Age-related macular degeneration (AMD) is the major cause of vision loss in developed countries, accounting for almost half of the number of severely sight impaired and sight impaired certification in the UK.1 It is estimated that over 1.5 million people in the UK are affected by macular disease of which approximately 600,000 are related to AMD and this is expected to increase by 15% until 2050. It is further estimated that 39,800 people develop neovascular AMD in the UK each year.
Treatment options and pathways continue to evolve with exciting new management approaches on the horizon. The purpose of this article is to provide an update on referral pathways and treatment strategies. An insight into some emerging management approaches is also provided.
Age-related Macular Degeneration
AMD presents as metamorphopsia (figure 1), central or paracentral scotoma and a reduction in central vision, which can progress over days or weeks; from onset in the case of wet AMD or years in dry AMD.
Figure 1: Metamorphopsia

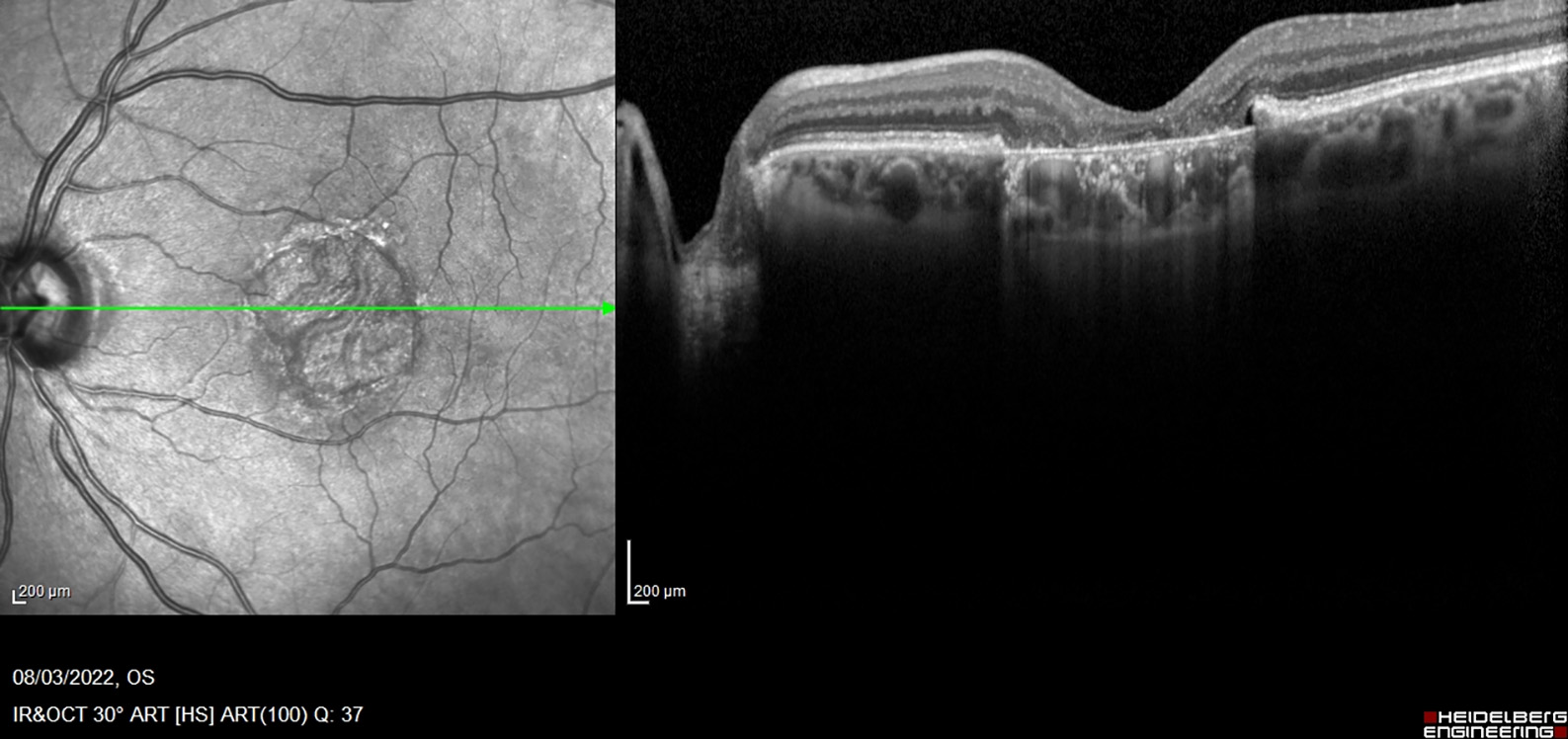
Dry (or atrophic) AMD is characterised by a thickening of Bruch’s membrane due to protein and lipid accumulation under the retinal pigment epithelium (RPE) called drusen (figure 2). The stages can be early where the patient is asymptomatic, intermediate where the patient may experience mild central blurring or poor visual acuity in low lighting through to late where there is geographic atrophy (figure 3) and patients will experience metamorphopsia, central scotoma and distortion of colour vision.
