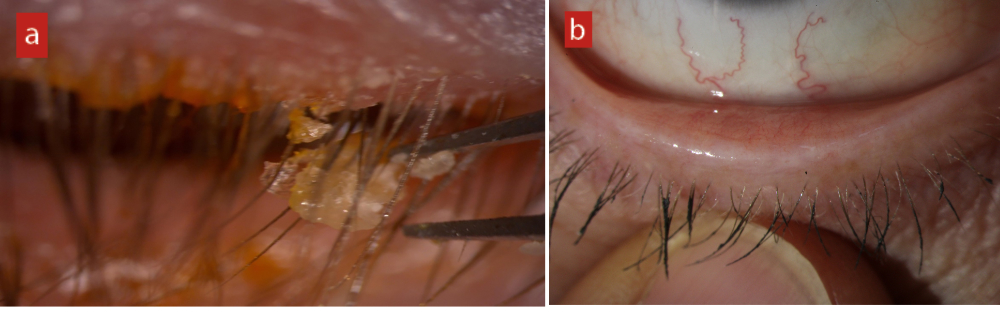
The lids are a perfect home for bacteria, with warm, moist, nutrient rich habitation in abundance. Blepharitis, whether frank and obvious or much more subtle and lacking any obvious crusts or collarettes (figure 2), results in overgrowth of normal skin bacteria and the resultant biofilm increases the level of inflammatory proteins and cytokines, triggering the classic inflammatory cascade.
The biofilm is, in essence, a slimy, sticky film of bacteria that coats the surface as a highly organised community. The bacteria in the community secrete a glycocalyx (in effect, a sugary coating), which allows them to stick to each other and influence each other’s behaviour. It is also a protective armour that is hard to penetrate.11 Eventually, the bacterial community achieves quorum-sensing; a type of communication that causes gene activation and releases virulence factors, such as the enzymes lipases and proteases.
It is also interesting to understand that not all strains of a species, such as Staphylococcus aureus (S.aureus), often found in blepharitis cases, are identical. Some strains of S.aureus are better designed to create a biofilm, one which matures rapidly and, in so doing, releases inflammatory toxins. These strains are more likely to be present in patients who get severe blepharitis and chalazia as a child. Other strains, however, may produce much slower-growing biofilms, ones that release less active virulence factors over a person’s whole lifetime and unlikely to lead to significant blepharitis at all. It all depends on the strain of S. aureus.11
Figure 2: (a) Frank and obvious anterior blepharitis. (b) Low grade, subtle anterior blepharitis
This interesting finding offers a possible explanation as to why, in clinic, we may see cases where the lid margins are riddled with blepharitis and yet the patient is completely asymptomatic and vice versa. However, another explanation might be a variation in the neuropathic response to dry eye; the balance between the sensitivity of the nerve endings to stimuli and the down-regulation of the nerves.
Saponification
