Metabolic syndrome
Metabolic Syndrome (MetS) is an umbrella term for a constellation of signs and risk factors that appear to increase the risk of cardiovascular disease (CVD) and type 2 diabetes. Specifically, MetS is associated with a two-fold increase in cardiovascular disease1 and a five-fold increase in developing diabetes.1-4 There is also evidence of an association between MetS and an increased risk of some types of cancer.
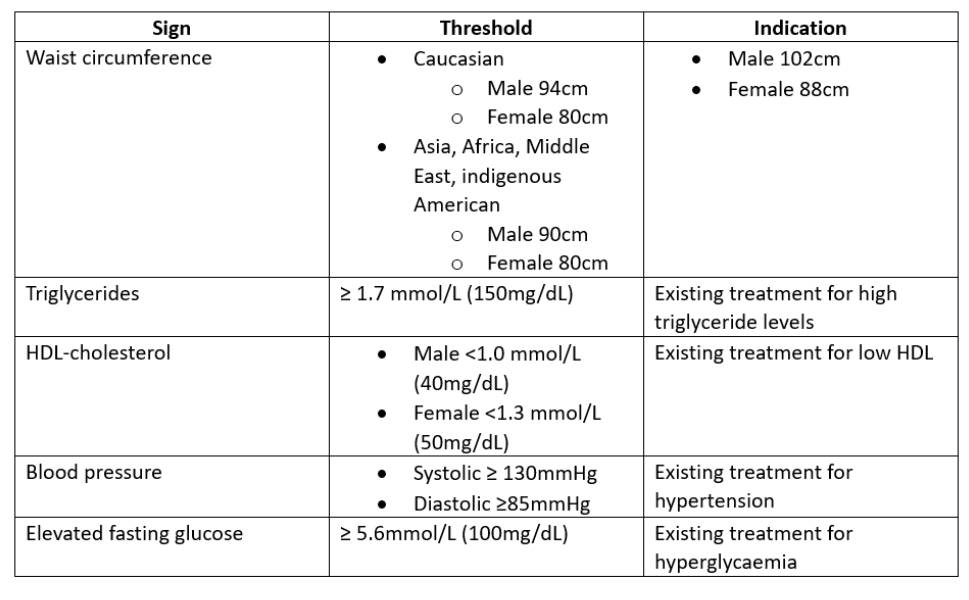
Table 1: Indicators for MetS. The presence of any three may confirm diagnosis5
Diagnosis
The origins of MetS as a distinct condition have been written about for over 100 years.3 The criteria for defining MetS are based around a number of abnormal metabolic components5 as shown in table 1 and summarised in figure 1. The key components including:
- Central obesity
- Hyperlipidaemia: raised levels of triglycerides, low levels of high-density lipoprotein (HDLs) and cholesterol
- Hyperglycaemia and insulin resistance
- Hypertension
Clinically, any three of these can be used to confirm the diagnosis of MetS.6
Figure 1: Checklist of risk factors for diagnosing metabolic syndrome
Epidemiology
MetS is thought to affect between 25% and 33% of adults over 50 years of age in the UK.7, 8 Worryingly, the prevalence of MetS among children in the UK is now thought to be as high as 10%, depending on criteria used, and has been cited as affecting 44% of overweight and obese children.9
The consequence of this is that 85% of obese pre-teens will become obese adults, and obese teenagers are 17 times more likely to become obese adults. While children with obesity rarely develop cardiovascular events during childhood, there is strong evidence for them undergoing accelerated atherogenesis, the arteries becoming narrowed and hardened due to a build-up of lipid plaques in the artery wall. Persistent obesity carries significant risk for developing type 2 diabetes, hypertension, dyslipidaemia and cardiovascular disease.
It is important to note that obesity can coexist with a healthy metabolism and, likewise, people of normal weight can have metabolic abnormalities. It is for this reason that some clinicians consider obesity to be a biomarker or risk factor that drives the development of MetS, rather than the primary cause of MetS.9 Although not yet fully understood, there appears to be an interplay between excess adiposity, insulin resistance and chronic inflammation.2, 9
The rest of this introductory article will now focus on each of the risk factors for MetS.
Obesity
Obesity is on the rise around the world and appears to be the driving force for a number of MetS components. The exact reasons for this obesity epidemic are widely debated and viewed from a number of perspectives. Factors cited as contributing to the increase include changes in nutrition, lack of physical activity and socioeconomic factors. The net effect of these factors is a chronic positive energy balance, with predictions of the prevalence of obesity reaching 30 to 50% of the UK population over the next two decades,10, 11 with associated increases in CVD and diabetes,12 and is projected to affect those between 60 and 69 years the most.9
Not all people with obesity have adverse metabolic profiles that predispose them to CVD or type 2 diabetes. Obesity confirmed without the other risk factors (hypertension, insulin resistance and hyperlipidaemia) has been termed metabolically healthy obesity (MHO).13-15 The prevalence of MHO is high, affecting between 12 and 17% of adults with obesity.16 These individuals are not immune from developing cardiometabolic diseases, but are likely to develop them at a lower rate than those with metabolically unhealthy obesity (MUO, where the obesity is accompanied by other risk factors) and at a higher rate than metabolically healthy, lean individuals.17 MHO can be considered transient for the majority of individuals, as they have a tendency to transition to MUO, thereby developing MetS.18 The implication is, therefore, that MHO is unstable and not a reliable indicator of future risk.
