The understanding of oxygen transport and consumption within the cornea while wearing contact lenses attached has matured over time and a series of basic measurements and observations has led to the development of complex predictive models.
The general history of development of contact lenses offers useful perspective in terms of the evolution of materials with increasing oxygen permeability. A basic property of the cornea is to become oedematous when exposed to oxygen concentrations less than standard atmospheric values. This was initially reported by Holden et al, by whom mean corneal swelling characteristics were recorded for a group of eight subjects for exposure over an eight-hour interval with oxygen concentrations of 1.0%, 2.5%, 4.9%, 7.5%, 10.1%, and 21.4%.1
The precorneal oxygen concentration required to avoid corneal oedema for the group as a whole was estimated at 10.1%; the equivalent to an oxygen tension of 74mmHg. There was considerable variation, however, in individual subject responses.
History of Contact Lens Development
A detailed account of the history of contact lens development from a variety of viewpoints is provided by Lamb and Bowden2 who record that, for a considerable period of time, the prime focus was upon contact lenses that included significant scleral contact. Initially, glass and PMMA plastic with zero oxygen permeability were the only materials available for manufacture of a range of contact lens devices and this limited the corresponding wear time.
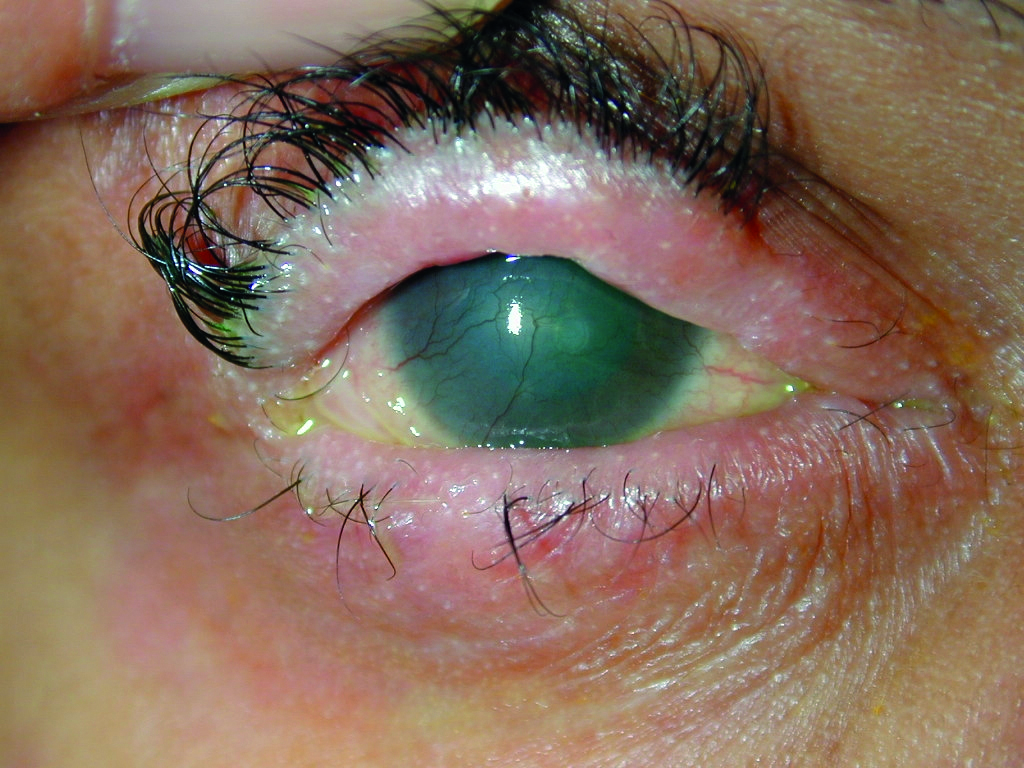
An early use of glass contact lenses, for example, was in the treatment of symblepharon (figure 1), a condition causing adhesion between the bulbar and palpebral conjunctiva. Treatment of this condition with a scleral lens maintained conjunctival separation while allowing no lens contact with the cornea.3 This condition may arise, for example, as a result of chemical burn injuries to the eye or with certain diseases, such as Stevens-Johnson syndrome.
