The era of laser technology is over sixty years old and, within this time line, there has been a consistent, continual development of such technology for ophthalmic applications. The emergence of multi-spot lasers, which rapidly deliver treatment patterns for retinal photocoagulation, has been a relatively recent development and so it would seem appropriate to review the clinical landscape where they now find application as regards relative clinical effectiveness and patient safety. As a prelude to this, however, it might be useful to reflect on the history of the use of intense light in the treatment of retinal disease.
Early Days
While Dr G Meyer-Schwickerath is credited with reporting the first successful use of focused sunlight to generate therapeutic retinal lesions,1 it was the Spanish ophthalmologist José Morón who had previously described his attempt to use focused sunlight to treat patients in his doctoral thesis, a statement he defended in 1946.2, 3
Meyer-Schwickerath had previously been aware of a retinal injury suffered by a student who had observed the solar eclipse of July 9, 1945, and so was inspired to make the link between incident light and retinal scarring. While the subsequent case studies from Meyer-Schwickerath describing the successful use of focused sunlight were significant in demonstrating the potential of the technique, such ‘solar treatments’ were not, however, practical to undertake as a working clinical tool.
A system based on the xenon lamp was subsequently developed for him by Dr Hans Littman (of Carl Zeiss, Oberkochen, Germany) and, on August 22, 1949, Meyer-Schwickerath carried out the first photocoagulation upon a patient with imminent retinal detachment using such a system.4 The xenon photocoagulation system thus developed, however, would not be commercially available until some years later.
During this period of working with Carl Zeiss, it was Meyer-Schwickerath who patiently developed the clinical expertise of xenon-based photocoagulation.5-8 The photocoagulation technique at this time was significantly limited by the large size of the lesions created by the incident light, being of a diameter of around 2000 microns, and so it was unsuitable for the targeting of small vessels. It would, however, have been appropriate for the treatment of specific, localised areas of ischaemia in diabetic retinopathy. The best results were, at first, obtained in the treatment of retinal tears and retinal tumours.
In a reflective address in 1972, Meyer-Schwickerath provided encouragement to innovators to endure the disinterest, scepticism and opposition of peers and colleagues to develop ideas for the improvement of patient treatments.9 The implication was, perhaps, that it was not enough to report interesting findings in a research-focused publication and that true progress came from practical application. Without his years of patient application and dedication, retinal photocoagulation would probably not have developed for another ten years with the arrival of the argon laser.
The Advent of Lasers
The world’s first laser was developed by Theodore Maiman of Hughes Research Laboratories in the USA in May 1960.10 The term ‘laser’ was coined as an acronym for light amplification by stimulated emission of radiation and, contrary to the common myth that its usefulness was not initially identified, it was soon recognised that lasers offered significant potential for ophthalmic application, though the prevalent wavelength at the time of 694 nm was poorly matched for targeting haemoglobin-rich tissues.
While various groups had endeavoured for some time to produce the first laser system, the actual development of the ruby laser by Maiman was somewhat unremarkable. While others had discounted ruby as a likely laser-producing candidate material, Maiman’s assessment was more positive. Placing a rod of ruby within a resonant cavity, he energised the rod using light pulses generated from a helical flashlamp coil. As higher and higher voltages were applied to the flashlamp, the characteristic emitted red wavelength of 694 nm appeared. There was a period where the xenon flashlamp and the ruby laser co-existed as a tool for retinal photocoagulation and comparisons were made of the scope of each technology.11, 12 The physics of laser light, however, offered significant advantages over the xenon flashlamp technology in terms of the ability of the laser to deliver smaller, better defined spot sizes.
While the emergence of the pulsed ruby laser was a significant breakthrough, the prime goal of the major corporations was to develop a continuous laser which would be more suited to telecommunications applications and assorted military uses, such as in range finders. It was the discovery of the argon laser, however, that would firmly establish the process of laser-based retinal photocoagulation.
The history of the argon laser is essentially one of serendipity. WB Bridges, a scientist with extensive experience working with electron tube devices while working at Hughes Research Laboratories (figure 1), was actively engaged in developing newer laser technology as a follow-on from the helium-neon laser developed by Bell Labs by Ali Javan and co-workers in December 1960. Initially, the helium-neon laser had been an infra-red laser, with a wavelength of 1152 nm. By changing the component mirrors to be red-reflecting, in 1962 Bell Labs were able to produce the characteristic visible red wavelength of 633 nm.
Figure 1: William B Bridges in his lab at Caltech with an argon ion laser, 2005
This was the first continuous power visible laser though, as yet, it was not suitable for clinical application since the output power could not easily be increased much above 100 mW. This significantly energised Hughes Research Laboratories (and many other research groups) to develop their own similar devices. Interest was sparked around this time by reports of the development of a mercury-neon laser by Spectra Physics. The introduction of argon as a background gas into a version of this laser by Bridges in his own laboratory led to the eventual creation of the argon laser, with its characteristic peak blue wavelength at 488 nm.13 What really enabled these developments was, essentially, the significant research and development funding provided by both the telecommunications sector and the US military. Hecht has published a useful summary of these various lines of development.14
A Clinical Tool
In 1968, an argon laser was developed by the Raytheon Corporation for clinical ophthalmic use which could, at last, provide effective photocoagulation of vascular retinal tissue. In this system, the emergent laser beam was directed using a series of flexible mirror stages, held within in an articulated arm rather than an optical fibre.15 By this time, a krypton laser had been developed and was known to provide usable lines at wavelengths of 531 nm (green) and 568 nm (yellow). However, the argon laser provided the easier system to produce and maintain. By 1970, an argon laser had been brought to market by Coherent that made possible the production of effective, relatively reliable and easy to use retinal photocoagulation systems. These first systems, however, relied on highly precise and specialised electrical and optical engineering and the laser tubes would, on occasion, require expensive replacement.