Figure 2: Drusen
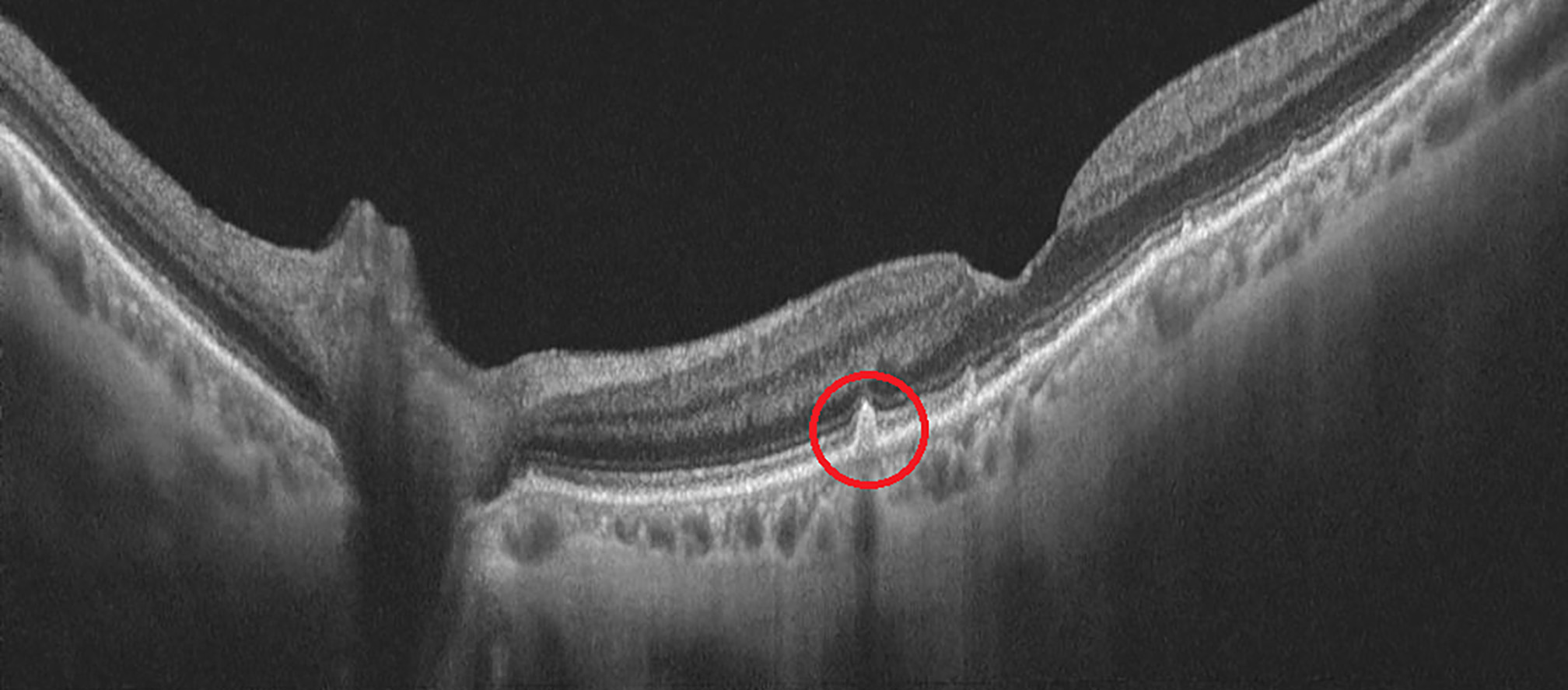
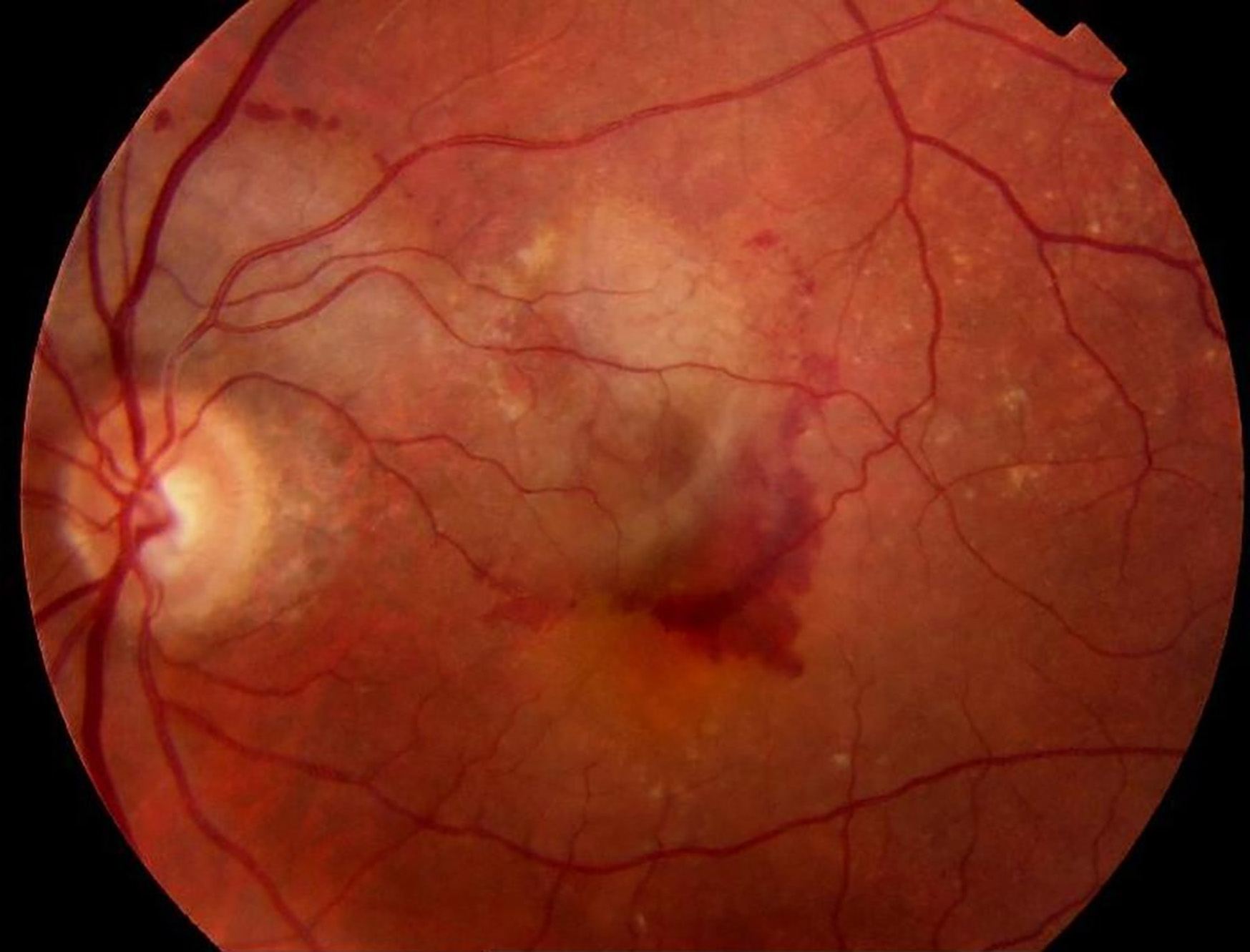
Approximately 10% of patients will go on to develop wet AMD. This is a result of immune cells responding to the damaged macula, causing secretion of proinflammatory and proangiogenic cytokines, particularly vascular endothelial growth factor (VEGF). This stimulates choroidal neovascularisation, which breaks through the neuroretina. This network of vessels leaks serous fluid, lipids, and/or blood in the subretinal or intra-retinal space (figure 4). This can lead to fibrovascular scar formation and death of the neurosensory retina.