One sign indicative of the interaction of bacteria with the ocular surface as part of a disease process is saponification. A key feature believed by this author to be a hallmark and clinically measurable sign of change in the local microbiome is the presence of froth, foam or bubbles. In the tear film, this is known as saponification. Some patients even attend the clinic aware of a white discharge which often, in my experience, appears to accumulate adjacent to the outer canthus along the lower lid margin (figure 3).
Figure 3: Classic appearance of a localised band of froth on the lower lid margin indicating saponification
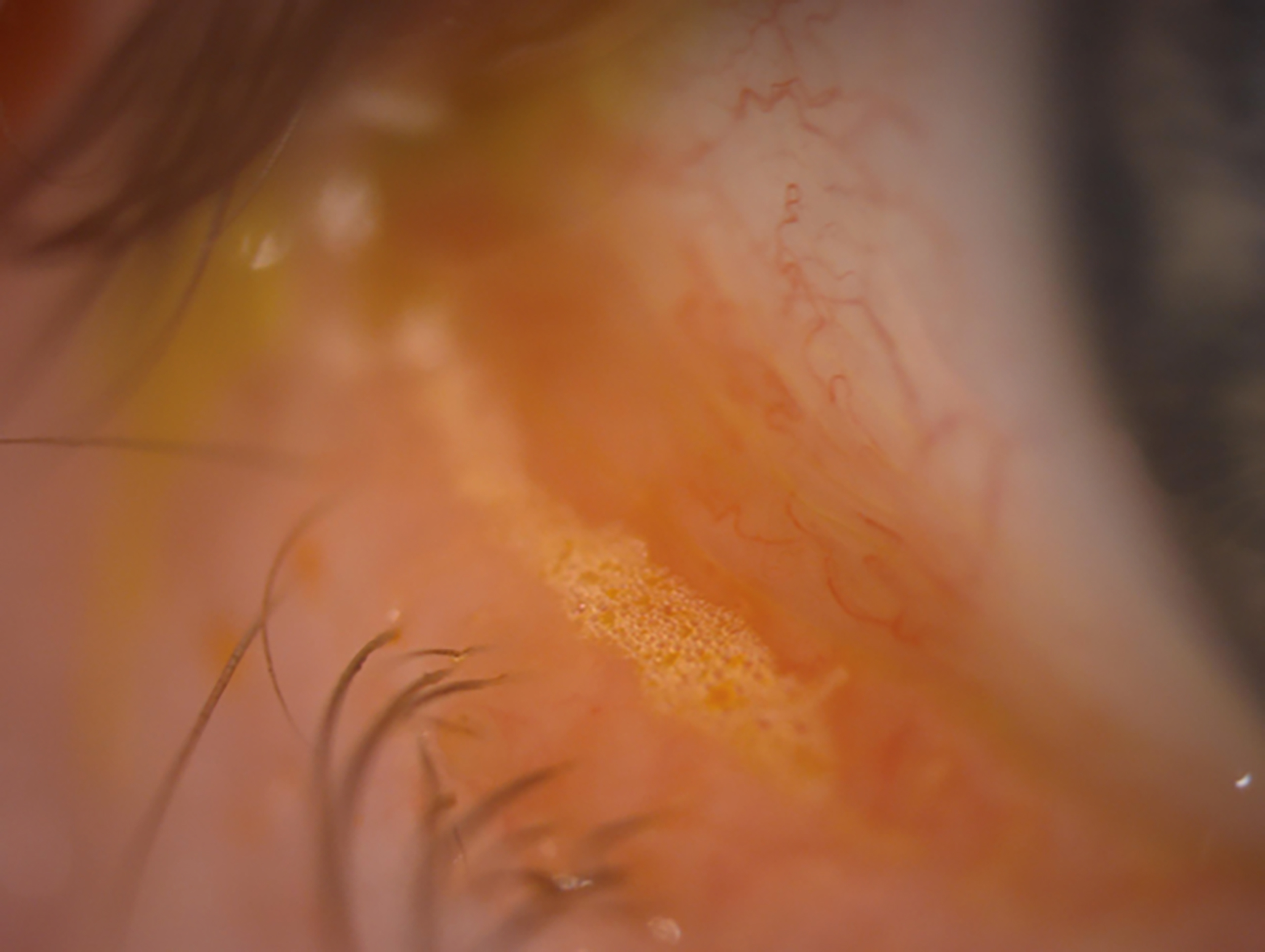
Bacteria grow best when there is an abundant food source. The saturated oils that result from MGD are a great food source and, with a warm moist environment, bacteria easily over-colonise the lid margin. These bacterial colonies secrete exotoxins to kill off their neighbours and these secretions can act as powerful inflammatory triggers. Bacteria also secrete enzymes, which help them to pre-digest their food and to better penetrate into host tissue to extend infection. Staphylococcus spp. are well known for their ability to secrete the enzyme lipase, which breaks down lipids. Saponification is the chemical process by which normal meibum lipids are broken down by bacterial enzymes into a surfactant when mixed with alkaline tears. This surfactant (or soap) has the effect on the ocular surface of further degrading the lipid layer and usually results in a burning and stinging sensation in the eye; quite literally, soap in the eye.
Figure 4 shows just how integral bacteria are in the vicious cycle of loss of homeostasis that occurs over time with blepharitis and MGD. This cycle can result in a bacterial load increase in blepharitis of some 14 times.12
Figure 4: The vicious circle of blepharitis, showing the influence of increased bacterial load and saponification
Managing the Microbiome
Total eradication of the offending microbes in blepharitis and MGD is simply not possible, but neither would it be the desired tactic. Allowing the body to redefine its healthy microbiome balance, by attempting to reset the system, is the fundamental role of any specific targeted therapy for microbiome imbalance in dry eye disease management. As with the gastrointestinal microbiome, regaining a synergistic balance that is key to successful management and this is often the way I explain any therapeutic strategy to the patients.