By today’s standards, the argon laser was highly inefficient and required water cooling to produce around three Watts of delivered power from 30 kW of electrical supply. At the heart of the laser was a laser tube which presented a challenging operating environment for the optical/materials/power management system. Control of pulse duration was achieved by use of a physical shutter and the beam intensity was controlled by selection of the appropriate optical filter to attenuate the main beam of known intensity.
This simple shutter system limited the duration of the shortest pulses that could be usefully delivered. The argon laser could, however, supply light at the two main wavelengths of light of 514 nm (green) and 488 nm (blue) known to be most suitable for retinal photocoagulation. That said, subtle observation with a spectroradiometer showed that the ‘mix’ of spectral wavelengths would alter with changing tube output power levels, though the user could typically select either ‘green’ or ‘blue’ wavelengths. Often, the task of checking outputs from argon and krypton lasers was a role for hospital-based scientific and technical services in order to ensure greater availability of systems for clinical use.16
The Nd:YAG laser, with a wavelength of 1064 nm, was developed at Bell Labs in 1964, yet it was not until 1988 that it was used in conjunction with a frequency-doubling crystal of potassium-titanyl-phosphate crystal (KTP) to generate the green 532 nm wavelength used for ophthalmic applications.17 This significantly reduced the size of photocoagulation systems, increased system reliability and, essentially, replaced argon laser systems for many ophthalmic treatments. A limited number of laser systems continued to be manufactured utilising the krypton 568 nm wavelength. More recently, the 577 nm wavelength has been produced by frequency-doubling the 1154nm wavelength produced by a laser diode. The trend continues to reduce the size of laser systems, as indicated in figure 2.
Figure 2: Pump laser module for ophthalmic applications. (Image courtesy of Credit Ferdinand-Braun-Institute (FBH), Berlin, Germany).
Wavelength Optimisation
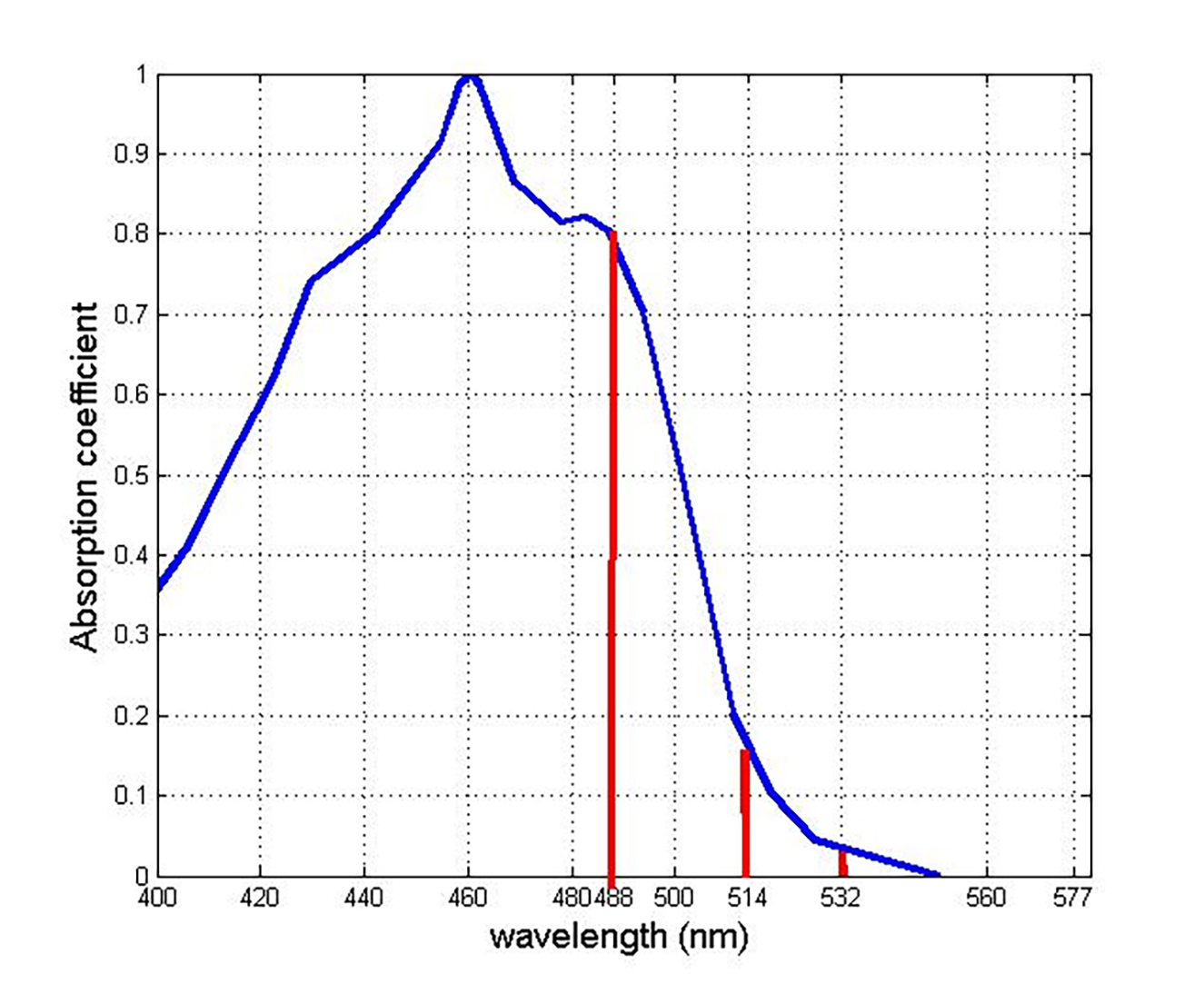
Laser photocoagulation has, for very good reason, tended to avoid any treatment within the macular region, where central vision is supported and the density of cone cells is highest. Any treatment of the paramacular region, however, is limited by light absorption by the protective macular pigments, lutein, zeaxanthin and meso-zeaxanthin.18 The peak absorption wavelength of macular pigment is around 460 nm. This matches closely the peak of the so-called blue light hazard, at around 440 nm. This has led to the use of wavelengths of light which facilitate the treatment of the peripheral macular region. Figure 3 shows the absorption curve of macular pigment alongside the key wavelengths used for retinal photocoagulation.