Figure 3: Geographic atrophy
Wet AMD appears to be a multifactorial disease with numerous risk factors. These include:
- Increasing age
- Female gender
- Elevated cholesterol
- Cardiovascular disease
- Smoking
- Positive family history
- UV light exposure2, 3
- Genetic predisposition to AMD, with at least 32 genetic loci linked to the disease4
Figure 4: Blood in the intra-retinal space
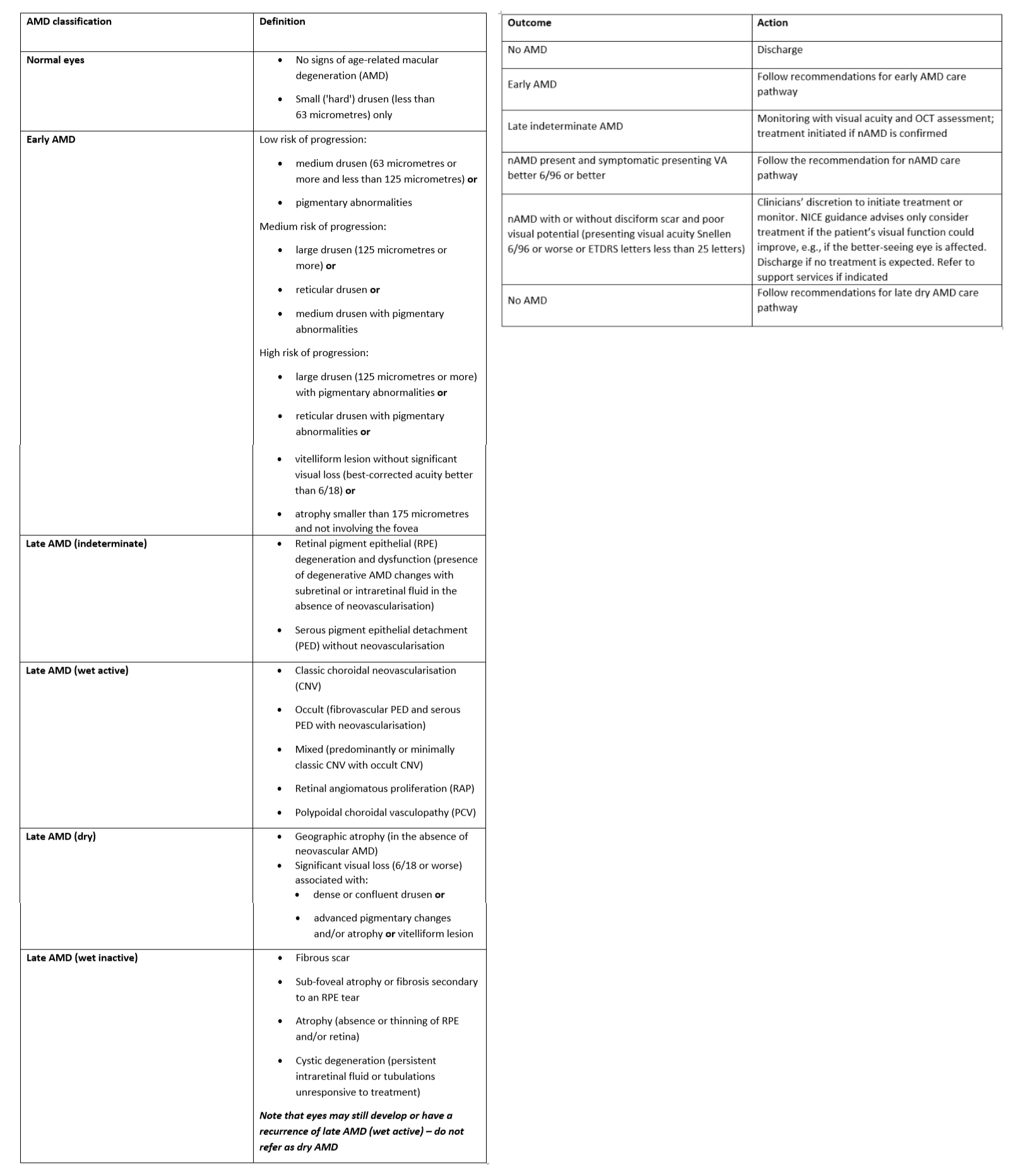
Wet AMD can be classified based upon the UK National Institute for Health and Care Excellence (NICE) guidance ng82, as seen in table 15 and the action as recommended by the commissioning guidelines on AMD set by the Royal College of Ophthalmologists seen in table 2.6
Table 1 & 2: (Left) Classification of wet AMD5; (right) Action and referral of AMD.6 AMD = age-related macular degeneration, ETDRS = early treatment diabetic retinopathy study, nAMD = neovascular age-related macular degeneration, NICE = National Institute for Health and Care Excellence, OCT = optical coherence tomography
Treatments for AMD
Dry AMD
Dry AMD treatments currently are limited to advice on diet including leafy greens, such as curly kale and Mediterranean diets, cessation of smoking, reducing UV exposure and with intermediate or worse disease the use of antioxidant supplements based on the ARED2 formula.7
In February 2023, the US Food and Drug Administration (FDA) approved pegcetacoplan (Syfovre), developed by Apellis Pharmaceuticals, as the first intravitreal drug for geographic atrophy. It is known that part of the immune system called the complement cascade is dysregulated in dry AMD patients, leading to inflammation-mediated cell damage, promoting the development of dry AMD and atrophy.
Pegcetacoplan targets the complement protein C3 to block the effects of this pathway on the macula as confirmed in the OAKS study.8 The intravitreal injections are administered once every 25 to 60 days. Apellis have made a submission to the medicines and healthcare products regulatory agency (MHRA) for the drug to be approved for use in the UK.
Iveric Bio has submitted a new drug application to the FDA for its drug avacincaptad pegol (Zimura) to treat dry AMD. This blocks the complement protein C5; which can slow down the degeneration of retinal cells, thus, slowing the advancement of AMD.
Another approach, which has reported results as a potential treatment for dry AMD, is the Valeda light delivery system (from Lumithera Inc). This uses photobiomodulation (PBM), a light-based technology that stimulates bioenergetic output in targeted tissues. Selected wavelengths of light in the far red to near infrared spectrum (500 to 1,000nm) modulate biologic function through direct and indirect cellular effects on mitochondrial respiratory chain components.
The retina is one of the most energy-demanding tissues in the body. PBM activation of photoacceptors in the mitochondria results in the improved generation of adenosine triphosphate (ATP) and the modulation of the production of intracellular signalling molecules, such as reactive-oxygen species (ROS) and nitric oxide (NO). This triggers secondary effects that produce sustained changes in cell function and viability. These effects can reduce inflammatory mediators, oedema, or drusen deposition. Modest improvement in visual function and increase in visual acuity has been demonstrated in early trials on dry AMD patients.9
Visual cycle modulators are drug treatments that stop toxic material (lipofuscin) from accumulating in the retina and, therefore, may be able to slow down vision loss in patients with dry AMD. Two drug treatments trialled are emixustat and fenretinide. When compared to a placebo, it was reported emixustat had little or no effect in slowing the progression of dry AMD. Fenretinide may slow progression of dry AMD, however further studies are required to confirm its efficacy.
As part of the FOCUS trial,10 the world’s first in-human gene therapy for dry AMD was performed in 2019. The technique involves injecting copies of a gene producing a propriety protein, called GT005 and developed by Gyroscope Therapeutics, into the subretinal space. This protein counteracts the inflammation caused by protein cells of the complementary over-acting immune system on retinal cells. A phase II Study (EXPLORE) to evaluate the safety and efficacy of two doses of GT005 is currently in progress.11
Two other possible treatments for dry AMD, which are in early clinical trials, are Oracea, an oral antibiotic (doxycycline) with anti-inflammatory properties, and metformin, which is commonly used for the control of diabetes as it seems to have an anti-inflammatory property which could lower the risk of developing dry AMD.