The overall message is that MHO should not be considered a healthy condition, and interventions should be considered in all individuals with obesity. The type of intervention advocated varies from encouraging weight loss to one of maintenance of cardiometabolic parameters.17,18
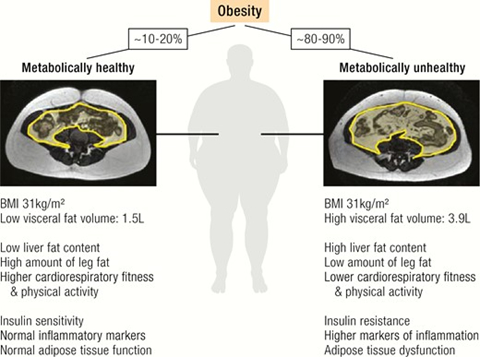
Figure 2 shows the main metabolic differences between MHO and MUO. Individuals with MHO are characterised by lower liver and visceral fat mass, higher leg fat content, greater cardiorespiratory fitness and physical activity, insulin sensitivity, normal inflammation markers and preserved adipose tissue function compared to patients with metabolically unhealthy obesity. MRI scans show greater visceral fat deposition associated with MUO.54
Figure 2: Differences between metabolically healthy (left) and metabolically unhealthy (right) obesity
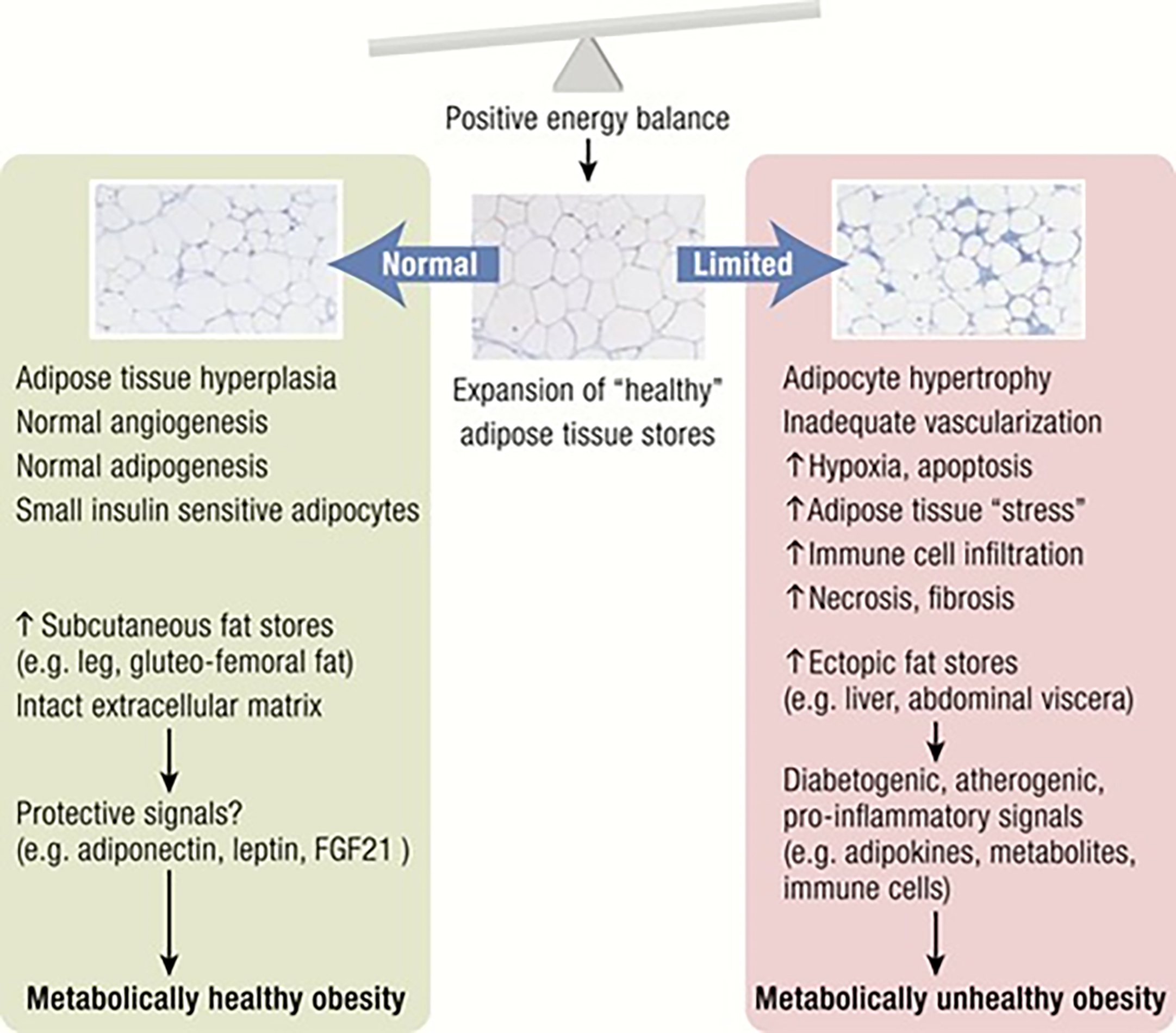
Another way of distinguishing between MHO and MUO is shown in figure 3. A chronically positive energy balance requires expansion of adipose tissue (AT) to store excess energy. Adipose tissue responds to higher storage demands by increasing the adipocyte number through adipogenesis from precursor cells (hyperplasia) and through adipocyte hypertrophy. If the expansion of healthy fat stores, such as subcutaneous leg fat, and the ability of AT to respond to excess calorie intake with healthier hyperplasia are impaired, AT dysfunction may develop. This is characterised by ectopic fat deposition (in the liver, abdominal visceral depots, skeletal muscle and pancreas) and a sequence from adipocyte hypertrophy through to fibrosis results. Adipose tissue dysfunction leads to the release of pro-inflammatory, diabetogenic, and atherogenic signals, such as adipokines, fatty acids from increased lipolysis, other metabolites and immune cells). This may contribute to end organ damage (such as in the liver, skeletal muscle, pancreas, vasculature) and the development of metabolically unhealthy obesity.
Figure 3: Differentiation of MHO and MUO by the nature of the expansion of adipose tissue.
In contrast, healthy expansion of AT leads to metabolically healthy obesity through an increased AT storage capacity, which serves as a safe ‘metabolic sink,’ and the secretion of a beneficial adipokine profile.
The expansion of adipose depots can be driven either by the increase in adipocyte size (hypertrophy) or by the formation of new adipocytes from precursor differentiation in the process of adipogenesis (hyperplasia). Notably, adipocyte expansion through adipogenesis can offset the negative metabolic effects of obesity, and the mechanisms and regulators of this adaptive process are now emerging.
Over the past several years, we have learned a considerable amount about how adipocyte fate is determined and how adipogenesis is regulated by signalling and systemic factors. We have also gained appreciation that the adipogenic niche can influence tissue adipogenic capability. Approaches aimed at increasing adipogenesis over adipocyte hypertrophy can now be explored as a means to treat metabolic diseases.
Limitations of the body mass index (BMI)
The body mass index (BMI) is a simple index of weight:height and is commonly used to classify overweight and obesity and is defined as a persons weight (in kg) divided by the square of their height (in metres) and is expressed in kg/m2 (table 2).
Table 2: Interpreting body mass index
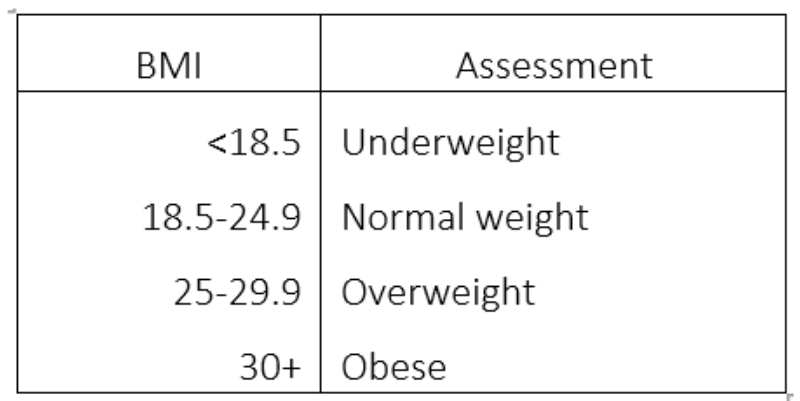
While BMI is undoubtedly a useful tool, it does have certain limitations. Specifically, it does not give an accurate measure of body fat as it does not differentiate between weight caused by muscle or fat and does not differentiate between visceral and peripheral fat distribution types.19,20 It has been stated that BMI alone is not enough to assess CVD risk and that waist circumference should be added to the assessment of overweight or obese individuals.21
Adipose tissue distribution
Non-visceral fat is found just below the skin in a region called the hypodermis. This subcutaneous fat is not related to many of the classic obesity-related pathologies, such as heart disease, cancer and stroke, and some evidence even suggests it might be protective.