For many years, it has been widely understood that the various methods of lid hygiene, both in practice and at home, have a key role in reducing the microbial load on the lid margins, as well as physically removing crusts and debris. Reducing the food source as well as debulking the biofilm and bioburden plays a key part in why lid hygiene is so important and explains the effectiveness of lid treatments if complied with. A comprehensive review of the management and therapy of contributors to dry eye disease was published as part of the TFOS DEWS II project.13
In-Practice Treatments
Physical debridement
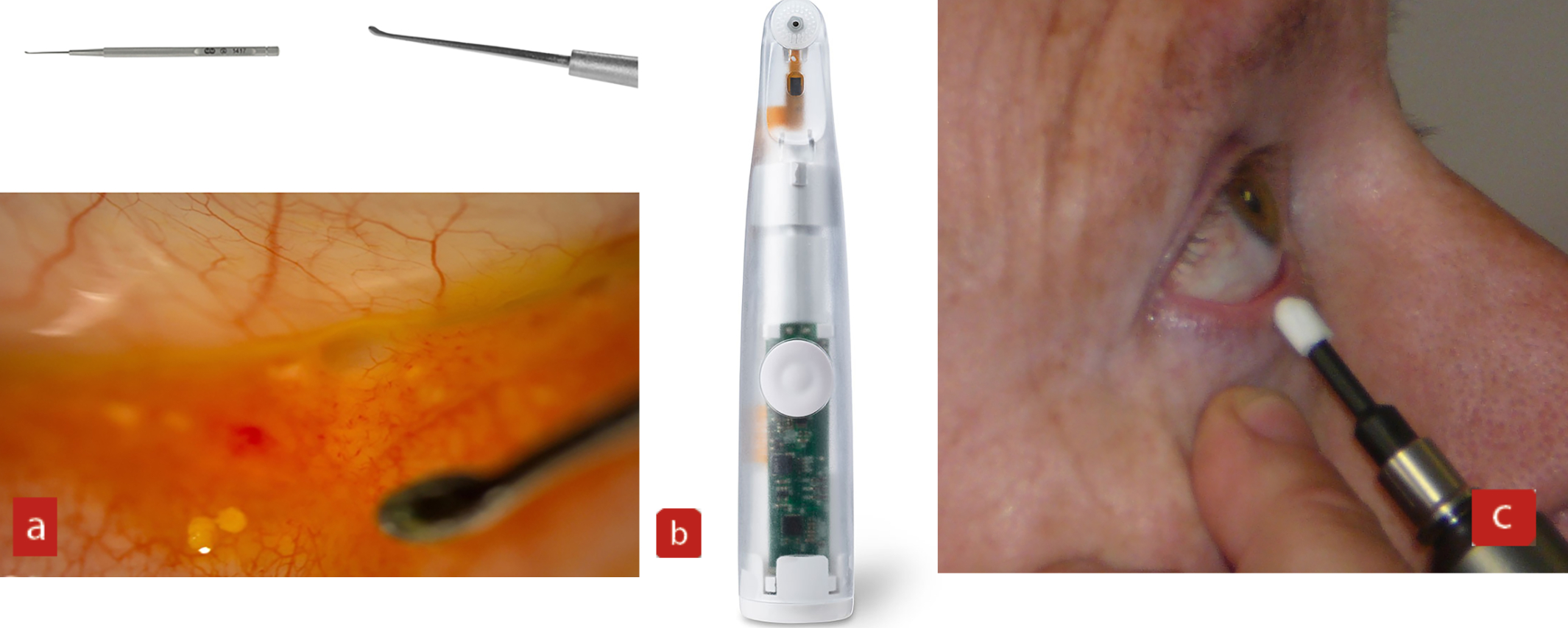
In-practice treatments, such as physical debridement (figure 5), whether with a golf club spud, micro-blepharo-exfoliation with Blephex or nulids, is a quick way of reducing bacteria, biofilm, mites and debris. This is a proven method for effective debulking, which often has a very visible and long-term effect in reducing inflammation around the lid margins, as well as removing visible crusting, collarettes and debris.
Figure 5: Debulking of the lid margin through debridement using; (a) A golf club spud (from specialisedophthalmicservices.com); (b) nulids; (c) Blephex. Each may be used to debride the lid margin, to remove any physical debris that could be obstructing the gland orifices, and for debulking the biofilm adherent to the lid margin itself
Lid cleansing measures at home wipe away bacteria and deposits from lid margins and lead to improved signs and symptoms in the majority of individuals.14 At-home therapies which have a direct role in microbiome management clearly include any method of regular lid hygiene, which I will not discuss in detail here as there are many options which are all commonly understood.13 An interesting point in the campaign to avoid baby shampoo as this is another surfactant, which will also destabilise the tears (just like the surfactant property of saponification).14
Hypochlorous Acid Spray