Current wet AMD treatment
Before 2003, patients with wet AMD had no treatments available and vision loss was likely. In 2003, NICE recommended photodynamic therapy (PDT) using a photosensitising drug called verteporfin. This drug attached to the inner surface of abnormal proliferating blood vessels and, when activated by non-thermal laser light at 689nm, generated highly reactive short-lived oxygen radicals which, in turn, damaged the walls of the abnormal blood vessel. The TAP study showed limited benefit in slowing reduction from baseline visual acuity in the order of 25% in classic lesions.12
Following NICE approval in 2008, an anti-VEGF drug ranibizumab (Lucentis) became available. This was recommended for patients with visual acuity between 6/12 and 6/96, with no permanent structural damage to the central fovea and a lesion size of less than 12-disc areas (figure 5).13
Figure 5: The development of intravitreal anti-VEGF injections greatly improved the visual prognosis in wet AMD
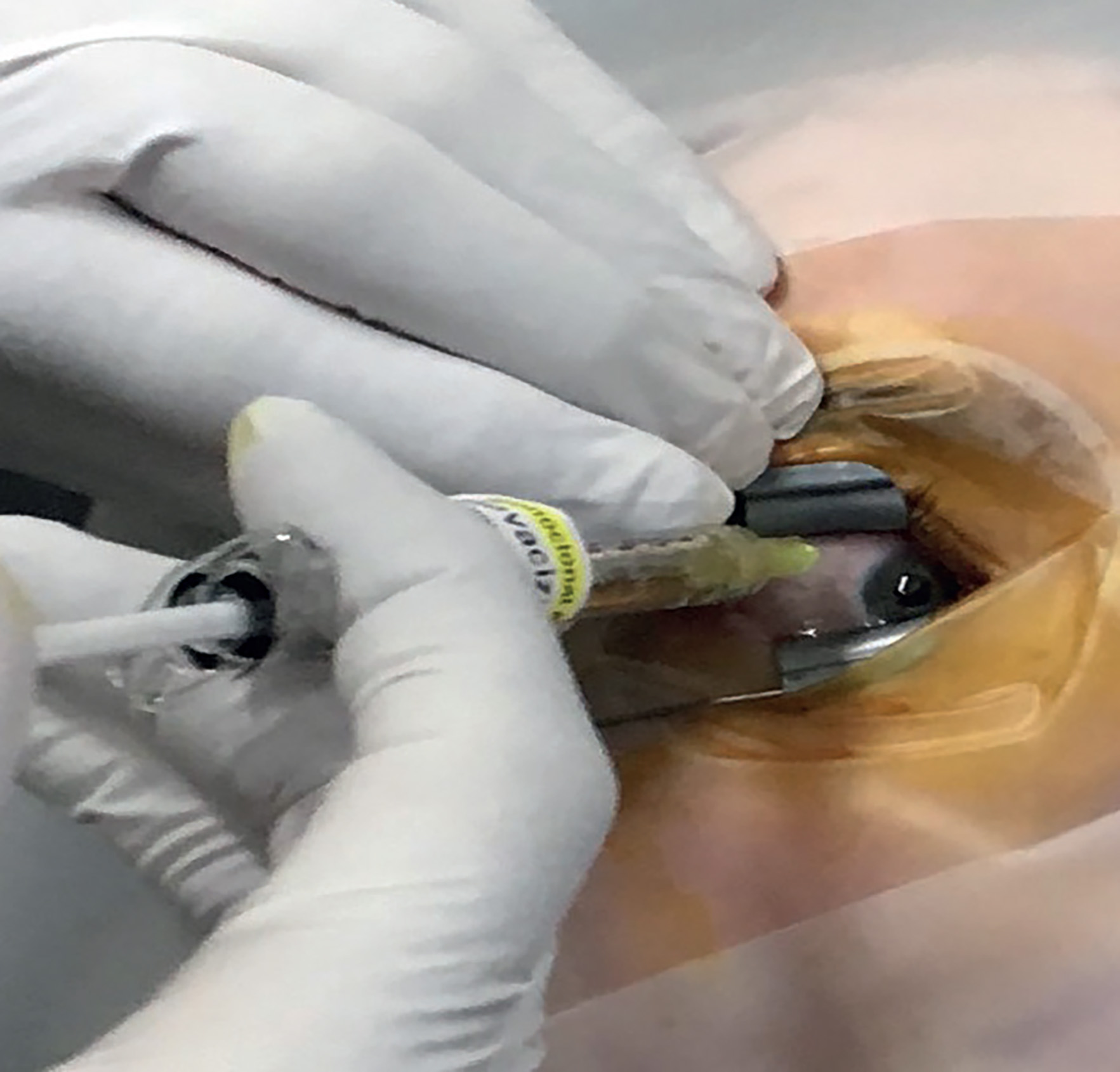
A number of studies reported on the efficacy of ranibizumab. The ANCHOR study 2006,14 compared the efficacy of ranibizumab injected monthly against PDT (verteporfin) and showed that ranibizumab-treated eyes lost less than 15 letters from baseline in 90% of cases and up to a 20-letter improvement over a two-year period compared to PDT in predominantly classic lesions.
In the same year, the MARINA study compared ranibizumab injected monthly to a sham control injection in a two-year, double-blind, sham-controlled study. The result showed up to a 20 letter gain with ranibizumab compared to the sham at the end of two years.
As monthly treatment was not thought to be sustainable on a population scale further studies were carried out to determine ways of maintaining the effects with less frequent dosing. The HORIZON Extension study, in 2009,15 showed that the gains from the ANCHOR and MARINA study were reduced with less frequent ‘as required’ ranibizumab dosing.
The PIER16, 17 and EXCITE18 studies compared fixed monthly and quarterly dosing. In both studies, best corrected visual acuity (BCVA) was maintained, but the monthly group achieved greater gains. Further subgroup analysis showed that there was a variability in response which could be used to individualise treatment.
The PRONTO19 and SUSTAIN20 studies looked at monthly review following the initial three monthly injections. The retreatment was based on central retinal thickness on OCT increasing > 100 microns and greater than five letter change in visual acuity from best achieved and, additionally with the PRONTO study, new onset of CNV, new haemorrhage or persistent fluid. They achieved good results in each study, although not quite as good as the monthly dosing in the SUSTAIN study, which also found that 20% of patients did not require an additional dose after the initial three. National audits showed that this type of approach was less effective in routine clinical practice possibly due to a difficulty in providing consistent capacity for monthly review appointments.21
Another individualised approach is known as ‘Treat and Extend’ (T&E). This is where a patient is treated at all clinic visits and, when the disease is inactive, the period between visits is extended at two-week intervals up to 12 weeks for ranibizumab. Conversely, when there is an increase in activity, the period between visits is reduced to a minimum of four weeks. This approach benefits the patient with fewer clinic visits and treatments in good responders and improves capacity for the clinic, but there will be some cases of treating patients where there is no disease activity.