Visceral adipose tissue (also known as organ fat or intra-abdominal fat) is located inside the abdominal cavity and is packed between the organs (the stomach, liver, intestines, kidneys and so on). An excess of visceral adipose tissue (VAT) is known as abdominal obesity in which the abdomen protrudes excessively. Epicardial adipose tissue is a particular form of VAT deposited around the heart and found to be a metabolically active organ that generates various bioactive molecules, which might significantly affect cardiac function.
In overweight or obese individuals, the level of VAT, rather than the level of subcutaneous AT, appears to correlate more with the risk development of CVD and diabetes.22, 23 Obesity is the most common cause of hypertension, impacting 65% of women and 78% of men,24 and VAT is a better predictor of increased blood pressure than subcutaneous fat, regardless of the level of BMI.23
Ectopic fat is the storage of triglycerides in tissues other than adipose tissue, tissues that are supposed to contain only small amounts of fat, such as in the liver, skeletal muscle, heart and pancreas. Ectopic fat is stored in relatively high amounts around the organs of the abdominal cavity, but is not to be confused with visceral fat. In the pancreas, this can interfere with cellular function and hence organ function and is associated with insulin resistance in type 2 diabetes.25 In the liver, the result can be ‘fatty liver’.
Though this does not appear to represent an independent risk factor for cardiovascular disease, liver fat can present in the absence of excess VAT, and appears to be associated with a two-fold increased risk of type 2 diabetes.26 The specific cause for the accumulation of ectopic fat is unknown. The cause is likely a combination of genetic, environmental and behavioural factors that are involved in excess energy intake and decreased physical activity.
To summarise:
- Excess visceral adipose tissue, with or without liver fat, increases the risk of cardiovascular disease
- Excess liver fat without visceral adipose tissue increases the risk of type 2 diabetes
Another association with VAT is the deposition of excess lipid in the skeletal muscle, something that is not found in individuals with subcutaneous obesity.27 This lipid deposition has been linked to insulin resistance.
Interventions
Both VAT and ectopic fat respond positively to lifestyle modification via diet quality alteration and increased physical activity. This may be independent of overall weight loss,28 leading researchers to suggest that loss of VAT and ectopic fat should be the main focus of obesity interventions, rather the magnitude of weight loss overall.19 It has been suggested that a modest weight reduction of 5 to 10% can account for up to a 30% loss of VAT and a concomitant improvement in the CVD risk profile,28 and that this could be considered more achievable goal to maintain.20
Since a number of MetS components are lifestyle driven, it appears clear that they are rarely assessed in a clinical setting.29 The usefulness of targeting lifestyle metrics in the clinical setting has, consequently, gained some attention in recent years. It has been proposed that healthcare professionals might consider the following four factors:
- Waist circumference
- Cardiorespiratory fitness
- Overall diet quality
- Level of physical activity
An enlarged waist circumference increases morbidity and mortality risk, regardless of BMI,30 and is highly predictive of excessive visceral adipose tissue31, 32 and elevated triglycerides, one of the MetS risk factors.31
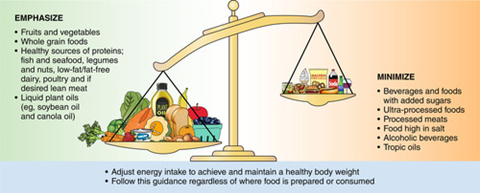
It is known that a good diet is important to overall health and wellbeing and limiting the likelihood of MetS.33 There is a shift towards food-based recommendations from clinicians,34 encouraging individuals to eat more of certain food groups and less of others (figure 4). The adoption of the Mediterranean diet has been shown to be beneficial.35 Increased physical activity is also known to be beneficial to health and well-being and positively impacts upon many of the MetS risk factors.
Figure 4: Shifting the dietary balance of patients is an increasingly useful tool employed by clinicians in maintaining health33
To summarise the key points for eye care professionals (ECPs):
- Reduced waist circumference is more useful than focusing on BMI and a good indicator of positive change in VAT
- Waist circumference is sensitive to lifestyle changes
- Discuss a healthy diet and adopting a Mediterranean diet
- Discuss the importance of physical activity
Triglycerides
Triglycerides are lipids found in the bloodstream. They represent the main source of energy and are essential for good health. However, an excess within the blood increases the risk of heart disease and CVD, while very high levels can cause serious medical conditions such as pancreatitis.36
Triglycerides (TGs) are derived from ingested lipids as well as through the conversion of excess sugar and alcohol. They are our main source of energy and come from two sources:
- Ingested food, entering the blood stream after a meal and carried in lipoproteins called chylomicrons
- Synthesised by the liver and carried in the blood stream in a different lipoprotein called VLDL very low density lipoprotein (VLDL)
After food consumption, TGs are absorbed in the small intestine where they combine with proteins called chylomicrons and enter the blood stream to be either used as an energy source, through conversion to glucose (gluconeogenesis) in the liver, or stored for later use in adipose tissue.