Figure 3: Absorption curve of macular pigment (blue) and the three key wavelengths used for retinal photocoagulation (red)18
Pattern Lasers
Developments within optical delivery systems have allowed the production of so-called pattern lasers. These can deliver a sequence of pulses rather than a single directed pulse. The duration of a specific directed laser treatment is limited, to some extent, by the risk of reflex movement of the eye being treated. The longer the duration of a specific treatment, the greater the chance that energy will be delivered ‘off target’ due to patient movement. In single spot treatments, maximum pulse durations of typically between 100milliseconds (ms) and 200ms will be selected, the delivered power during the pulse being an independent variable. The actual parameters of pulse duration and set power required to achieve effective photocoagulation at any given spot size can vary with the degree of absorption in the transit of light to the retinal surface. This can vary between patients.
Where, for example, a 3 x 3 array of 9 discrete sequential pulses is being delivered by a pattern laser, an individual pulse duration of 20 ms will result in a total treatment time of at least 180 ms. To maintain a pulse duration of 100 ms for the pattern laser, for example, a total delivery time of at least 900 ms would be needed. This increases the risk of patient movement which would decrease the precision of the target impact. The requirement to use pulses of shorter duration with a pattern laser requires the output power to be increased in order to achieve effective photocoagulation for any given spot size. The technology behind such pattern delivery of laser spots adds a significant degree of complexity to the successful use of such a delivery system.