The dosing approaches mentioned can all be used to individualise treatment. There is a greater adoption of the T&E approach, and this was supported by a recent meta-analysis demonstrating that T&E can achieve similar functional and anatomical outcomes compared to fixed or PRN dosing regimens.22
While ranibizumab was in development, another agent known to be chemically similar had been in use worldwide from around 2005; bevacizumab (Avastin). This was a drug that was developed to treat colorectal cancer. By scaling down the vial intended for intravenous use into the volume injected into the eye, when used off label in this way to treat wet AMD, similar efficacy was shown to ranibizumab in both the US CATT study23 and UK IVAN study.24 Its usage in the UK has been limited, due to concerns about the off-label status, while other NICE approved options are available and funded.
In 2011, Bayer launched aflibercept (Eylea), which was approved by NICE for treating wet AMD in July 2013. This is a VEGF trap, forming a stable and inert 1:1 complex with VEGF dimers.25 The VIEW studies25 showed that an equivalent effect could be achieved to monthly ranibizumab when aflibercept was administered bi-monthly after the initial three loading doses. Further studies showed equivalent benefit using a treat and extend approach with extension interval up to 16 weeks.26-28 At the time of writing, aflibercept is the most commonly used anti-VEGF in the UK.
Brolucizumab (Beovu) was approved by NICE in the UK in February 2021, with the expectation there could be fewer injections while maintaining vision and reducing retinal fluid as demonstrated in the HARRIER study.29 Brolucizumab inhibits the interaction of VEGF-A with its receptors on endothelial cells, preventing endothelial proliferation, vascular permeability, and neovascularisation. Despite studies showing the potential for a majority of patients to maintain visual gains with 12 weekly dosing after the loading stage, its usage has been limited by a relatively higher inflammation rate (4.6%) compared to other agents (0.8% with aflibercept) and rare cases of severe vision loss caused by retinal vascular occlusion.30
Faricimab (Vabysmo) was approved by NICE in June 2022. This has a combined effect of blocking both VEGF-A and Angiopoietin 2. This combined molecule has an anti VEGF-A component to reduce neovascularisation and vascular leakage and an anti-Ang2 component to reduce inflammation and stabilise the vessels to reduce leakage. This drug is showing greater potential for widespread adoption because it shows the potential for a significant proportion of patients to achieve 12- and 16-weekly dosing without the inflammation risks associated with brolucizumab.31
Future wet AMD treatments
The RETINA study group has suggested some areas to consider for future individualisation of treatment.20 Do we need a loading phase and if so, how long?
The VIEW trial21 demonstrated intra-retinal fluid (IRF) reduces within a week after injection compared to three months for sub-retinal fluid (SRF). Recent studies22, 26 have found that baseline SRF has little negative effect on visual acuity outcomes, and that up to 200 microns of SRF at the fovea was not detrimental to visual acuity outcome. IRF, on the other hand, seems to consistently be associated with poorer visual outcomes.
Another modification to the treatment plan could be based on the type of lesion. Type 3 (retinal angiomatous proliferation) lesions are more sensitive to anti-VEGF therapy, compared to the more resistant type 1 choroidal neovascular membranes. Tolerating some SRF in type 1 lesions may have little effect on visual outcome prediction but, in type 3 lesions, SRF at baseline is one of the most significant negative predictors for visual outcome. Reduction or extension between treatments could perhaps in future be tailored to the type of retinal fluid (SRF or IRF) or type of lesion (Type 1 or 3).
Starting to become available in 2023 are a number of biosimilar drugs. These are injectables based on ranibizumab due to its patent expiring. It is hoped that adoption of these will help reduce the national expenditure on anti-VEGF drugs, although first line usage may be limited by the benefits of newer agents and in the latest national guidance there is an expectation that there would be a low threshold for switching to other agents if they are not showing sufficient response to the biosimilar.32
There is also a recent development in the delivery of ranibizumab using a port delivery system (PDS), called Susvimo (developed by Genentech). This device had FDA approval but, more recently, due to septum dislodgement in the implant, it has been recalled. It is a 2.6mm by 8.4mm silicone coated polysulfone drug reservoir capsule which is implanted into the eye at the pars plana through a 3.5mm scleral incision and anchored within the sclera by an extra-scleral flange (figure 6). This allows sustained delivery of ranibizumab through its micro fenestrated titanium release control element. Drug release is by passive diffusion into the vitreous cavity via a concentration gradient.
Figure 6: The Susvimo port delivery system (from Roche/Genentech)
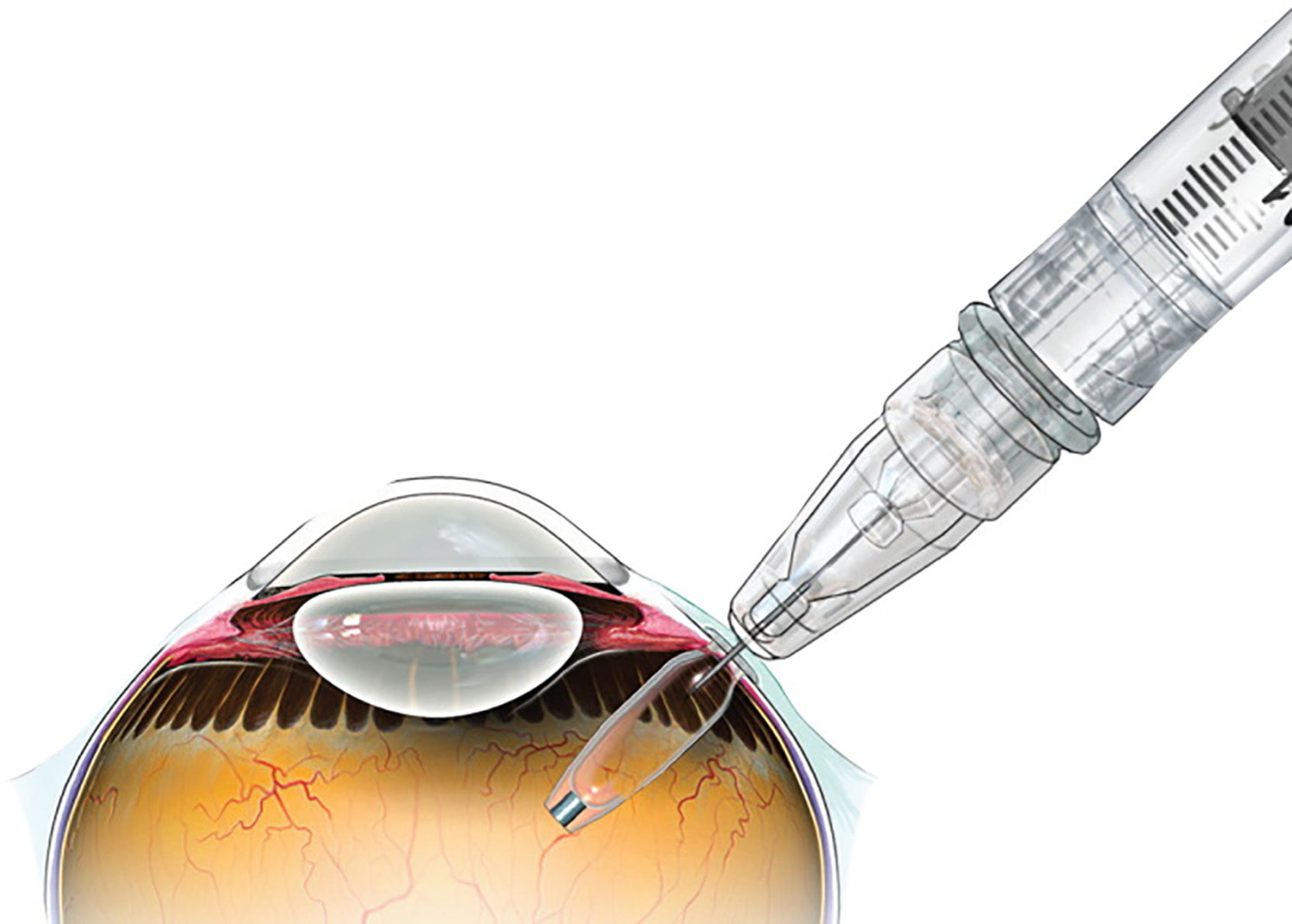
The LADDER trial33, 34 demonstrated an improvement in BCVA of 2.9 letters using the 100mg/ml concentration in the reservoir, which was consistent with monthly 0.5mg ranibizumab injections. The time to refill was 8.7 months, 13 months and 15.8 months for the 10mg/ml, 40mg/ml and 100mg/ml arms of the study, indicating a potential for a long-lasting effect. It is envisaged that issues relating to the dislodgement will be resolved to allow this form of treatment to be made available in future.