Triglycerides are a combination of:
- Three fatty acids (saturated, unsaturated fat or both)
- Glycerol, a form of glucose
Both TGs and cholesterol are types of fat that need to be attached to protein in order to pass through the body. These proteins, or lipoproteins, are of five types, dependant on the amount of protein in the lipoprotein (figure 5). The nature of the lipoprotein influences their impact upon overall cardiovascular health.36
Figure 5: Classification of lipoproteins
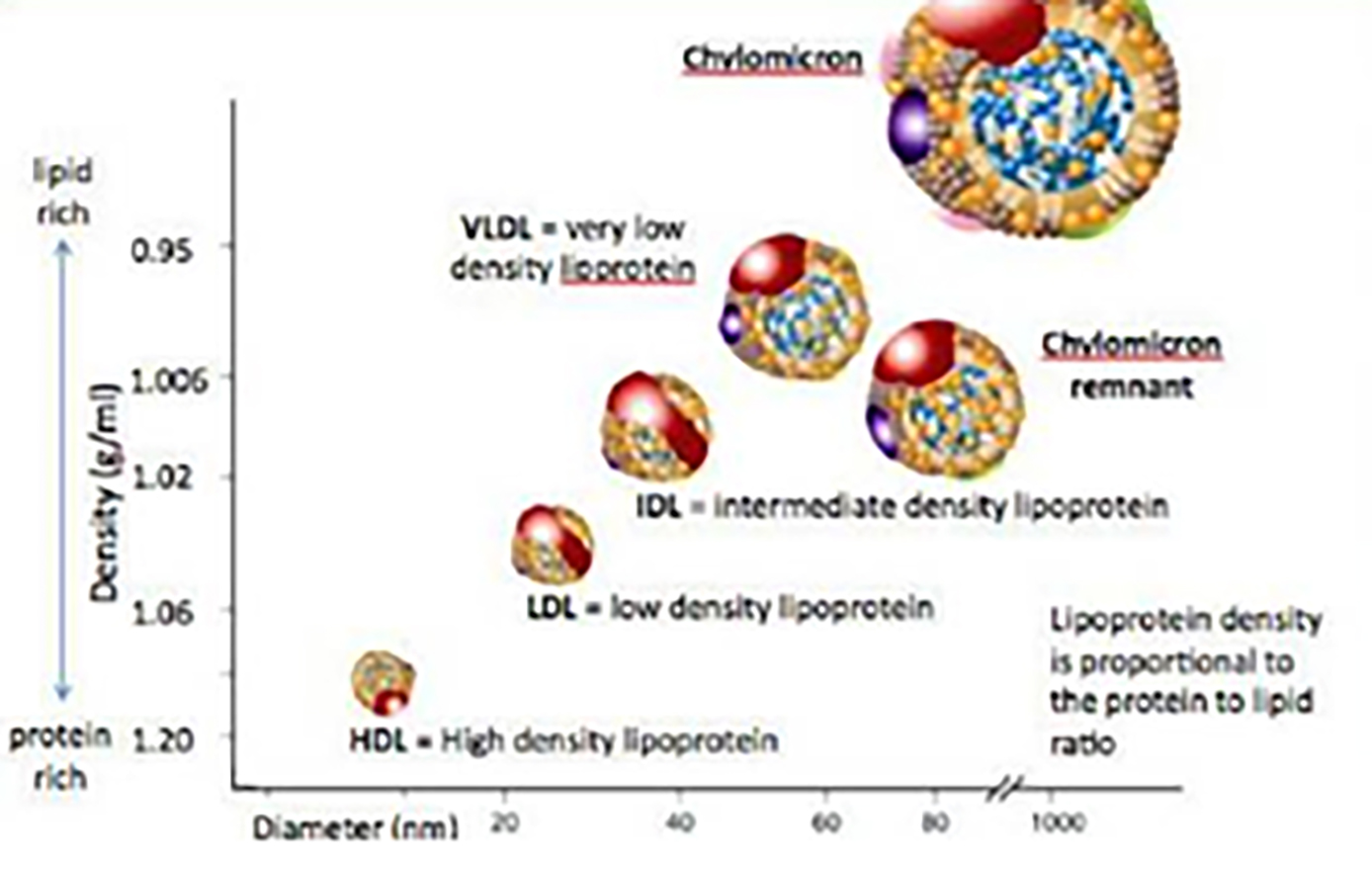
- Low density lipoproteins (LDL): These LPs transport cholesterol to the cells and have a lower protein to cholesterol ratio and so are considered low density. Too much LDL in the blood can result in clogging up blood vessels, or atherosclerosis, and hence LDL-bound cholesterol is described colloquially as ‘bad cholesterol’.
- Very low-density lipoprotein (VLDL): These LPs have a very low protein to lipid ratio. They carry TGs, and some cholesterol, from the liver to other cell sites around the body. As with LDL, too much VLDL-bound cholesterol in the blood can promote atherosclerosis.
- High density lipoproteins (HDL): These lipoproteins contain a high protein: cholesterol content, and carry cholesterol away from the cells and back to the liver, thereby lowering the cholesterol in the body. For this reason, LDL is described colloquially as ‘good cholesterol’.
- Intermediate density lipoprotein (IDL): These LPs carry cholesterol and TGs and are considered VLDL after some of the TGs are removed. The amount of lipid bound is between that found in VLDL and LDL.
- Chylomicrons: These are the largest LPs. They carry TGs from the gut to the liver after a meal. They are broken down in the liver where the lipids are reassigned to one of the other lipoproteins.36
As there is a clear association between the blood levels of different bound lipoproteins, tables 3 and 4 summarise the guides used for healthy cholesterol and TG levels. These is an association of elevated TG with lowered HDL (good cholesterol) leading to heart disease.
Table 3: Healthy triglyceride levels
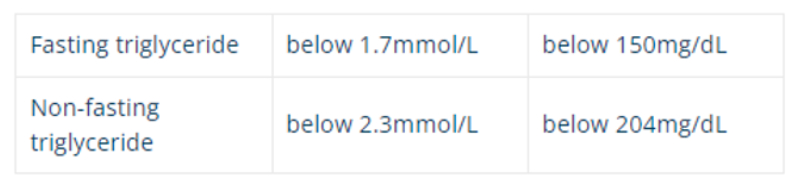
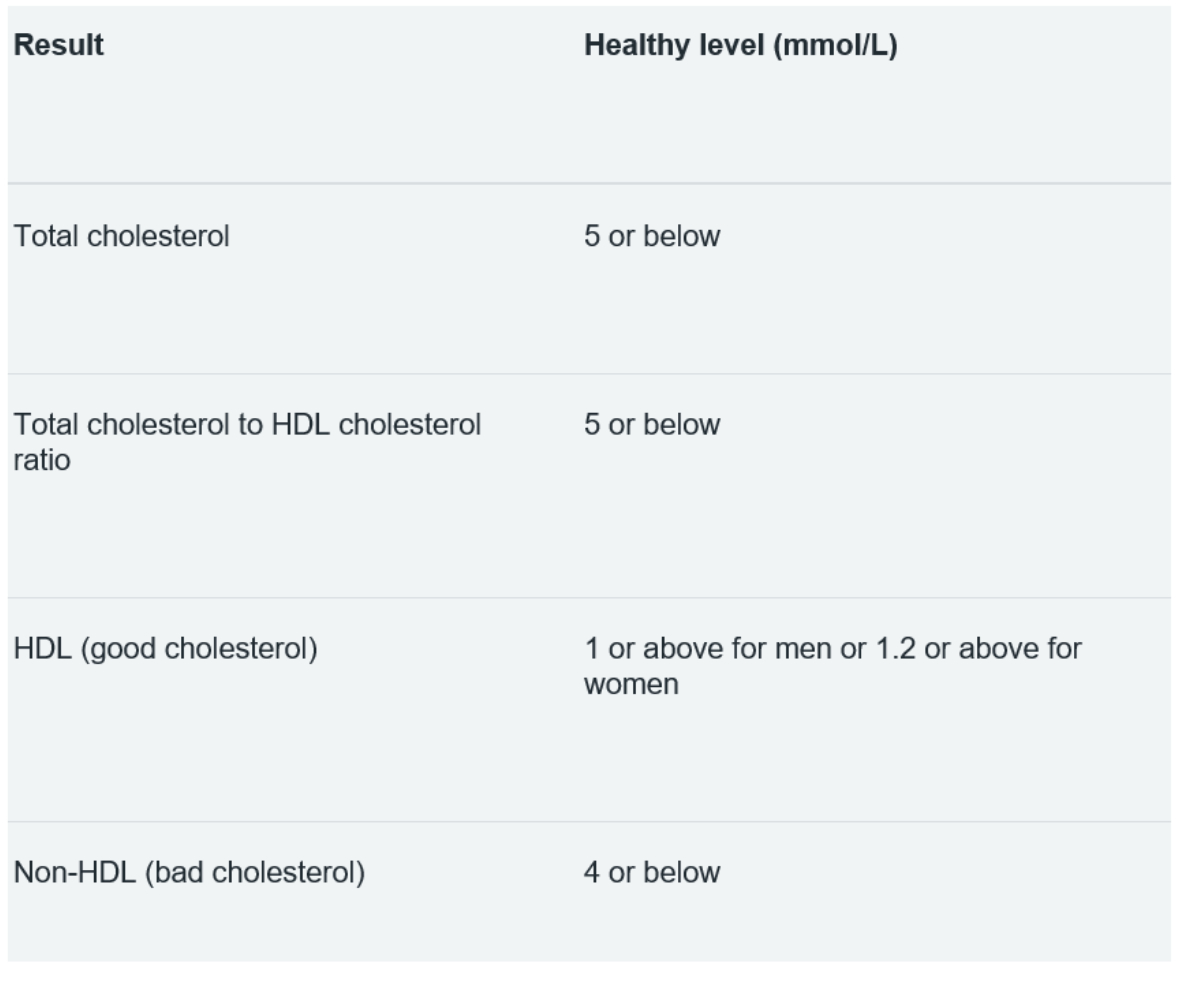
Table 4: Healthy cholesterol levels37
Elevated levels of TG can be due to primary (inherited) and secondary causes. The more common causes of secondary TG elevation are due to the following:
- Lifestyle:
- Sedentary lifestyle
- Unhealthy diet; particularly those diets high in saturated fats and added sugar
- Alcohol intake in certain people
- Medical Conditions:
- Kidney disease
- Non-alcoholic fatty liver disease
- Pregnancy
- Underactive thyroid
- Medications:
- Some diuretics: Furosemide, bumetanide and torsemide38
- Oestrogen therapy (oral tablets)39
- Beta-blockers40
An excellent resource for ECPs about all matters triglyceride can be found at www.cuh.nhs.uk/patient-information/dietary-advice-for-management-of-high-triglycerides.
There are a number of approaches for the management of elevated levels of triglycerides.41
- Lifestyle: Lifestyle changes are considered the cornerstone of treatment and TG levels respond well to changes in diet and lifestyle. Maintaining a healthy weight can significantly reduce harmful blood lipid levels.42 Intermittent fasting is gaining popularity for weight loss and there is increasing evidence suggesting it may reduce the risk of MetS.43 Smoking cessation has a beneficial effect on cardiovascular risk, resulting in improvements in HDL levels in particular.44
- Medication: this can be considered if lifestyle change is insufficiently effective. Different types of medicine can improve triglyceride levels, including the following:
- Fibrates
- Nicotinic Acid
- Prescription Omega-3
- Statins
Hypertension