The conventional method for single spot retinal photocoagulation has been to leave evidence of treatment by way of a visible lesion as a marker at the treatment site. This assists the laser operator to distinguish areas which have been treated previously from those remaining areas still requiring treatment. Pattern lasers, however, have been developed to provide an option to deliver treatments that do not require a marker. As an example of this, the PASCAL range of pattern lasers (manufactured by Iridex and distributed by Topcon; figure 4) can deliver patterns of coagulation as a mixture of so-called marker sites and sub-threshold sites.
Figure 4: PASCAL Synthesis laser system. (Image courtesy of Hiroyuki Nomoto, MD, Nomoto Eye Clinic)

Initially, the threshold for visible photocoagulation for a given spot size is determined using a single laser burn. The relative level of the sub-threshold delivery can be set, for example, at a given percentage of the marker level, such as 50% of the threshold visible burn level. In a 3 x 3 rectangular pattern, the corner spots can act as marker locations with the remaining 5 spots being delivered as sub-threshold types. Similarly, for a 4 x 4 pattern, there would be 4 marker spots and 12 sub-threshold spots. The marker spots assist the operator to deliver appropriately within a target area, preventing repeat treatments and also areas of undertreatment. The sequence of delivery locations of such pattern lasers is typically designed to minimise local heat build-up.
Figure 5: Detail of PASCAL laser pattern configuration screen for a rectangular array. (Image courtesy of Hiroyuki Nomoto, MD, Nomoto Eye Clinic)
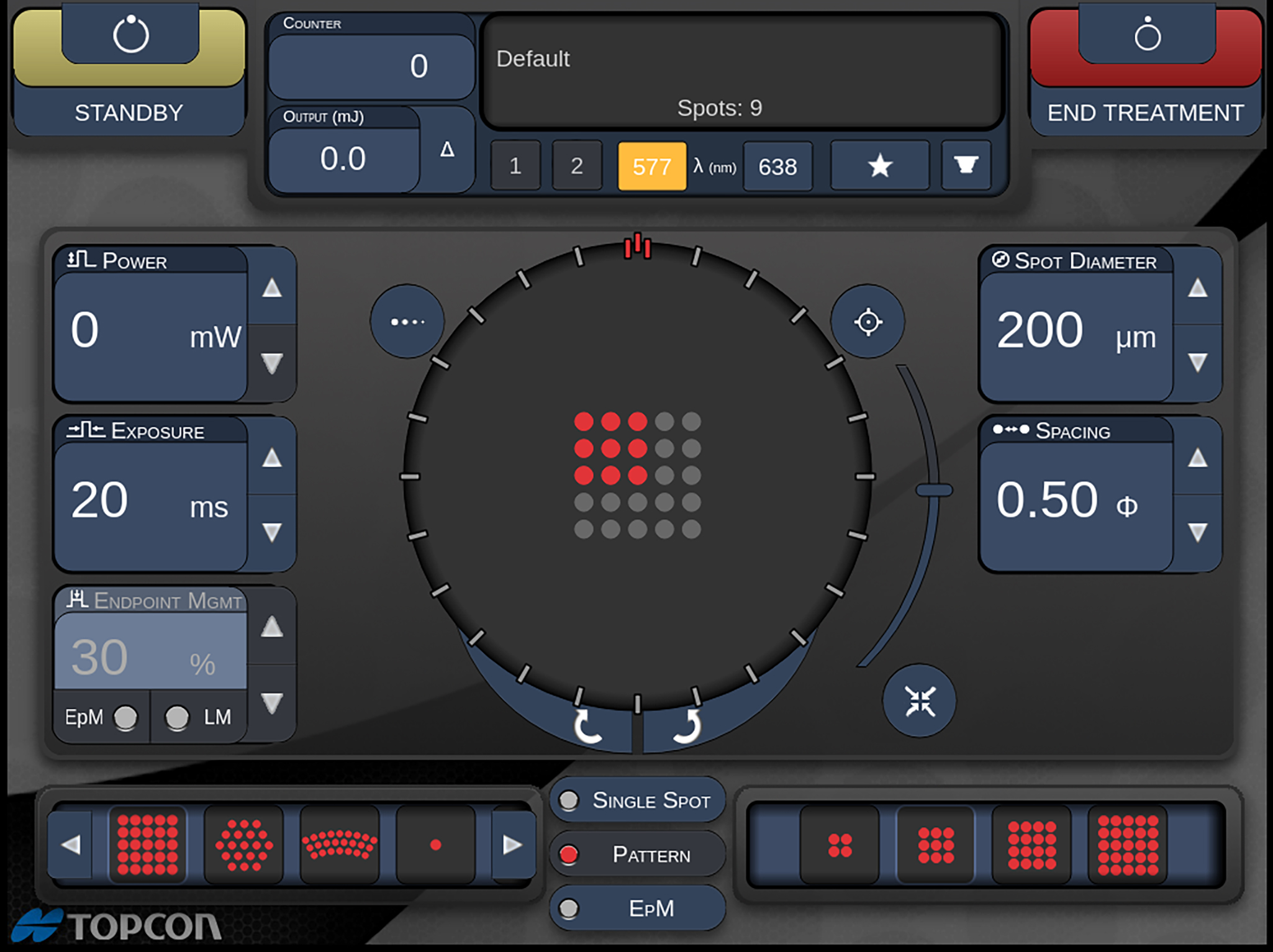
An immediate benefit of the pattern laser is the more rapid delivery of laser treatments. Conventional retinal coagulation for diabetic retinopathy utilises sufficient energy to create visible lesions. Figure 5 shows a PASCAL laser pattern configuration screen for a rectangular array. The control module is designed for intuitive use. Figure 6 shows a PASCAL laser pattern configuration screen for treating a sector around the macular area. Figure 7 shows the delivered burn pattern round a retinal tear, where the coagulation burns are directed to stabilise the retinal structures.