There are also early trials looking at eye drop or oral routes for drug administration. The difficulty is that current anti-VEGF biologics are large peptides, making it difficult to cross the ocular tissue membranes.
Other possible new treatments on the horizon to look out for include the following:
- Sunitinib malate; this is an injectable polylactic-co-glycolic acid (PLGA) developed by Greybug Vision. It is injected into the eye six-monthly and forms a deposit which slowly biodegrades over time into lactic acid and glycolic acid. This inhibits intracellular tyrosine kinases and inhibits VEGF receptors 1,2 and 3 and other growth factors preventing neovascularisation. The ALTISSIMO trial showed that 48% of patients did not require supportive therapy for at least six months and 62% for at least four months.35
- Gene therapy treatments; these use an adeno-associated virus (AAV) carrying the anti-VEGF gene into the host’s DNA. This stimulates the body to make its own anti-VEGF. One therapy under trial is RGX-314, which needs the AAV to be injected under the retina and could be effective for a number of years. Another is ADVM-022, which is delivered as a usual intravitreal injection and could last six months.36
Referral Pathways
In Manchester, like many other parts of the UK, referral for wet AMD is direct to the Ophthalmology AMD Service, via the rapid access form developed by RNIB and Royal College of Ophthalmologists, College of Optometrists, and the Macular Society. This followed the initial work by National Institute for Health and Care Excellence (NICE) document TA68, published on September 24, 2003. This was superseded by NG82 in January 2018.5 This report classifies the stages of age-related macular degeneration and its clinical signs as listed in table 1.
A study looking at referrals to a wet AMD clinic from community optometry found that there was agreement on symptomatology in 57.4% of cases, but scored less on clinical signs of neovascular age related AMD, with the poorest signs being for macular oedema (44.4%) and drusen (51.9%).37 Overall, 37% of patients referred had neovascular age-related AMD needing treatment, which is consistent with Optegra’s audit of referrals to treatment of 30% (data on file).
There are different approaches to managing the increasing numbers of patients referred to the Hospital Eye Service (HES), with some traditionally seeing the patients for initial face-to-face consultations and others using community optometry or imaging clinics/hubs to collect the OCT scans, enabling the hospitals to use virtual review to triage cases and increase capacity.
Local Optical Committee Support Unit (LOCSU) has developed a pathway, called the Maculopathy Referral Filtering and Monitoring Pathway, which facilitates referral filtering for people presenting with signs and symptoms of wet maculopathy but also for the monitoring of patients who have late-stage disease (figure 7).
Figure 7: The LOCSU Maculopathy Referral Filtering and Monitoring Pathway
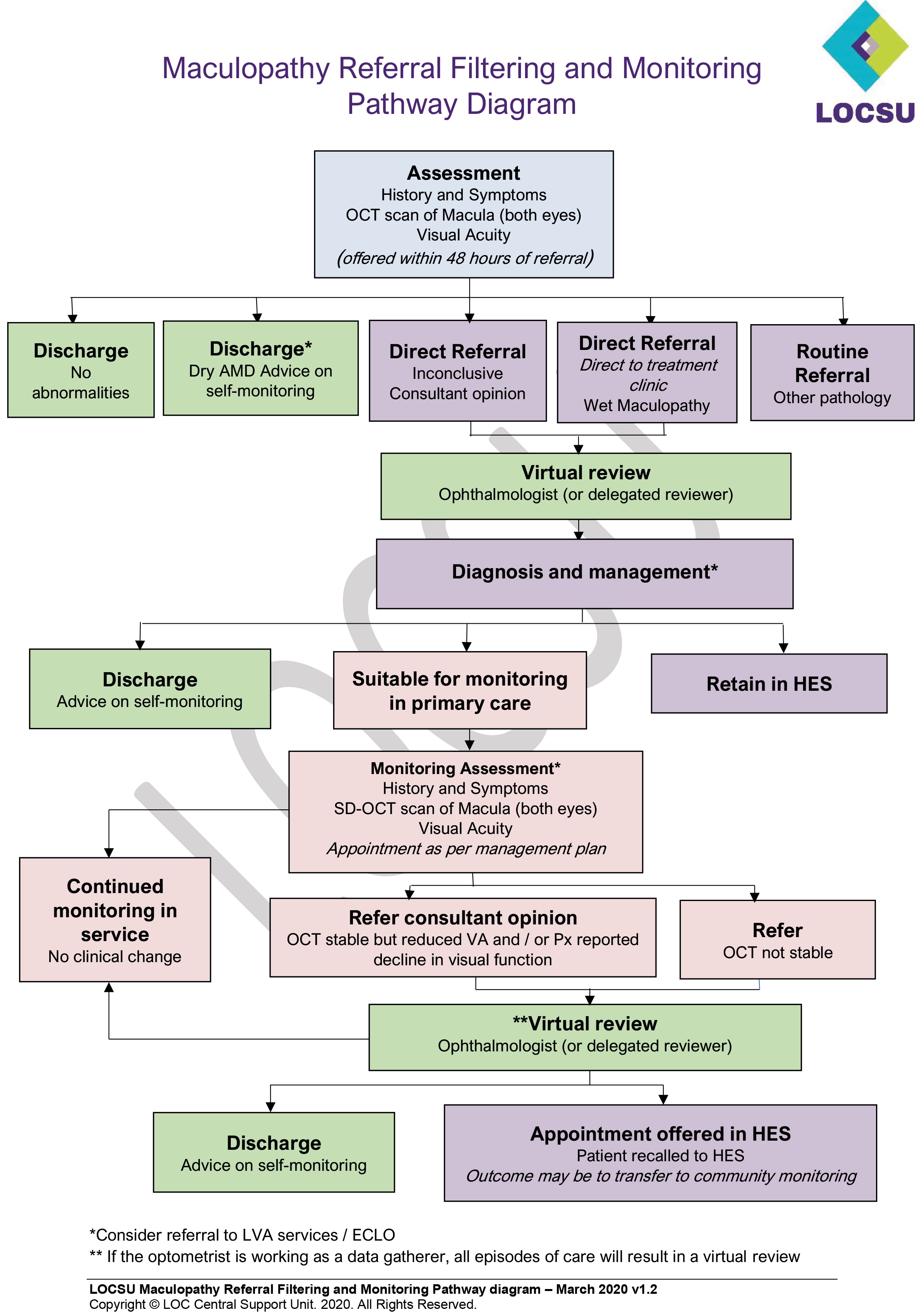
The pathway details how routine monitoring can be delivered within optical practice. Patients with late AMD who have identified a change following self-monitoring are also included. Patients following routine diabetic screening who require further checks to rule out wet maculopathy can also be seen within the service, reducing the numbers attending the HES.