Hypertension or raised blood pressure, is a common finding in individuals with MetS.45 It is present in up to 85% of MetS patients,46 and is more common in obese individuals, particularly those with excess VAT.23 Although not totally understood, the precise mechanisms by which obesity promotes HTN are complex and covered in detail elsewhere.22, 23
As a general guide:
- High blood pressure is considered to be from 140/90mmHg (or an average of 135/85mmHg at home) – or 150/90mmHg (or an average of 145/85mmHg at home) if over the age of 80
- Ideal blood pressure is usually considered to be between 90/60mmHg and 120/80mmHg, while the target for over-80s is below 150/90mmHg (or 145/85mmHg at home)
- Blood pressure readings from 121/81mmHg to 139/89mmHg could mean a risk of developing high blood pressure if steps are not taken to keep your blood pressure under control
As with many other individual components of MetS, lifestyle modification plays an important role in the management of hypertensive patients with MetS, minimising the condition and improving the efficacy of hypertensive medication where prescribed.23 The use of lifestyle therapies, particularly diet and exercise, have been considered as a first step in the management of MetS.23
The Mediterranean diet has been advocated for improving BP and reducing the risk of CVD. The Dietary Approaches to Stop Hypertension (DASH) diet has been shown to be beneficial in reducing blood pressure.47 Interestingly, the Mediterranean Diet and DASH diets appear to be more effective in younger adults (<45 years), suggesting a tailored management approach by age group may be needed.48 The inclusion of physical activity into lifestyle discussions appears more important than diet in older adults in whom it has the most positive effect on BP.48
Useful information about blood pressure is available at www.nhs.uk/conditions/high-blood-pressure-hypertension/treatment.
When BP remains high in spite of lifestyle modifications, then antihypertensive medications should be considered in MetS patients with raised blood pressure. Such treatment is generally with the following classes of medications:
- Angiotensin Converting Enzyme Inhibitors (ACEi): This family of medication inhibits the formation of the enzyme, angiotensin II, which normally promotes constriction of the blood vessels. Blocking this enzyme results in relaxation of the vessels and a reduction in BP. Commonly prescribed examples in the UK include:
- Ramipril
- Lisinopril
- Enalapril
- Perindopril
- Angiotensin-II Receptor Blockers (ARB): This family of medications blocks the angiotensin II receptors, thereby reducing blood vessel constriction and aiding relaxation of the blood vessels, and reducing BP. They are often considered alongside ACEi in the way they cause side effects. Commonly prescribed examples in the UK include:
- Losartan
- Candesartan
- Valsartan
- Olmesartan
- Irbesartan
- Calcium Channel Blocker (CCB): Vasoconstriction is reduced through the action of blocking calcium entry into the vascular smooth muscle, with a resulting reduction in BP. Commonly prescribed examples in the UK include:
- Amlodipine
- Felodipine
- Nifedipine
- Diltiazem
- Verapamil
- Diuretics: This class of medication works in the kidneys and enhance the expression of fluid out of the body, thereby reducing BP. Commonly prescribed examples in the UK include:
- Bendroflumethiazide
- Indapamide
- Others include Amiloride and Spironolactone
Other families of medication used in the management of hypertension include:
- Beta blockers: This family of medications reduce BP by slowing the heart and with less force. They are less commonly used nowadays as other medications are considered more effective, although can be considered if other treatments have not worked. Examples include:
- Atenolol
- Bisoprolol
- Alpha blockers: These achieve their blood pressure lowering effect through relaxing blood vessels. The most commonly used example is doxazosin
The ocular implications of hypertension and its treatment are to be considered in more detail in part two of this series.