Figure 1: Stevens-Johnson syndrome with symblepharon
The first practical demonstration of a device in direct contact with the eye’s surface to correct refractive errors is attributed to Adolf Gaston Eugen Fick in 1887 (figure 2) where direct contact was achieved with the sclera and coupling to the pupil achieved via a dextrose solution.4 Shortly after, in 1888, the Müller brothers in Wiesbaden developed a technique of a blown glass scleral contact lens manufacture.5 This procedure, however, required exceptional craft skills.
Figure 2: Adolf Gaston Eugen Fick. Photo: College of Optometrists (BOA Museum)

The role of Carl Zeiss in the development of contact lenses of various types was significant over a time where a range of contact lens prototypes were manufactured by the company for various ophthalmologists, using available facilities for precision lens manufacturing. The solid glass lenses in contact with the cornea, however, could only be tolerated for relatively short periods of time.
A step forward in contact lens manufacturer, one which produced better fitting devices, was developed by Joseph Dallos (figure 3), a Hungarian ophthalmologist who, in the 1930s, developed a technique for replicating the profile of the corneal surface using an alginate-like material for the manufacture of glass scleral lenses.6
This technique was further developed to take impressions using alginate products that could be used in cold impression forming procedures. Such impression products remain in extensive use in dentistry, and was still used for customised specialist lens design until recent years.
Fortunately, Joseph Dallos was persuaded to leave Hungary and relocate to London in anticipation of the rise of Nazi Germany. He never retired and continued to attend his contact lens practice till his death in 1979.
Figure 3: Joseph Dallos. Photo: College of Optometrists (BOA Museum)

The use of glass for scleral lenses was always going to be a limiting factor for their production. The invention of polymethylmethacrylate (PMMA) in 1931, under the trade name of Perspex, provided a much more convenient material for lens manufacture. In 1938, Dr István Gyröffy in Hungary first used the material in Europe in sheet form for the manufacture of scleral lenses. Also around this time, CW Dixey & Son in London manufactured PMMA lenses from solid blocks of the material using a specially developed precision lathe system.
Scleral lenses designed for the refractive correction of the cornea began to be manufactured from PMMA from around this time onwards, with various developments taking place to improve the quality of fit, though issues of limitation of wear time and corneal hypoxia remained. In some designs, the technique of fenestration, initially implemented by Joseph Dallos, was adopted to minimise central corneal oedema.7
Fisher et al investigated the potential benefit of fenestrations incorporated into scleral lenses to reduce corneal oedema using open-eye wear.8 While marginal benefits were noted, the authors indicated that these would be more significant for patients with conditions of compromised corneas.
The first actual use of thin profile plastic lenses solely for correcting corneal refraction errors with no scleral contact is generally attributed to Kevin Tuohy in the USA.9 This ‘discovery’ came about when the central corneal section of a scleral lens, which was being manufactured from PMMA, broke away from its supporting scleral section.
Tuohy investigated the suitability of the remaining circular corneal lens section on his wife, whose prescription was close to that of the ‘accidental’ corneal lens, with presumably some success as he filed a patent in February 1948, which was granted in June 1950. The lighter, more compact, lens was found to be better tolerated than larger glass lenses for sole corneal use as previously manufactured by Zeiss.
This marked the start of the development of plastic contact lenses purely for the correction of corneal refractive defects but with no scleral contact.
Diligent researchers of contact lens history, however, recount that a certain Dr Dennis England with a practice in Ohio filed a patent in December 1945 for specifically manufactured contact lenses from PMMA, lenses entirely equivalent to the serendipitous device of Kevin Tuohy. The initial patent application was rejected in October 1946 and, following two lengthy and costly petitions for review, the application was subsequently withdrawn.
A major step forward in corneal contact lens development was the discovery of the so-called soft lenses by the Czech chemists Otto Wichterle and Drshoskzv Lim in 1959,`10 though it was not until 1971 that the FDA in the USA approved such materials for corneal contact lens use. This first approved lens was the Softlens product, developed by Bausch and Lomb.
Extensive product development, however, had to be undertaken to achieve a stable, marketable product. This provided beneficial levels of oxygen permeability (compared with PMMA) and a much greater degree of user comfort. The value of oxygen permeability of such material is considered as directly proportional to hydrogel water content.11
Efron and Morgan indicated, however, that dehydration of hydrogel contact lenses during wear can reduce levels of oxygen transmissibility by significant amounts.12 This effect is likely to be more problematic for thinner lenses.