Optegra Eye hospitals have developed an AMD treatment pathway and a one-stop macular assessment refinement service (MARS) similar to that seen in many other centres. Hospitals triage the referrals coming into the hospital, as separate arrangements are made for any non-AMD-related macular conditions. At the first visit, patients are seen by health care technicians (HCTs) who perform an assessment of the following:
- Visual acuity; in logMAR ETDRS letters
- Intraocular pressure
- Pupils dilation
- OCT and OCT-Angiography (OCT-A)
The patients are then seen by a consultant ophthalmologist for a full ophthalmic examination and review.
If there is treatable neovascular age-related AMD, the patient is counselled about the condition and given supporting material. They have the treatment plan explained, along with the risks and benefits, and if they are ready to start treatment, a consent form is signed.
The patient will then be treated, on the same day, with an anti-VEGF injection. This is administered by a qualified nurse injector (or in some other centres by optometrist injectors), who again will explain the treatment and answer any questions or concerns the patient has before proceeding. The number of nurse and optometrist injectors is expected to increase in an attempt to meet the increasing demand for medical retina services.
Once treated, the patient is advised of any red flags to watch for, such as a drop in visual acuity, the onset of flashes or floaters, or a red or painful eye. All patients have a clinic booklet, which includes contact information for the clinic in the day or out of hours. Before leaving, the patient is booked for review for their next dosing injection at a date based on the protocol for that anti-VEGF.
Following the dosing stage, based on the outcome seen on the visual acuity and OCT, the patient is placed on one of the three treatment plans. These are:
- Pro re nata (PRN, ‘according to circumstance’); following the dosing stage, if the retina is dry, treatment is stopped and they are monitored four weekly and only receive further treatment should there be a reoccurrence
- Fixed dosing; the most effective period between injections is found and the patient maintained on this long term
- Treat and extend; the more widely accepted plan. A protocol is followed based on patients’ visual acuity, OCT to assess levels of SRF, IRF and subretinal hyper-reflective material (SHRM) to monitor retinal disease activity. If the patient has retinal fluid that is improving then their treatment interval is maintained. If there is an increase in activity, then the injection interval is reduced and, if the retina is essentially dry, the interval can be extended. The extension interval will depend on the drug used.
On subsequent visits and after the dosing stage, the patients have similar assessments with the HCTs and are then seen by one of the macular clinic specialist optometrists to review the test results, discuss with the patient if there has been any health and medication changes or if there are any concerns. Their eyes are examined, the results discussed with the patient and a decision made on whether to treat or not or if the injection interval is maintained, reduced or extended.
This service is offered by most NHS hospital sites and by some private hospitals offering NHS medical retina services and aims to triage and assess the patient and offer treatment if needed within two weeks. This timescale is specified by NICE and states if wet AMD is suspected the referral should be made within one working day of the patient being seen in community optical practice to a macula service and that the hospital should assess, confirm diagnosis, and offer treatment within 14 days of the referral.
Summary
AMD remains a leading cause of visual impairment and the incidence is expected to continue to increase. Timely referral for appropriate treatment greatly improves the prognosis for those affected therefore it is important that primary eye healthcare practitioners are aware of local referral pathways and guidance. Treatment options continue to evolve with exciting new management approaches on the horizon. This article has provided an update on current treatment strategies and given a brief insight into emerging management approaches.
- Nigel Burgess is a therapeutic optometrist, Mr Sajjad Mahmood is a consultant ophthalmologist, and Dr Nabila Jones is an optometrist and research associate; all at Optegra Eye Sciences. Dr Clare O’Donnell is Head of Eye Sciences & Optometry at Optegra Eye Sciences, and Reader Aston University.
References
- The Royal College of Ophthalmologists. New Guidance for Commissioning Age Related Macular Degeneration Services. www.rcophth.ac.uk, 2021
- Tomany SC, Wang JJ, Van Leeuwen R, Klein R, Mitchell P, Vingerling JR, et al. Risk factors for incident age-related macular degeneration: pooled findings from 3 continents. Ophthalmology. 2004;111(7):1280-7
- Katsi VK, Marketou ME, Vrachatis DA, Manolis AJ, Nihoyannopoulos P, Tousoulis D, et al. Essential hypertension in the pathogenesis of age-related macular degeneration: a review of the current evidence. Journal of hypertension. 2015;33(12):2382-8
- Black JR, Clark SJ. Age-related macular degeneration: genome-wide association studies to translation. Genetics in medicine:official journal of the American College of Medical Genetics. 2016;18(4):283-9
- NICE. Age-related macular degeneration. 2018. Available at: www.nice.org.uk/guidance/ng82
- Chandra S, McKibbin M, Mahmood S, Downey L, Barnes B, Sivaprasad S. The Royal College of Ophthalmologists Commissioning guidelines on age macular degeneration: executive summary. Eye (London, England). 2022;36(11):2078-83. Available at: https://www.nature.com/articles/s41433-022-02095-2
- National Eye Institute. Benefit of supplements for slowing age-related macular degeneration 2022 Available from: https://www.sciencedaily.com/releases/2022/06/220602121430.htm
- Steinle N, Heier J, Singh RP, Boyer DS, Lad EM, Metlapally R, et al. A phase 1b multi-center, open label, single-arm safety study of intravitreal pegcetacoplan supporting the phase 3 DERBY and OAKS studies for geographic atrophy: A solution to “tarmac delay”. Investigative Ophthalmology & Visual Science. 2021;62(8):278-.