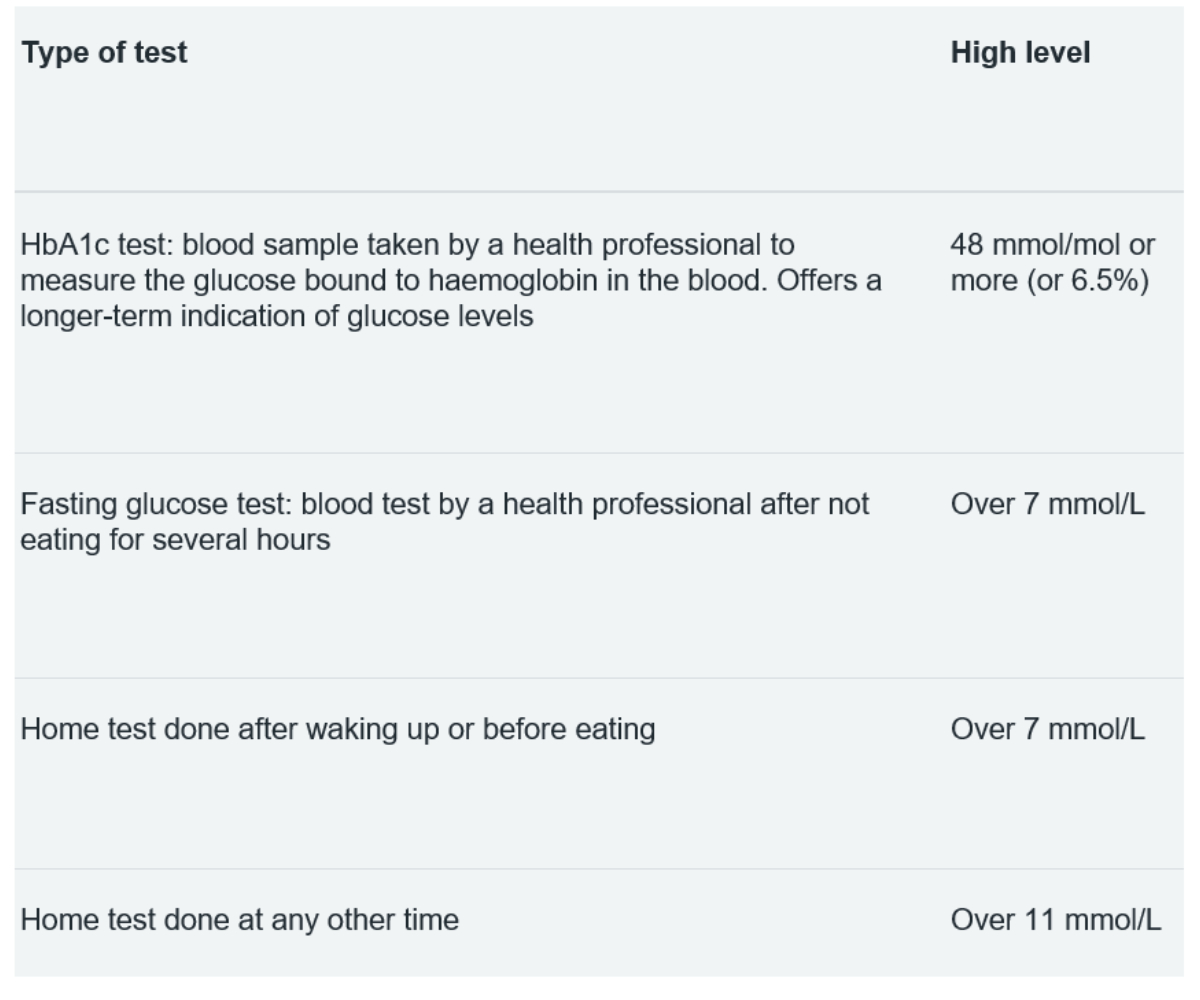
Table: 5 Glucose tests and results
Hyperglycaemia
Optometrists are familiar with diabetes mellitus and its ocular effects, and these will be summarised in part three of this series. The concept of insulin resistance is perhaps less familiar, and its importance as a risk factor for MetS is worth discussing. Insulin resistance is a process whereby our cells do not respond appropriately to the effects to the hormone insulin.49 This can ultimately lead to type 2 diabetes. Let us take a step back and look at the process in a little more detail.
The role of insulin is to promote the entry of glucose into the cells to be used for energy or stored as fat. Figure 6 shows how glucose enters the cell via a glucose channel. The majority of our cells need the presence of insulin to facilitate the entry of glucose, including those within the retina.50 It is worth noting that approximately one third of our cells use glucose without the need for insulin, for example beta cells in the pancreas and various cells within the brain.51
Figure 6: The mediation of glucose entry to a cell by insulin
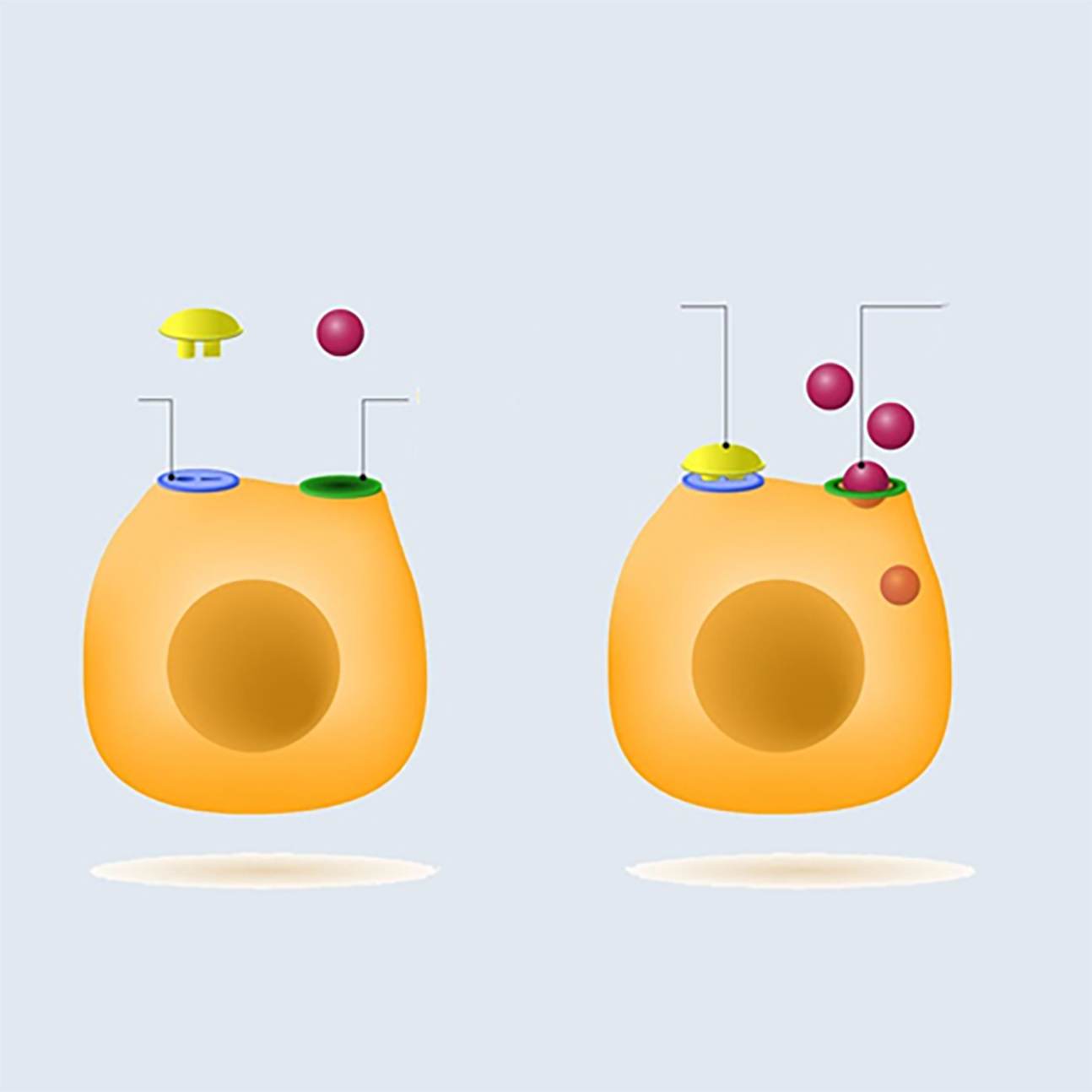
When insulin is present, it binds to specific receptors on the surface of the cell and initiates a set of mechanisms within the cell that allows glucose to enter the cell. The implication is that, if the internal mechanisms are not working appropriately, the glucose channel remains closed, so preventing glucose entry and a net accumulation of glucose outside of the cell and circulating in the blood, described as hyperglycaemia.
Beta cells in the pancreas are heavily perfused and monitor how much glucose is in the blood, and release insulin accordingly. These pancreatic beta cells are bathed in glucose and produce more insulin in response, unaware that the peripheral cells are not responding to insulin. The beta cells eventually cease working and this results in hyperglycaemia.
This begs the question as to why the cell is no longer responding to insulin? It is believed that the internal mechanism for facilitating glucose entry within the cells is impacted by altered lipid metabolism (notably free fatty acids and triglycerides), which in turn links back to obesity.52, 53
Table 5 summarises the different tests for assessing blood glucose levels and the healthy limit for each measurement. Just as it is useful for each patient to know their glucose profile, it is also useful for ECPs to be familiar with these figures when assessing their patients.