Advances had been made in manufacturing rigid gas permeable plastics (RGPs) during this time,13 with the inclusion of silicon offering increased oxygen permeability. Rigid corneal lenses still required a greater adaptation period from the wearer, but had the advantage that they maintained higher quality optical profiles compared to hydrogel types. Their rigidity allowed a precorneal tear lens to correct any corneal deformation, even with lens rotation on the eye.
The next major development in corneal lens technology was the development of silicon-based hydrogel lenses in 1998 by Ciba Vision with yet higher values of oxygen permeability.14
The historical perspective of contact lens development, however, reveals the key roles of talented individuals driven by the desire to improve the quality of vision of patients requiring specialist optical prescriptions. More details of the evolution of contact lens materials are provided by Musgrave and Fang.15
Elements of this historical record can be found in the British Optical Association Museum at the College of Optometrists in London.
Oxygen Permeability Coefficient and Oxygen transmissibility Coefficient
In clinical applications, oxygen permeability describes how readily oxygen passes through particular tissues or materials. In the context of the properties of contact lenses, oxygen transmissibility is the value of permeability divided by the lens thickness.
Thus, thinner lenses of a given material will have higher values of oxygen transmissibility. Also, because of the thickness profile of corrective lenses, transmissibility will be reduced at the edge of negatively powered lenses and in the centre of positively powered lenses.
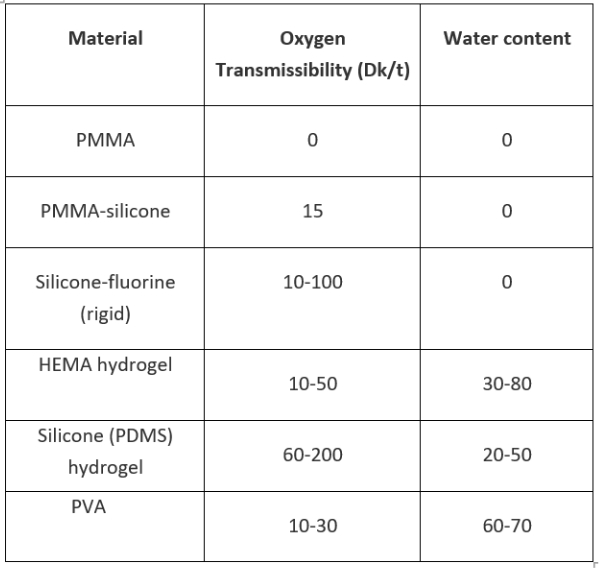
Table 1 summarises the oxygen properties of some contact lens materials.
The permeability coefficient (Dk) represents the product of diffusion (D) and solubility (k) and is typically expressed in the Barrer unit, which can be expressed in sub units as:
10-10 (cmSTP3.cm)/(cm2.s.mm.Hg)
This describes the volume of gas flow across a given area of membrane per second for a given pressure difference of oxygen across the membrane for a given thickness of membrane.
The volume cmSTP3 relates to the gas volume referenced to 0° C and pressure of 105 Pa (or 1 bar). The Barrer unit is associated with the distinguished New Zealand-born chemist Richard Barrer who made significant contributions to membrane science.
On a practical basis, values of Dk for contact lenses are made by measuring the oxygen transfer rates through finite thicknesses of such lenses within specific cross-sectional areas. The method developed for hydrogel materials uses the so-called polarographic measurement cell16 and is applicable to samples having an oxygen permeability from 0 to 145 Barrer (figure 4).