We also have the option to recommend the use at home of hypochlorous acid spray on to the periorbital skin (figure 6). Hypochlorous acid (HOCl) is a useful weapon to fight germs. It is even effective against pathogens like methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa. This powerful weapon is safe, chemical free and non-toxic. HOCl is a weak acid that occurs naturally in our body. Neutrophils are white blood cells that are the first to arrive on site when an invading pathogen is detected. Neutrophils chase down and engulf the pathogen through phagocytosis. Upon contact, neutrophils release a burst of bactericidal chemicals including a powerful oxidizing agent; HOCl. This kills the pathogen by destroying cell membranes and proteins.15 Interestingly, before antibiotics became widely available, HOCl was used to irrigate and disinfect wounds in World War I. HOCl has been found to be effective against epidemic keratoconjunctivitis (EKC) in one case study but was not shown to be effective against Demodex.16
Figure 6: Hypochlorous acid is available as a spray for application upon the periorbital skin (image courtesy of Purifeyes)

Low Level Light Therapy (LLLT)
Near infrared low level light therapy (LLLT) is a method of photobiomodulation originally developed for dermatological uses (figure 7). It is athermal and causes non-traumatic cellular photoactivation aimed at improving cellular function in normal cells and producing an anti-inflammatory effect.17 The photon interference triggered by the LEDs emitting specific wavelengths allows penetration below the skin. The resultant photoactivation is thought to trigger the repair of compromised cells and improve normal functioning of healthy cells. LLLT also has a localised warming effect, to melt meibum as well as offering the potential to reduce Demodex mite infestation via increased phagocyte activity.18 When considering the implications for MGD management, LLLT alone has been shown to produce significant improvements in tear break-up time.19
Figure 7: Low level light therapy (LLLT) using the EyeLight system (Espansione, Italy)
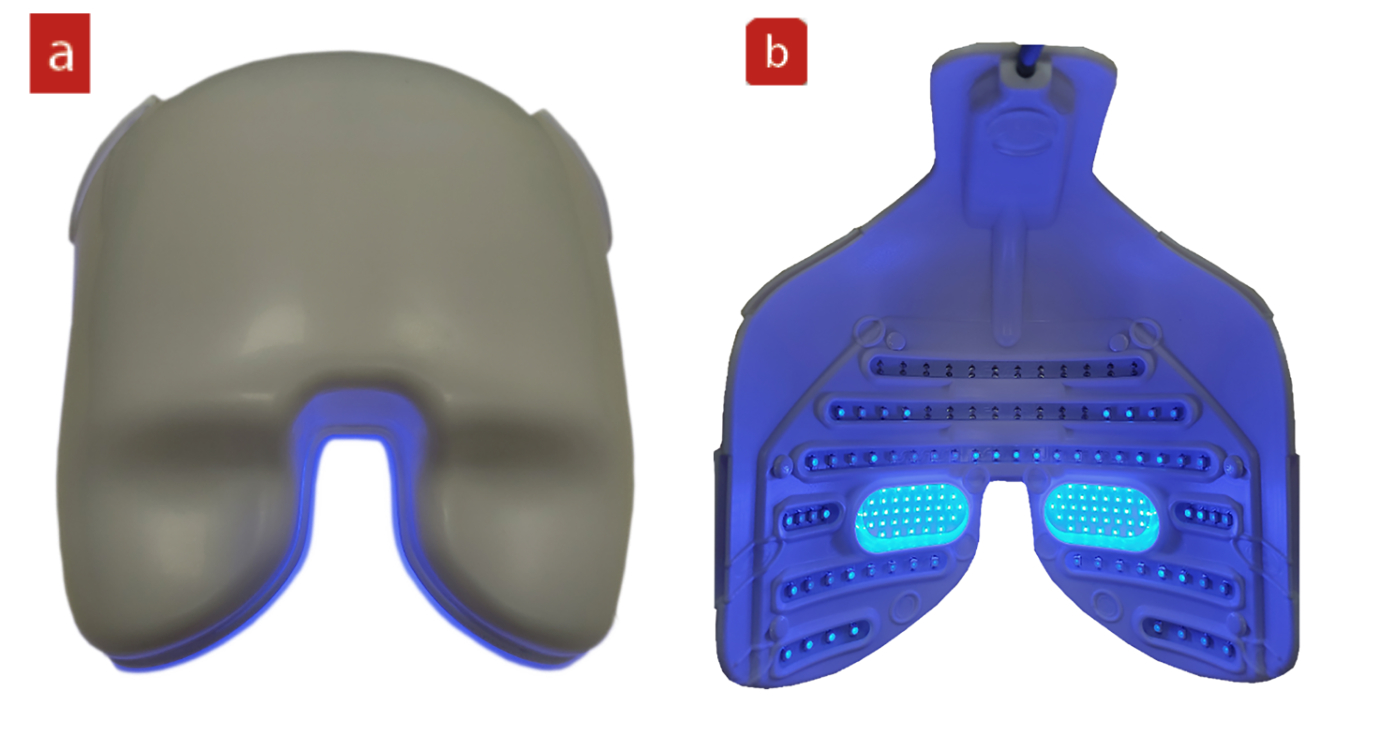
A more recent introduction to the LLLT therapeutic range available using the Eye-Light device is a short-wave blue option (figure 8). This is aimed at targeting Gram-positive bacteria on the skin in the treatment area.