Diet and exercise modification is usually sufficient to control blood sugar levels where glucose resistance has been identified. Nevertheless, there is a wide variety of anti-diabetes drugs that can be prescribed when the situation requires and without the need for recourse to insulin treatment. The decision to prescribe is based on both the safety of the drug treatment and its predicted effectiveness, as well as patient tolerance of the drug, any relevant comorbidity, and potential interactions with other existing medications. Medications used include the following:
- Metformin hydrochloride: One of the most used drugs used for treating type 2 diabetes. This acts by lowering both the basal and post-prandial (after eating) blood glucose concentrations. It is the first choice for initial treatment for all patients with type 2 diabetes and, according to NICE, also has a ‘positive effect on weight loss, reduced risk of hypoglycaemic events and the additional long-term cardiovascular benefits associated with its use.’
- Sulphonylureas: Another group of drugs used for the treatment of type 2 diabetes. They are potent osmotic agents
- Thiazolidinediones (TZDs): These are drugs that ameliorate both peripheral and hepatic insulin resistance and are considered to be an effective glucose-lowering treatment for patients with type 2 diabetes. They are only used as a second or third-line therapy, and are prescribed in combination with other oral agents or insulin.
Other anti-diabetes drugs include:
- Meglitinides
- Gliptins
- Sodium glucose co-transporter 2 inhibitors
- Glucagon-like peptide-1 receptor agonists
Conclusion
Metabolic syndrome is a common disorder representing the presentation of a number of signs and underlying health imbalances that can increase the subsequent risk of major health problems. Lifestyle drives a lot of the MetS risk factors, yet there appears little in the way of lifestyle questioning in a clinic setting. There is a growing body of evidence to promote raising awareness in medical settings of waist circumference, physical activity level and nutrition quality to supplement blood results.
ECPs are in a position to assess health and offer advice to prevent future problems. Also, some of the key warning signs for MetS are also strongly associated with ocular changes. These will be the focus of the next two parts of this series, while the final article will discuss how best the ECP might use this information in helping to minimise the potential for MetS health concerns.
- Dr Rohit Narayan is a senior lecturer at Anglia Ruskin University, Cambridge and a therapeutic optometrist with an interest in holistic optometric care.
- Anoushka Narayan is a final year medical student at Aston University.