Figure 4: Polarographic method for determining Dk/t for hydrogel contact lens material where the oxygen electrode is placed directly in contact with the test surface
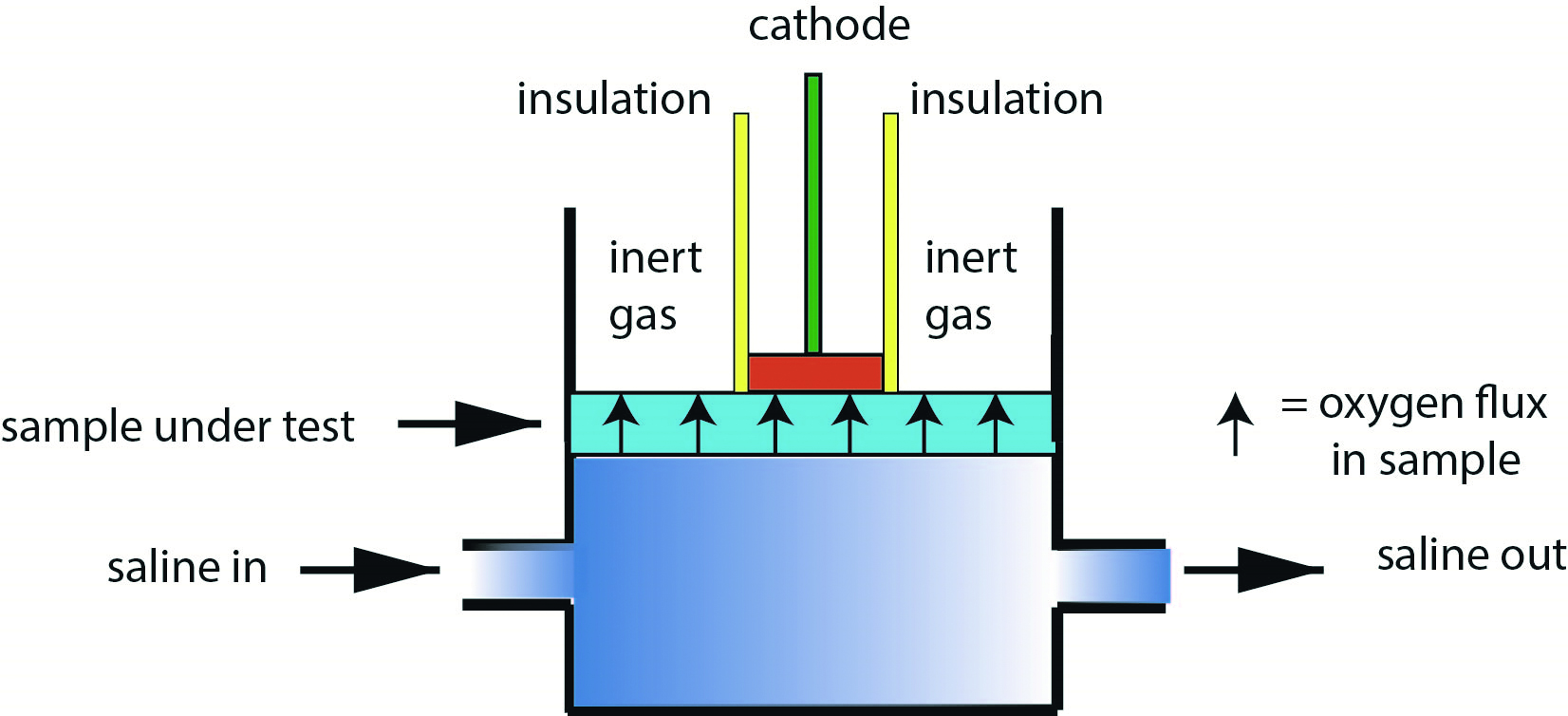
Saline with dissolved oxygen percentage of 21% is circulated on one side of the test lens. Figure 4 shows the technique of using an inert gas (typically nitrogen) in areas where the oxygen electrode surface and insulation are not in contact with the sample material.