Blue light energy is absorbed by molecules called porphyrins found within bacteria and results in photosensitisation. Exposure to the light can result in photodynamic inactivation, a process resulting in the death of the bacteria. On exposure to the blue light, the membrane-bound porphyrin molecules generate singlet oxygen radicals that damage or disrupt the cell walls of Gram-positive bacteria and lead to cell death. The relatively weak defence mechanism in bacteria against singlet oxygen-induced damage contributes to the high efficiency of the photodynamic inactivation.20
Figure 8: Short wavelength LLT mask from EyeLight seen(a) from the clinician’s view and (b) the patient view
Putting It All Together – Case Review
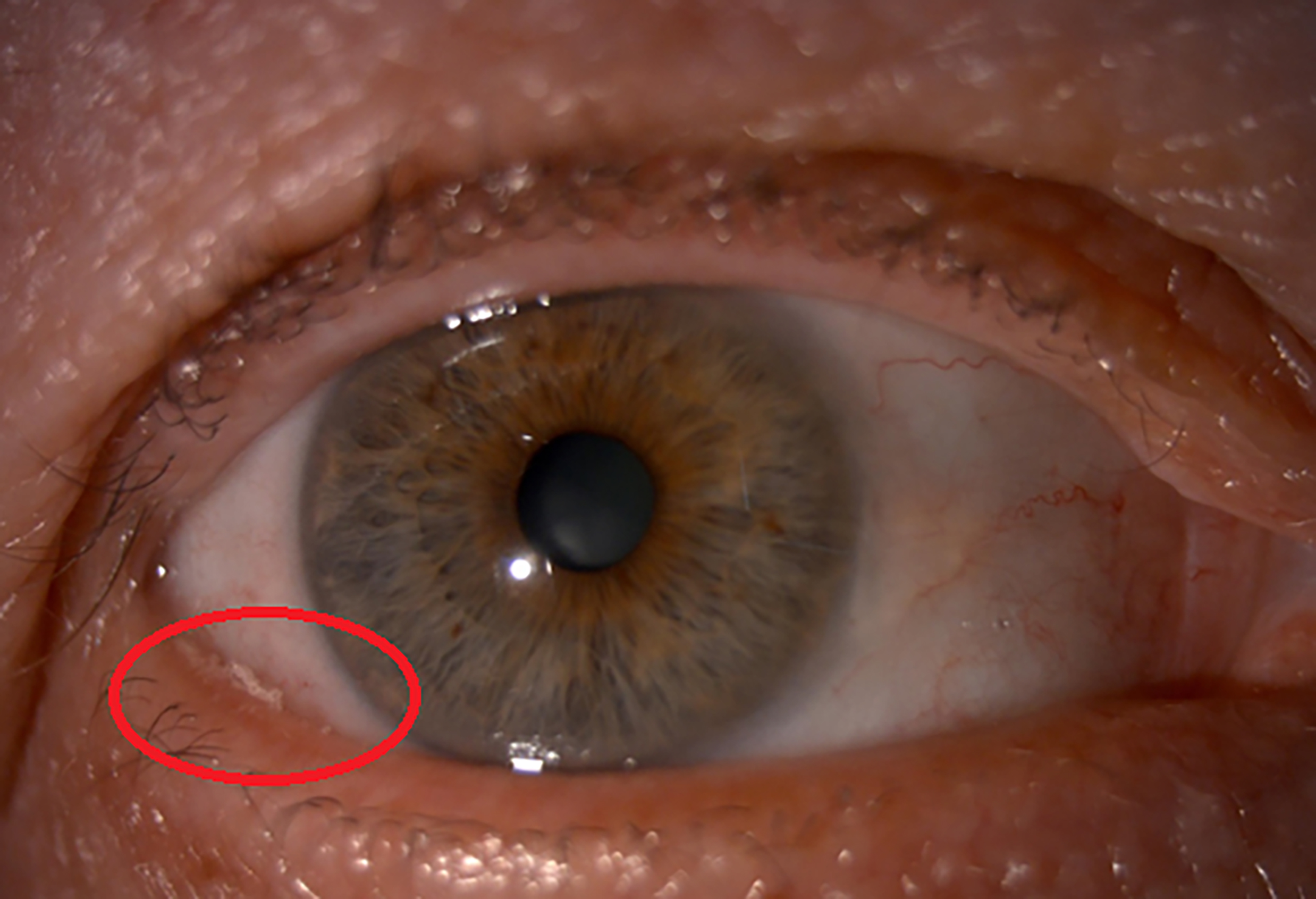
Last week (Optician 03.02.23), I highlighted a patient I saw recently in my clinic with exacerbated ocular surface issues and dry eye disease and an associated saponification (figure 9).
Figure 9: Saponification (red circle)
The initial diagnosis was Demodex blepharitis and meibomian gland dysfunction.
The initial management plan was as follows:
In practice:
- Forced meibomian gland expression (MGX)
- Lid margin debridement (manual and Blephex)
At home:
- Warm compresses therapy (twice a day)
- Omega eye capsules (omega-3 supplement of fish oil derivation): daily
- Blephasol lid cleansing (micellar solution for removing oily debris and crusted matter): daily
- Blephademodex (tea tree oil lid wipes): weekly
- Purifeyes (hypochlorous spray): twice a day
On follow-up, the patient showed greater signs of saponification and persistence of the Demodex blepharitis (figure 10).