Figure 6: Detail of the PASCAL laser pattern configuration screen for a sector around the macular area. (Image courtesy of Hiroyuki Nomoto, MD, Nomoto Eye Clinic)
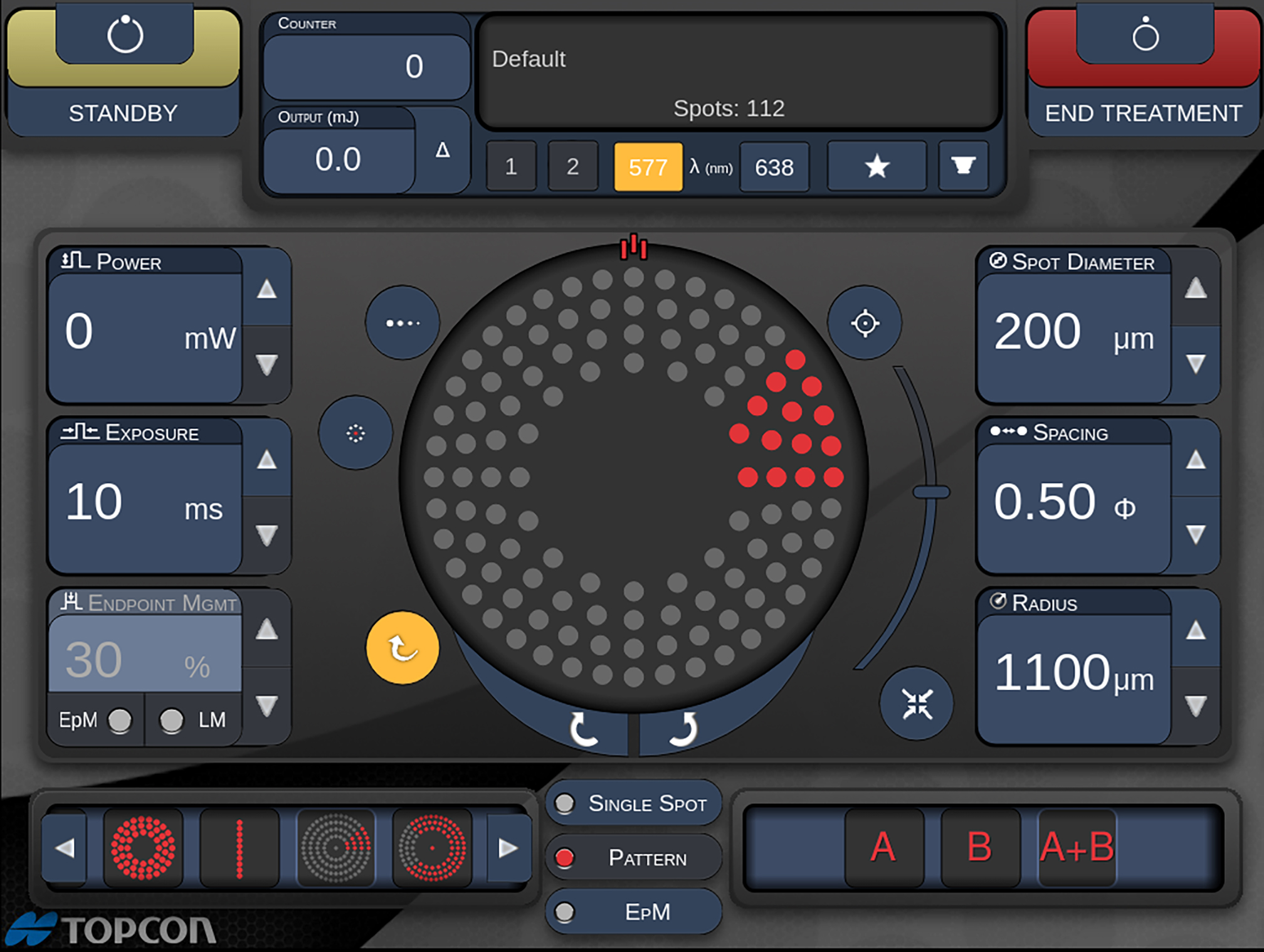
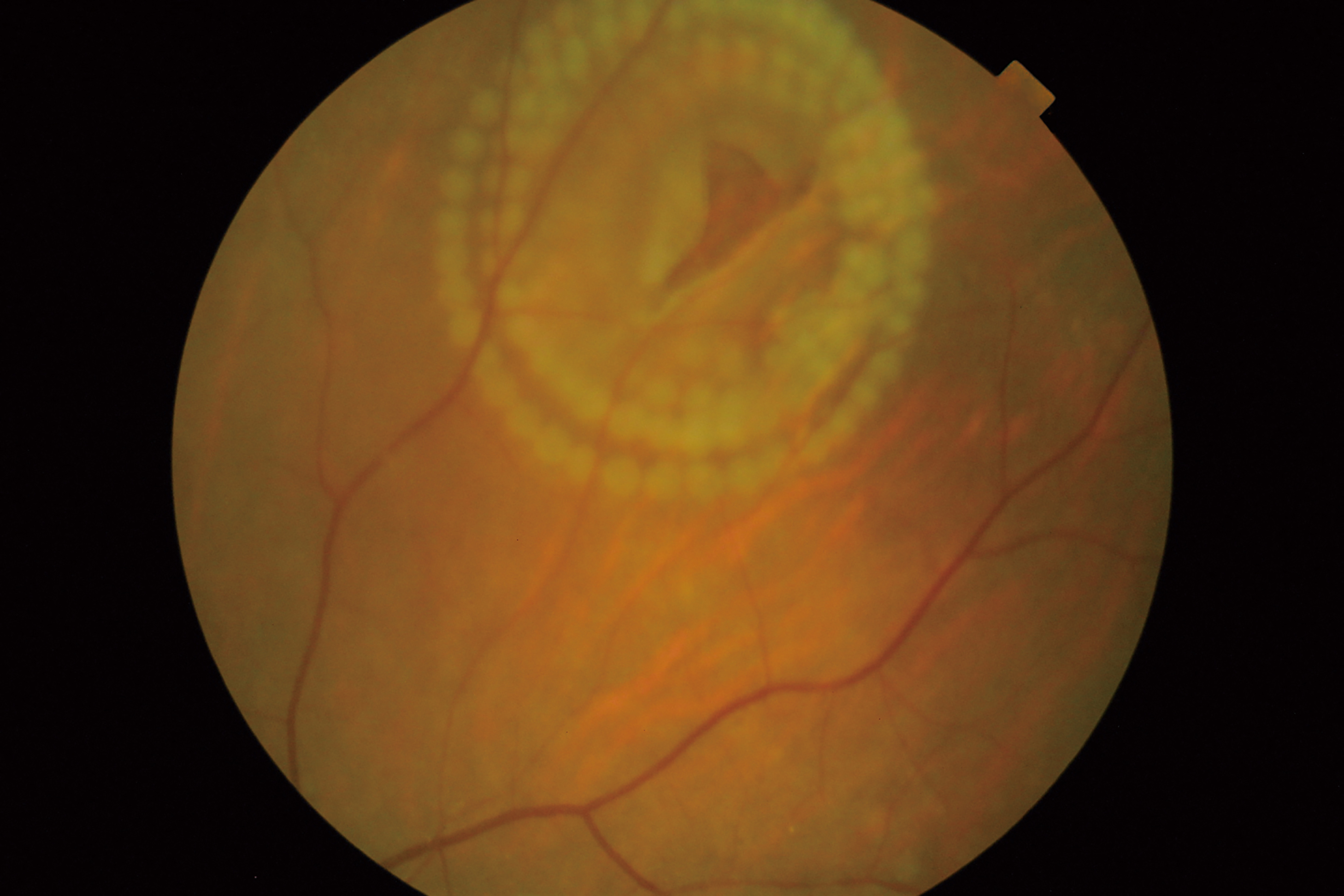
Figure 7: Image of delivered burn pattern round a retinal tear using PASCAL laser system. (Image courtesy of Hiroyuki Nomoto, MD, Nomoto Eye Clinic)
Pulse Width Modulation of Pascal Lasers
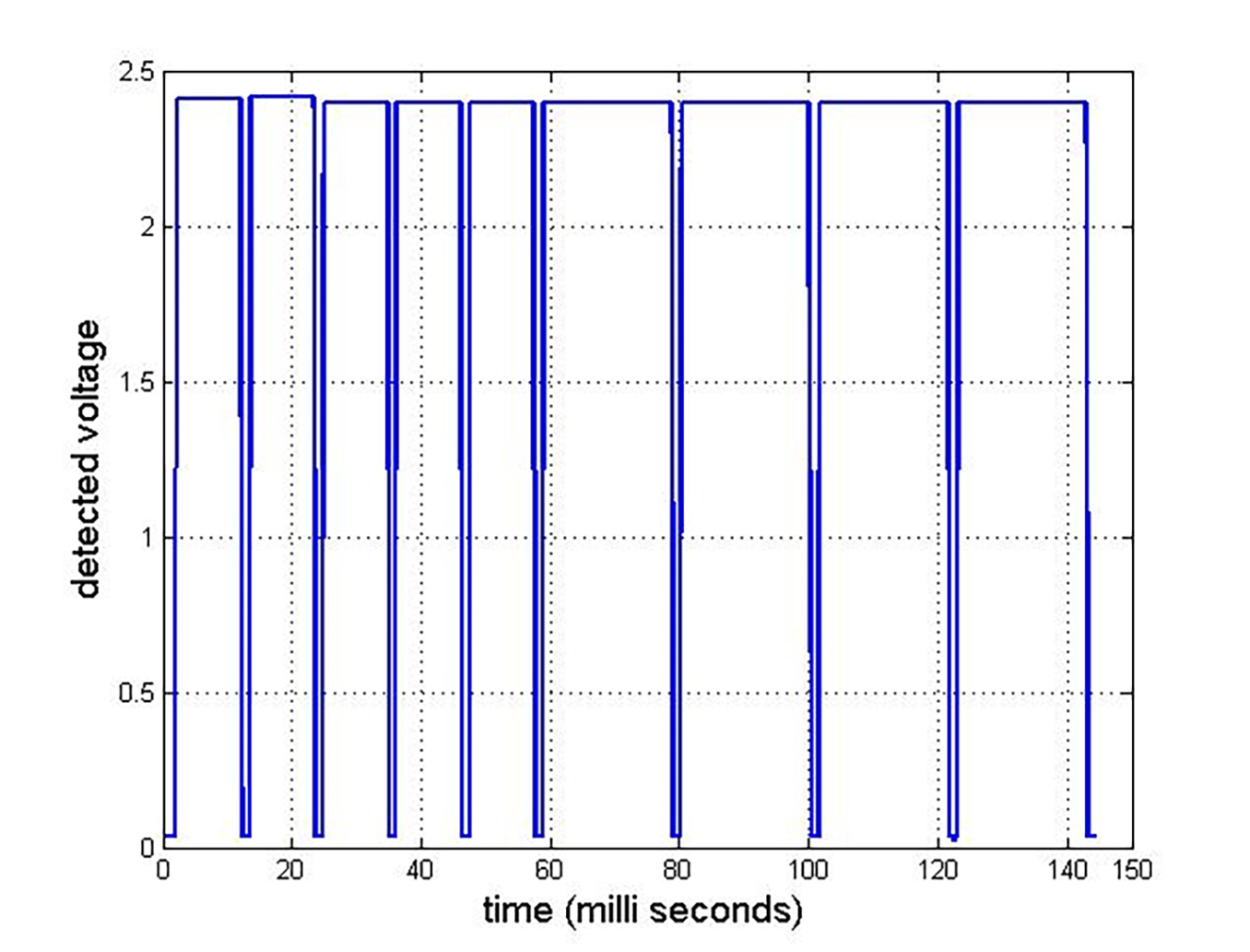
Specific pattern lasers utilise a range of different technologies for pattern projection. In terms of the energy delivered within specific target sites, the PASCAL laser systems modulate this by variation of the pulse width at constant power level. This feature is shown in figure 8. The output from a pattern array is detected using a circuit incorporating a photodiode detector.19 The time gap between successive pulses is around 1.5 ms.
Figure 8: Pulse train of five, 50% pulses and four ‘marker’ pulses for a 3 x 3 array sequence19
When using pattern lasers, care is required in matching the intended plane of focus of the laser energy with the targeted retinal structures. Where, for example, a 5 x 5 array is projected towards the peripheral retina, the laser energy will be delivered at greater depth than intended in some locations while, at others, it will be delivered above the retinal surface and be of little effect.
Single Spot Versus Pattern Laser