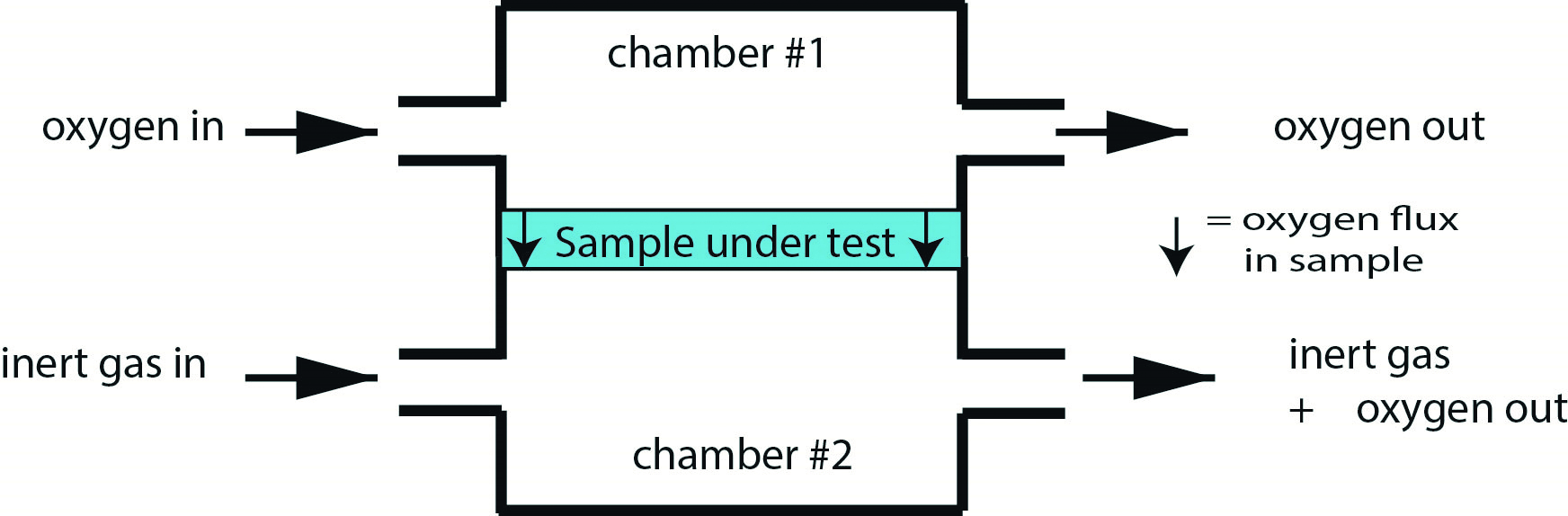
The coulometric method17 measures oxygen transfer across the test material by detecting trace oxygen levels within a pure gas flow of inert gas, again typically nitrogen (figure 5). The various methods are specifically defined in ISO 18369-4 (2017)18 where the use of such standards greatly assists the adoption of consistent measurement protocols.
It is the value Dk/t that is most often used for the comparison of oxygen transmission values of different contact lenses. Thin lenses of materials with low Dk values can have equivalent oxygen transmission values of thicker lenses made from material with higher Dk values. Most manufacturers cite a value relating to the centre of a lens of -3.00DS power.
Figure 5: Coulometric method for determining Dk/t for non-hydrogel contact lens material where measurements relate to the concentration of oxygen exiting the measurement cell (chamber #2) relative to the base flow of inert gas (typically nitrogen)
It is also important to realise that with variation in the thickness of a contact lens across its diameter, the local value of oxygen transmission will change accordingly. Negative-powered contact lenses are thinner in the centre than in the periphery, and vice versa with positive-powered lenses.
Models
Models have been developed to simulate the oxygen concentration within the cornea under various conditions, such as the normal eye over the diurnal cycle and after various extents of contact lens wear. The basic derived system based on the Monod kinetics model is illustrated in figure 6, where tear layers are identified between the corneal epithelium and the rear of the lens (tear film #1) and the front of the lens (tear film #2).19 In open eye conditions the tear layer forms between the lens surface and air and in closed eye condition between the lens surface and the palpebral conjunctiva.