Figure 10 (a) Saponification. (b) Persistent Demodex blepharitis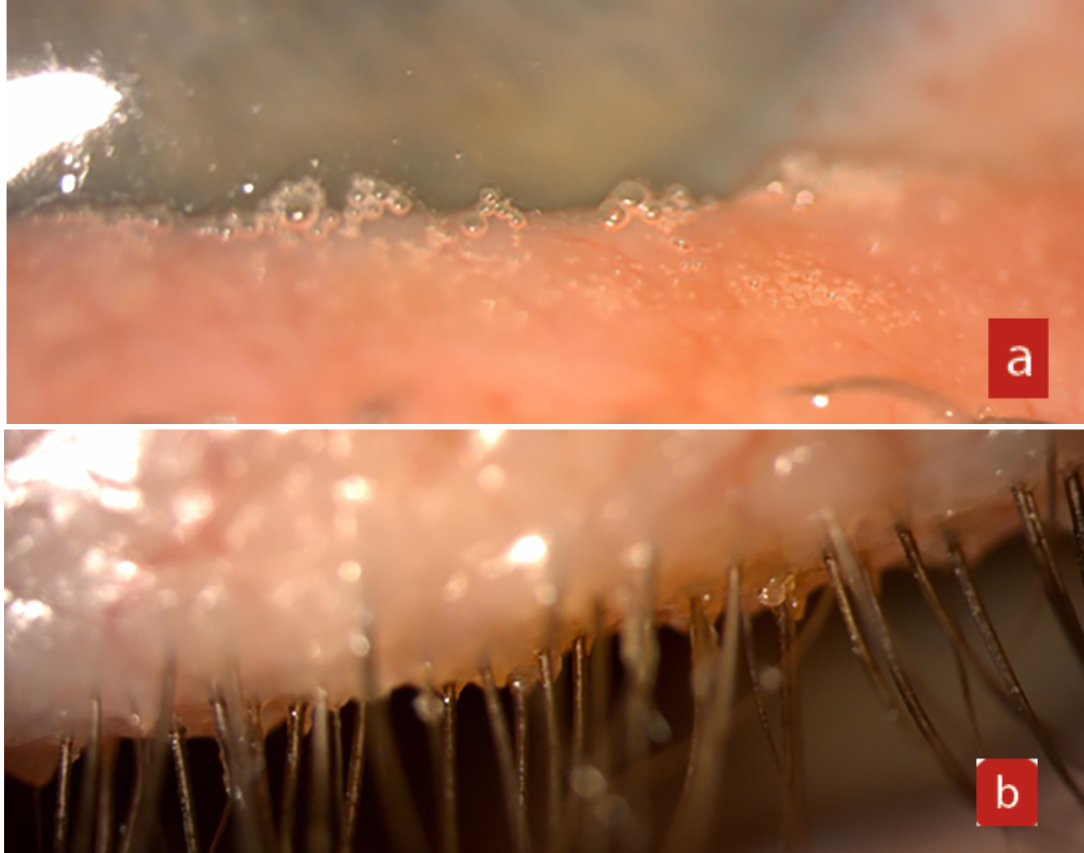
The adapted management plan was introduced as follows:
In practice:
- MGX
- Lid margin debridement
Also:
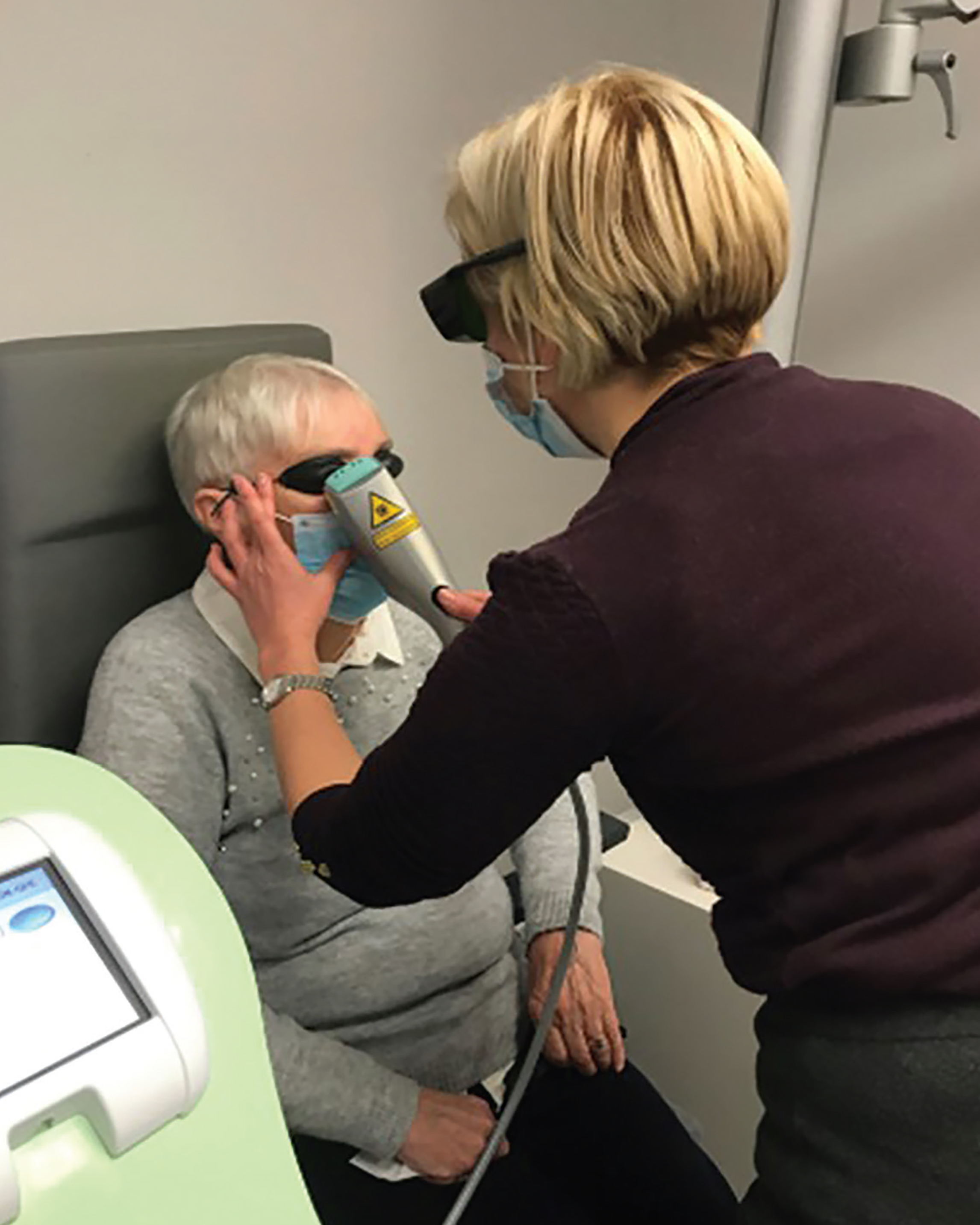
- Intense-pulsed light (IPL, figure 11)
- Near infrared (NIR) and short wavelength blue (SWB) low level light therapy (LLLT)
Figure 11: Intense pulsed light (IPL) therapy
This was carried out as four separate sessions, each with all three treatment types (IPL and NIR and SWB LLLT) one month apart each time.