The conventional single spot retinal photocoagulation treatment has long been established as approved practice.20 One study by Nemcansky et al is a comparison of the effectiveness of pattern scanning laser and conventional laser for the management of diabetic retinopathy.21 A group of 30 patients with diabetic retinopathy underwent treatment in one eye with a pattern scanning laser, while the other eye was treated conventionally. The two techniques were found to be equally effective in managing the progression of diabetic retinopathy.
It was, however, noted that the patient cohort reported a significantly lower perception of pain when treated with the pattern laser when compared with conventional treatment. The authors indicate, however, that caution is required in interpreting similar observations from comparable studies on account of variations in the number of spots delivered and the possible inclusion of the distant peripheral retina, an area where pain thresholds are lower. The authors also indicate that variations between studies relating to the number of burns delivered with each technique make comparisons of treatment outcomes problematic. Poorer results with pattern lasers may, in fact, be due to delivery of an insufficient number of lesions.
One associated factor is that pulses of shorter duration, typical of pattern lasers, tend to have reduced ‘creep’ outwards from the outer edge of the original lesion, resulting in a reduced area of thermal damage. This effect has been described by Jain et al based on observations of lesions produced in rabbit eyes and analysed by histology and image analysis.22 Thus, a moderate lesion produced at 100 mW and pulse duration 20 ms was observed to have a clinically confirmed lesion diameter of 101microns, compared with a confirmed comparable lesion of 120microns for a pulse duration of 50 ms; the spot size was 132microns in both situations. While the energy delivered within the pulse is a key variable, the pulse duration affects the extent of heat conducted away from the immediate zone of energy delivery.