References
- Roddy, G.W., 2020. Metabolic syndrome is associated with ocular hypertension and glaucoma. Journal of Glaucoma, 29(9), pp.726-731
- Fahed, G et al. Metabolic syndrome: Updates on pathophysiology and management in 2021. International Journal of Molecular Sciences, 2022, 23(2), p.786
- Nilsson, P.M., Tuomilehto, J. and Rydén, L. The metabolic syndrome–what is it and how should it be managed? European Journal of Preventive Cardiology, 2019, 26(2_suppl), pp.33-46
- Eckel RH. The metabolic syndrome. Chapter 32, Harrison’s Cardiovascular Medicine, McGraw-Hill Education
- Alberti, K.G.M.M. and Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Medicine, 1998, 15(7), pp.539-553
- Bosomworth, N.J. Normal-weight central obesity: Unique hazard of the toxic waist. Canadian Family Physician, 2019, 65(6), pp.399-408
- https://www.heartuk.org.uk/genetic-conditions/metabolic-syndrome (Accessed 09.05.2023)
- https://www.nhs.uk/conditions/metabolic-syndrome (Accessed 09.05.2023)
- Baranowski, E.S. and Barrett, T.G. Metabolic syndrome: unravelling or unravelled? Paediatrics and Child Health, 2019, 29(7), pp.297-302
- Janssen, F., Bardoutsos, A. and Vidra, N. Obesity prevalence in the long-term future in 18 European countries and in the USA. Obesity Facts, 2020,13(5), pp.514-527
- McPherson, K., Marsh, T., Brown, M. and Britain, G. Tackling obesities: future choices: Modelling future trends in obesity and the impact on health. 2007, Department of Innovation, Universities and Skills
- https://www.diabetes.org.uk/about_us/news/1-10-adults-living-diabetes-2030 (Accessed 09.05.2023)
- Wildman, R.P et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004). Archives of Internal Medicine, 2008, 168(15), pp.1617-1624
- Ortega, F.B., Lavie, C.J. and Blair, S.N. Obesity and cardiovascular disease. Circulation Research, 2016, 118(11), pp.1752-1770
- Lavie, C.J. et al. Healthy weight and obesity prevention: JACC health promotion series. Journal of the American College of Cardiology, 2018, 72(13), pp.1506-1531
- van Vliet-Ostaptchouk, J.V. et al. The prevalence of metabolic syndrome and metabolically healthy obesity in Europe: a collaborative analysis of ten large cohort studies. BMC Endocrine Disorders, 2014, 14(1), pp.1-13
- Blüher, M. Adipose tissue dysfunction in obesity. Experimental and Clinical Endocrinology & Diabetes, 2009, 117(06), pp.241-250
- Mongraw-Chaffin, M. et al. Metabolically healthy obesity, transition to metabolic syndrome, and cardiovascular risk. Journal of the American College of Cardiology, 2018, 71(17), pp.1857-1865
- Piché, M.E. et al. Obesity phenotypes, diabetes, and cardiovascular diseases. Circulation Research, 2020, 126(11), pp.1477-1500
- Padmanabhan, P. et al. Metabolically healthy obesity: An eye-opener. Gastroenterology, Hepatology and Endoscopy Practice, 2023, 3(1), p.1
- Ross, R. et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men: a randomized, controlled trial. Annals of Internal Medicine, 2000, 133(2), pp.92-103
- da Silva, A.A. et al. Role of hyperinsulinemia and insulin resistance in hypertension: metabolic syndrome revisited. Canadian Journal of Cardiology, 2020, 36(5), pp.671-682
- Ihm, S.H. Pathophysiology and optimal management of hypertension in patients with cardiometabolic syndrome. Cardiometabolic Syndrome Journal, 2021, 1(1), pp.46-65
- Mendoza, M.F. et al. Hypertension in obesity. Current Opinion in Cardiology, 2020, 35(4), pp.389-396
- Snel M et al. Ectopic fat and insulin resistance: pathophysiology and effect of diet and lifestyle interventions. International Journal of Endocrinology, 2012: 983814
- Mantovani, A. et al. Non-alcoholic fatty liver disease and risk of incident diabetes mellitus: an updated meta-analysis of 501 022 adult individuals. Gut, 2021, 70(5), pp.962-969
- Kelley, D.E. and Goodpaster, B.H. Skeletal muscle triglyceride. An aspect of regional adiposity and insulin resistance. Clinical Diabetology, 2001, 2(4), pp.255-266
- Neeland IJ et al. Empagliflozin reduces body weight and indices of adipose distribution in patients with type 2 diabetes mellitus. Diabetes & Vascular Disease Research, 2016, Mar;13(2):119-26
- Lévesque, V. et al. Relation between a simple lifestyle risk score and established biological risk factors for cardiovascular disease. The American Journal of Cardiology, 2017, 120(11), pp.1939-1946
- Cerhan JR et al. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clinical Procedures, 2014, Mar;89(3):335-45
- Lemieux, I. et al. Hypertriglyceridemic waist: a marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein B; small, dense LDL) in men? Circulation, 2000, 102(2), pp.179-184
- Nazare, J.A. et al. Usefulness of measuring both body mass index and waist circumference for the estimation of visceral adiposity and related cardiometabolic risk profile (from the INSPIRE ME IAA study). The American Journal of Cardiology, 2015, 115(3), pp.307-315
- Lichtenstein, A.H. et al. and American Heart Association Council on Lifestyle and Cardiometabolic Health; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; and Stroke Council, 2021. 2021 dietary guidance to improve cardiovascular health: a scientific statement from the American Heart Association. Circulation, 144(23), pp.e472-e487
- Liu, A.G. et al. A healthy approach to dietary fats: understanding the science and taking action to reduce consumer confusion. Nutrition Journal, 2017, 16(1), pp.1-15
- Estruch, R et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. New England Journal of Medicine 378, no. 25 (2018): e34
- https://www.heartuk.org.uk/cholesterol/triglycerides (Accessed 09.05.2023)
- https://www.nhs.uk/conditions/high-cholesterol/cholesterol-levels (Accessed 09.05.2023)
- Anisman, S.D., Erickson, S.B. and Morden, N.E. How to prescribe loop diuretics in oedema. BMJ, 2019, Feb 21;364:l359
- Simpson, E.R. et al. Estrogen—the good, the bad, and the unexpected. Endocrine Reviews, 2005, 26(3), pp.322-330
- Borghi, C. et al. Hypertension and dyslipidemia combined therapeutic approaches. High Blood Pressure & Cardiovascular Prevention, 2022, 29(3), pp.221-230
- Mach, F. et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). European Heart Journal, 2020, 41(1), pp.111-188
- Zomer, E. et al. Interventions that cause weight loss and the impact on cardiovascular risk factors: a systematic review and meta-analysis. Obesity Reviews, 2016, 17(10), pp.1001-1011
- Varady, K.A. et al. Clinical application of intermittent fasting for weight loss: progress and future directions. Nature Reviews Endocrinology, 2022, 18(5), pp.309-321
- Maeda, K. et al. The effects of cessation from cigarette smoking on the lipid and lipoprotein profiles: a meta-analysis. Preventive Medicine, 2003, 37(4), pp.283-290
- Katsimardou, A. et al. Hypertension in metabolic syndrome: novel insights. Current Hypertension Reviews, 2020, 16(1), pp.12-18
- Duvnjak, L. et al. Hypertension and the metabolic syndrome. Diabetologia Croatica, 2008, 37(4), pp.83-89
- Saneei P et al. The Dietary Approaches to Stop Hypertension (DASH) diet affects inflammation in childhood metabolic syndrome: a randomized cross-over clinical trial. Annals of Nutrition & Metabolism, 2014;64(1):20-7
- Park, Y.M.M. et al. Mediterranean diet, Dietary Approaches to Stop Hypertension (DASH) style diet, and metabolic health in US adults. Clinical Nutrition, 2017, 36(5), pp.1301-1309
- Faiq, M.A. et al. Ocular manifestations of central insulin resistance. Neural Regeneration Research, 2023,18(5), pp.1139-1146
- Sánchez-Chávez, G. et al. Insulin stimulated-glucose transporter Glut 4 is expressed in the retina. PloS one, 2012, 7(12), p.e52959
- Wilcox, G. Insulin and insulin resistance. Clinical Biochemist Reviews, 2005, 26(2), p.19
- Sears, B. and Perry, M. The role of fatty acids in insulin resistance. Lipids in Health and Disease, 2015, 14(1), pp.1-9
- Kraegen, E.W. et al. Triglycerides, fatty acids and insulin resistance-hyperinsulinemia. Experimental and Clinical Endocrinology & Diabetes, 2001, 109(04), pp.516-526