At the six-month review, the symptoms had much improved, with no burning sensation reported and the patient stated this to be the most comfortable she had been ‘for years’. Her DEQ-5 score was now seven, and all at-home therapies had been complied with. Figure 12 shows the improved appearance.
Figure 12: No saponification is visible at all
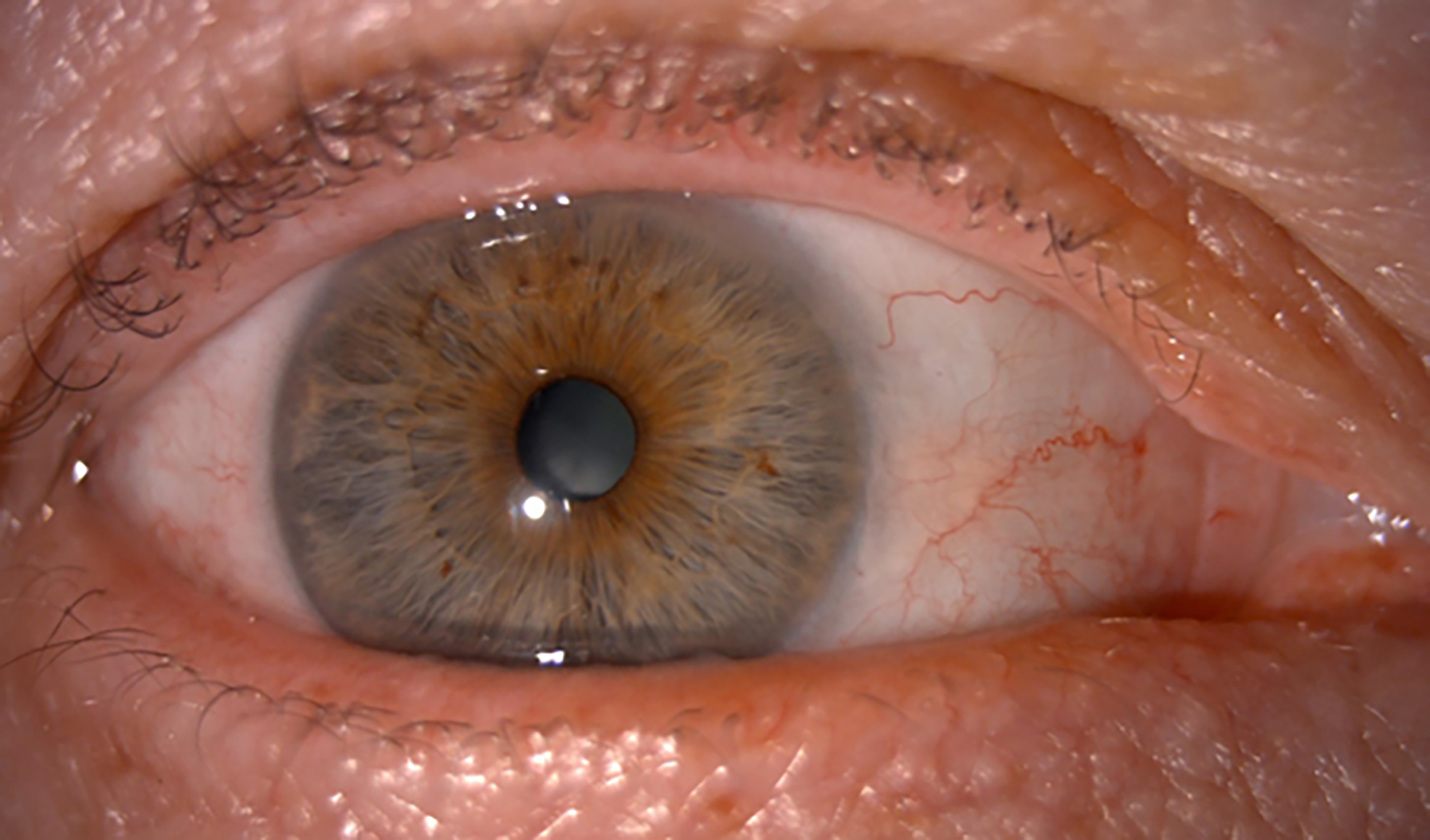
A revised plan was discussed as follows.