A more detailed and significant assessment of effects of various modes of retinal photocoagulation was provided by Lavinsky et al. Lesions of 400, 200 and 100microns size were produced at pulse durations of 100ms and 20ms and observed end points were monitored in a group of 22 patients over a period of 12 months.23 OCT was used to monitor the effective lesion size and validation of the lesion details was provided by cross reference to a separate linked animal study. It was found that all lesions contracted over time and there was a likely restoration of photoreceptor function resulting from migration of cells from regions unaffected by the initial photocoagulation. The degree of contraction was greater for the 20ms lesions, as shown in figure 9 for a target lesion size of 400 microns.
Figure 9: Dynamics of change of effective lesion size as determined by OCT for moderate initial 100ms and 20ms pulses over time for a target lesion size of 400 microns23
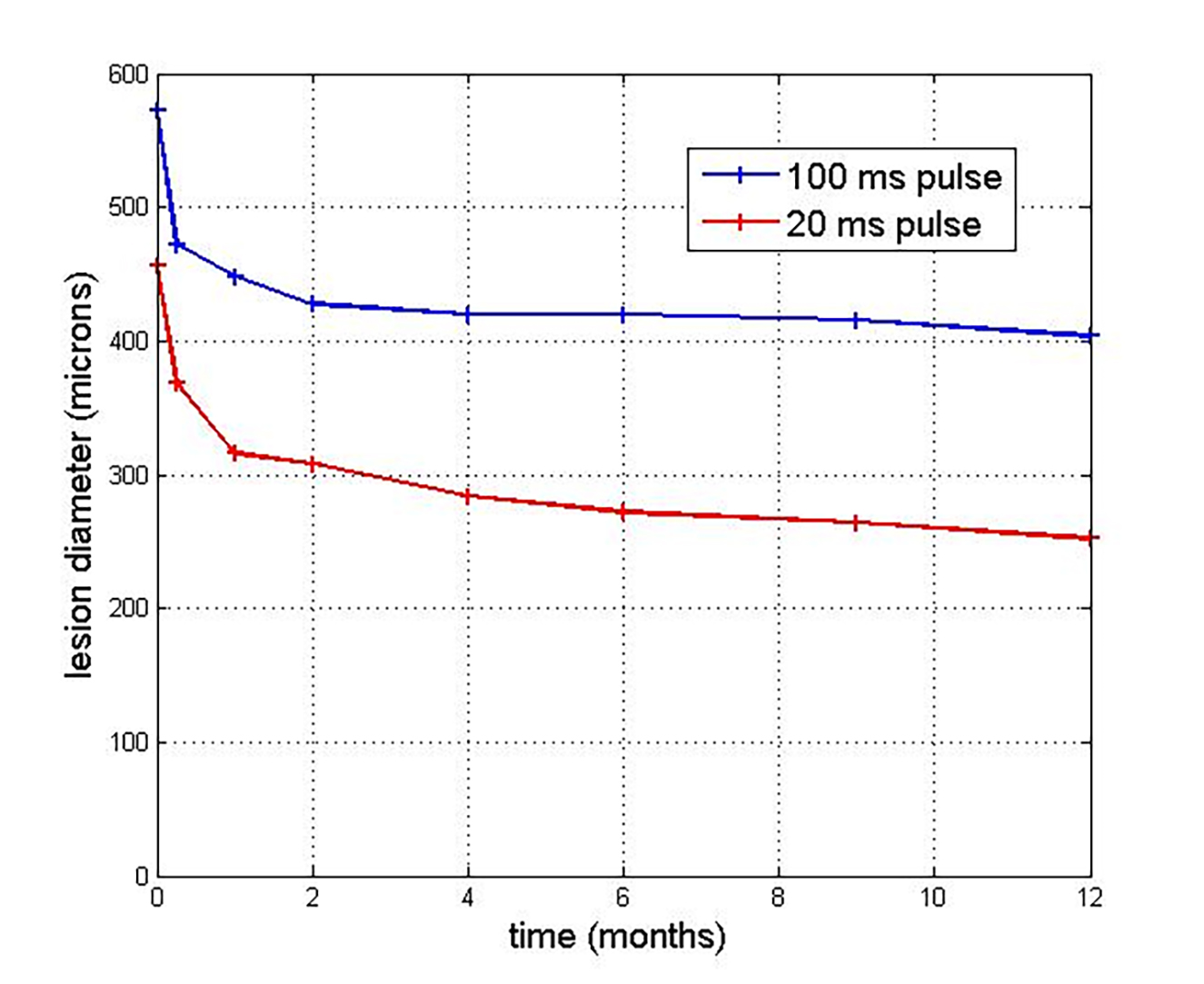
The process of contraction of lesions was observed for up to 12 months for the 20ms pulses, but for a shorter time for the 100ms pulse. A key finding was that shorter pulses were less likely to result in retinal scarring and damage to the nerve fibre layer. The authors concluded that additional burns would be required with pattern scanning lasers, such as the PASCAL type, to have the same clinical effect as conventional treatment due to smaller effective lesion size. This study was able to predict the range of parameters required to determine the effectiveness of pattern laser technologies.
Huang et al undertook a retrospective study involving 16 patients who received PASCAL treatment and 13 patients receiving conventional treatment by pan-retinal photocoagulation (figure 10).24 The clinical outcomes of the two groups were essentially similar, though the authors highlighted the requirement for more extensive studies. The PASCAL treatments, however, allowed a higher patient throughput which would have relevance for the rapidly increasing treatment workload for diabetic retinopathy in China, where this research was undertaken.