- Burton B, Parodi MB, Jürgens I, Zanlonghi X, Hornan D, Roider J, et al. LIGHTSITE II randomized multicenter trial: Evaluation of multiwavelength photobiomodulation in non-exudative age-related macular degeneration. Ophthalmology and Therapy. 2023;12(2):953-68.
- Nielsen J, MacLaren RE, Heier JS, Steel D, Ivanova T, Sivaprasad S, et al. Preliminary results from a first-in-human phase i/ii gene therapy study (focus) of subretinally delivered gt005, an investigational aav2 vector, in patients with geographic atrophy secondary to age-related macular degeneration. Investigative Ophthalmology & Visual Science. 2022;63(7):1504-.
- GTL. EXPLORE: A phase II study to evaluate the safety and efficacy of two doses of GT005, 2022 Available from: https://clinicaltrials.gov/ct2/show/NCT04437368
- Group ToA-rMDWPTS. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: One-Year Results of 2 Randomized Clinical Trials - TAP Report 1. Archives of Ophthalmology. 1999;117(10):1329-45
- NICE. Ranibizumab and pegaptanib for the treatment of age-related macular degeneration 2008 [cited 2023. Available from: https://www.nice.org.uk/guidance/ta155
- Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology. 2009;116(1):57-65.e5
- Singer M, Wong P, Wang P-W, Scott L. HORIZON extension trial of ranibizumab (LUCENTIS) for neovascular age-related macular degeneration (AMD): Two-year safety and efficacy results. Investigative Ophthalmology & Visual Science. 2009;50(13):3093
- Regillo CD, Brown DM, Abraham P, Yue H, Ianchulev T, Schneider S, et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. American Journal of Ophthalmology. 2008;145(2):239-48
- Abraham P, Yue H, Wilson L. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER study year 2. American Journal of Ophthalmology. 2010;150(3):315-24.e1
- Schmidt-Erfurth U, Eldem B, Guymer R, Korobelnik JF, Schlingemann RO, Axer-Siegel R, et al. Efficacy and safety of monthly versus quarterly ranibizumab treatment in neovascular age-related macular degeneration: the EXCITE study. Ophthalmology. 2011;118(5):831-9.
- Lalwani GA, Rosenfeld PJ, Fung AE, Dubovy SR, Michels S, Feuer W, et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. American journal of ophthalmology. 2009;148(1):43-58.e1
- Holz FG, Amoaku W, Donate J, Guymer RH, Kellner U, Schlingemann RO, et al. Safety and efficacy of a flexible dosing regimen of ranibizumab in neovascular age-related macular degeneration: the SUSTAIN study. Ophthalmology. 2011;118(4):663-71
- Tufail A, Johnston R, Akerele T, McKibbin M, Downey L, Natha S, Chakravarthy U, Bailey C, Khan R, Antcliff R, Armstrong S, Varma A, Kumar V, Tsaloumas M, Mandal K, Bunce C. The neovascular age-related macular degeneration database: multicenter study of 92 976 ranibizumab injections: report 1: visual acuity. Ophthalmology. 2014;121(5):1092-101
- Rosenberg D, Deonarain DM, Gould J, Sothivannan A, Phillips MR, Sarohia GS, et al. Efficacy, safety, and treatment burden of treat-and-extend versus alternative anti-VEGF regimens for nAMD: a systematic review and meta-analysis. Eye (London, England). 2023;37(1):6-16.
- Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388-98.
- Dakin HA, Wordsworth S, Rogers CA, Abangma G, Raftery J, Harding SP, et al. Cost-effectiveness of ranibizumab and bevacizumab for age-related macular degeneration: 2-year findings from the IVAN randomised trial. BMJ Open. 2014;4(7):e005094
- Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, Brown DM, Chong V, Nguyen QD, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121(1):193-201.
- Chaudhary V. Treat & extend in neovascular age-related macular degeneration: how we got here and where do we go next? Eye (London, England). 2023;37(4):581-3.
- Mitchell P, Holz FG, Hykin P, Midena E, Souied E, Allmeier H, et al. Efficacy and safety of intravitreal aflibercept using a treat-and-extend regimen for neovascular age-related macular degeneration: The ARIES Study: A randomized clinical trial. Retina (Philadelphia, Pa). 2021;41(9):1911-20.
- Ohji M, Takahashi K, Okada AA, Kobayashi M, Matsuda Y, Terano Y. Efficacy and safety of intravitreal aflibercept treat-and-extend regimens in exudative age-related macular degeneration: 52- and 96-week findings from ALTAIR : A randomized controlled trial. Advances in Therapy. 2020;37(3):1173-87
- Dugel PU, Koh A, Ogura Y, Jaffe GJ, Schmidt-Erfurth U, Brown DM, et al. HAWK and HARRIER: Phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127(1):72-84.
- Monés J, Srivastava SK, Jaffe GJ, Tadayoni R, Albini TA, Kaiser PK, et al. Risk of inflammation, retinal vasculitis, and retinal occlusion-related events with brolucizumab: Post hoc review of HAWK and HARRIER. Ophthalmology. 2021;128(7):1050-9
- Heier JS, Khanani AM, Quezada Ruiz C, Basu K, Ferrone PJ, Brittain C, et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet (London, England). 2022;399(10326):729-40.
- SPS. Preparing to use ranibizumab biosimilar, 2022. Available from: https://www.sps.nhs.uk/articles/preparing-to-use-ranibizumab-biosimilar
- Khanani AM, Callanan D, Dreyer R, Chen S, Howard JG, Hopkins JJ, et al. End-of-study results for the ladder phase 2 trial of the port delivery system with ranibizumab for neovascular age-related macular degeneration. Ophthalmology Retina. 2021;5(8):775-87.
- Campochiaro PA, Marcus DM, Awh CC, Regillo C, Adamis AP, Bantseev V, et al. The Port Delivery System with ranibizumab for neovascular age-related macular degeneration: Results from the randomized phase 2 ladder clinical trial. Ophthalmology. 2019;126(8):1141-54.
- Delaney-Gesing A. Graybug Vision releases preliminary topline results of Phase 2b ALTISSIMO trial 2021 [cited 2023]. Available from: https://www.ophthalmologytimes.com/view/graybug-vision-releases-preliminary-topline-results-of-phase-2b-altissimo-trial
- Khanani AM, Thomas MJ, Aziz AA, Weng CY, Danzig CJ, Yiu G, et al. Review of gene therapies for age-related macular degeneration. Eye (London, England). 2022;36(2):303-11.
- Muen WJ, Hewick SA. Quality of optometry referrals to neovascular age-related macular degeneration clinic: a prospective study. JRSM short reports. 2011;2(8):64.