In practice:
- Manual and Blephex debridement
- Single top-up IPL and NIR LLLT and SWB LLLT (photobiomodulation) on an ongoing six-monthly basis (or sooner if required)
At home:
- As before
This case shows how important it is to understand the many influences upon a patient’s dry eye disease expression and how successful management needs an approach that embraces a range of options.
Summary
The common theme of dry eye disease and associated ocular disorders is the bioburden built up as a clear imbalance in the microbiome. Working up a logical and methodical approach to both diagnosis and management is always the key to break the vicious cycle for these patients and achieve tangible and significant results. The importance of bioburden and the resultant effect on the ocular surface inflammatory cycle and tear film in these types of cases must not be underestimated and needs to be addressed as a clearly defined strategy of good management.
- Sarah Farrant is a therapeutic optometrist with a specialist interest in dry eye disease and myopia management practising in Somerset, UK.
References
- Lim ES et al. Early life dynamics of the human gut virome and bacterial microbiome in infants. Nature Medicine, 2015;21:1228–34
- Dhar, D. and Mohanty, A. Gut microbiota and Covid-19-possible link and implications. Virus Research, 2020, p.198018
- Cavuoto KM et al. Relationship between the microbiome and ocular health. The Ocular Surface, 2019.
- den Besten G et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. Journal of Lipid Research, 2013;54(9):2325-2340
- Doan T et al. Paucibacterial microbiome and resident DNA virome of the healthy conjunctiva. Investigative Ophthalmology & Visual Science, 2016, 57(13), pp.5116-5126
- Ozkan J, Willcox MD. The ocular microbiome: molecular characterisation of a unique and low microbial environment. Current Eye Research, 2019, 44(7), pp.685-694
- Gomes JÁ et al. Ocular surface microbiome in health and disease. Asia-Pacific Journal of Ophthalmology, 2020, 9(6): pp 505-511
- Xiaotian L et al. Demodex infection changes ocular surface microbial communities, in which meibomian gland dysfunction may play a role. Ophthalmology and Therapeutics, 2021 Sep;10(3):601-617
- Changhao W et al. Composition and diversity of the ocular surface microbiota in patients with blepharitis in north western China. Frontline Medicine (Lausanne), 2021; 8: 768849
- Baudouin C et al. Revisiting the vicious circle of dry eye disease: a focus on the pathophysiology of meibomian gland dysfunction. British Journal of Ophthalmology, 2016;100:pp300-306
- Lappin-Scott H et al. Revealing a world of biofilms – the pioneering research of Bill Costerton. Nature Review of Microbiology, 2014;12(11):781–787
- Bezza Benkaouha I et al. Bacterial flora in blepharitis. Journal Française de Ophthalmologie, 2015 Oct;38(8):723-8
- Jones L et al. TFOS DEWS II Management and Therapy Report. Ocular Surface, 2017 Jul;15(3):575-628
- Craig JP et al. Commercial lid cleanser outperforms baby shampoo for management of blepharitis in randomized, double-masked clinical trial. Investigative Ophthalmology & Visual Science, 2017, Jun 23;58(8):2247
- Stroman DW et al. Reduction in bacterial load using hypochlorous acid hygiene solution on ocular skin. Clinical Ophthalmology, 2017,11:707-71
- Kabat AG. In vitro demodicidal activity of commercial lid hygiene products. Clinical Ophthalmology, 2019;13:1493-1497
- Kim WS, Calderhead RG. Is light-emitting diode phototherapy (LED-LLLT) really effective? Laser Therapy, 2011;20(3):205–215
- Pult H. Low-level light therapy in the treatment of meibomian gland dysfunction. Investigative Ophthalmology and Visual Science, 2020, 61.7:99
- Toyos R et al. The effects of red light technology on dry eye disease due to meibomian gland dysfunction. Journal of Japanese Ophthalmology,2017;3(5):555624
- Sulek A et al. Photodynamic inactivation of bacteria with porphyrin derivatives: effect of charge, lipophilicity, ROS generation, and cellular uptake on their biological activity in vitro. International Journal of Molecular Sciences, 2020, Nov 18;21(22):8716