Figure 10: Pan-retinal photocoagulation
Summary
The technology behind retinal photocoagulation has shown progressive development over 70 years of increasingly successful application. This has been greatly enhanced over the last 15 years with the introduction of pattern lasers which have offered novel treatment regimes. Subtle differences between conventional single spot treatments and pattern laser treatments continue to be identified and preference needs to consider both clinical effectiveness and patient safety.
A lack of large multicentre studies, however, has been a feature regarding the uptake of pattern lasers. This implies that a full understanding of the relative merits of the co-existing technologies remains some way off. Pattern lasers provide a treatment mode of greater complexity compared with conventional techniques, one which requires greater in-depth training to undertake effectively and safely. But pattern lasers have advantages and are here to stay.
- Dr Douglas Clarkson is a research fellow at the department of clinical physics and bio-engineering, Coventry and Warwickshire University Hospital Trust.
References
- Meyer-Schwickersth G, 1949, Kozguldtion der Netzhsut mit Sonnenlicht. Ber Dtsh Ophthalmol Ges 55: 256-259
- Morón J. Sobre etiologia y patogenic de los fototraumatismos retinianos. Doctoral dissertation. Madrid, Nov (Complutense University Library, Madrid, Spain).
- Ascaso FJ, Grzybowski A. José Morón was the first to introduce the retinal light photocoagulation. Acta Ophthalmologica, 2022 Mar;100(2):234-236
- Kirchhof B, Joussen A, Bornfeld N, Wessing A. Prof Dr med Dr hc mult. Gerd Meyer-Schwickerath inventor of light coagulation on his 100th birthday (July 10, 2020). Graefes Archives of Clinical & Experimental Ophthalmology, 2020 Sep;258(9):1837-1838
- Meyer-Schwickerath G. Lichtkoagulation; eine Methode zur Behandlung und Verhütung der Netzhautablösung [Light coagulation; a method for treatment and prevention of the retinal detachment]. Albrecht Von Graefes Archives of Ophthalmology, 1954;156(1):2-34
- Meyer-Schwickerath G. Prophylactic treatment of retinal detachment by light-coagulation. Transactions of the Ophthalmology Society, UK., 1956;76:739-50
- Meyer-Schwickerath MD. Correspondence: history and development of photocoagulation. American Journal of Ophthalmology, 1967;63:1812-1814
- Degenhardt AH. Light coagulation of the eye. British Journal of Physiological Optometry, 1976;31(4):11-8. PMID: 802753.
- Meyer-Schwickerath G. Twenty-five years of photocoagulation. Transactions of the American Academy of Ophthalmology & Otolaryngology, 1973 Jan-Feb;77(1):OP3-5. PMID: 4581059.
- Maiman, T. Stimulated Optical Radiation in Ruby. Nature 1960 187, 493–494
- L’Esperance FA Jr. Clinical comparison of xenon-arc and laser photocoagulation of retinal lesions. Archives of Ophthalmology, 1966 Jan;75(1):61-7
- Zaret MM, Breinin GM, Schmidt H, Ripps H, Siegel IM, Solon LR. Ocular lesions produced by an optical maser (laser). Science, 1961 Nov 10;134(3489):1525-6
- Bridges WB. Laser oscillation in singly ionized argon in the visible spectrum. Applied Physics, 1964 Lett. 4, 128
- Hecht J. A short history of laser development. Applied Opththalmology, 2010 Sep 1;49(25):F99-122
- L’Esperance FA Jr. An ophthalmic argon laser photocoagulation system: design, construction, and laboratory investigations. Transactions of the American Ophthalmology Society, 1968;66:827-904
- Clarkson, D.G., Brennen, S.E. Calibration and service procedures for argon and krypton ophthalmic lasers. Laser Medicine and Science, 1988 3, 277–285
- Jalkh AE, Pflibsen K, Pomerantzeff O, Trempe CL, Schepens CL. A new solid-state, frequency-doubled neodymium-YAG photocoagulation system. Archives of Ophthalmology, 1988 Jun;106(6):847-9
- Arunkumar R, Gorusupudi A, Bernstein PS. The macular carotenoids: A biochemical overview. Biochimica et biophysica acta. Molecular and cell biology of lipids, 2020 Nov;1865(11):158617.
- Clarkson DM, Makhzoum O, Blackburn J. Determination of pulse profile characteristics of multi spot retinal photocoagulation lasers. Medical Engineering & Physics, 2015 Oct;37(10):1027-31
- Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology, 1991 May;98(5 Suppl):766-85
- Nemcansky J, Stepanov A, Nemcanska S, Masek P, Langrova H, Studnicka J. Single session of pattern scanning laser versus multiple sessions of conventional laser for pan-retinal photocoagulation in diabetic retinopathy: Efficacy, safety and painfulness. PLoS One, 2019 Jul 16;14(7):e0219282
- Jain A, Blumenkranz MS, Paulus Y, Wiltberger MW, Andersen DE, Huie P et al. Effect of pulse duration on size and character of the lesion in retinal photocoagulation. Archives of Ophthalmology, 2008 Jan;126(1):78-85
- Lavinsky D, Cardillo JA, Mandel Y, Huie P, Melo LA, Farah ME, Belfort R, Palanker D. Restoration of retinal morphology and residual scarring after photocoagulation. Acta Ophthalmologica, 2013 Jun;91(4):e315-23
- Huang CX, Lai KB, Zhou LJ, Tian Z, Zhong XJ, Xu FB, Gong YJ, Lu L, Jin CJ. Long-term effects of pattern scan laser pan-retinal photocoagulation on diabetic retinopathy in Chinese patients: a retrospective study. International Journal of Ophthalmology, 2020 Feb 18;13(2):239-245