Figure 6: Representation of components of cornea/contact lens for simulation of oxygen transport and consumption after Moreno et al.20 (not to scale)
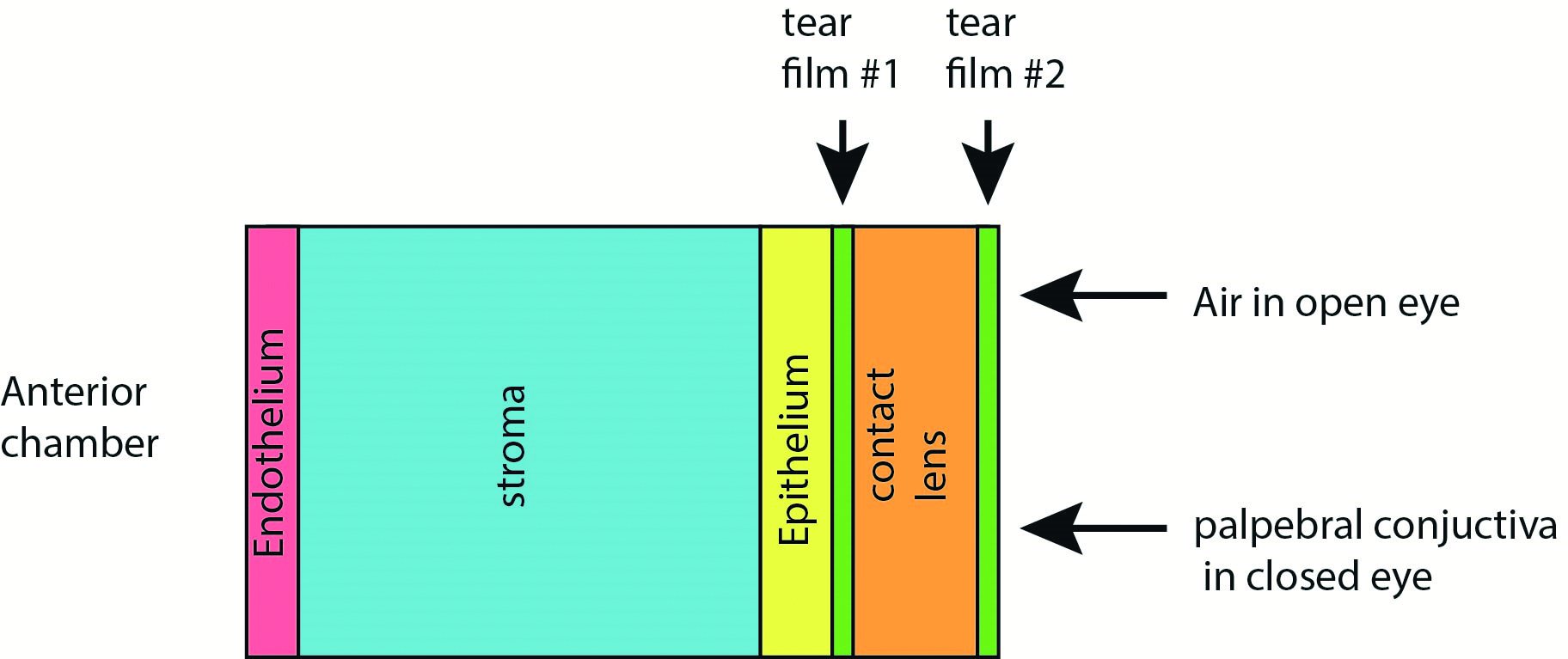
Although a compact physical system, the various distinct ‘compartments’ of the model give rise to a relatively large number of parameters to drive the model, as outlined in table 2 and where typical values are as described by Moreno et al.20 The partial pressures of oxygen establish a set of boundary conditions for the model, which is driven by a series of complex differential equations.
The model is driven at specific parameter values though the maximum oxygen consumption and layer permeability will, however, be subject to a range of values within any population group. This specific model was found to be successful in predicting oxygen levels at the corneal/lens interface for a range of contact lenses.
Table 2: Summary of key parameters included in model and illustrative values20
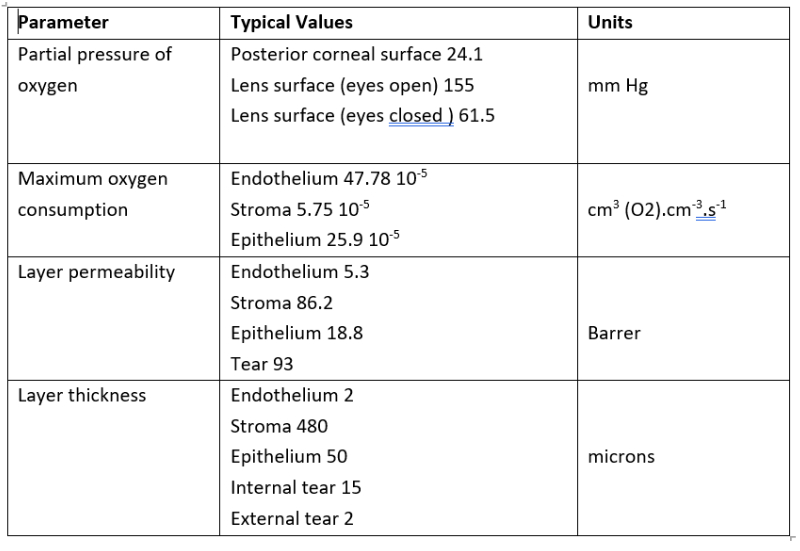
In terms of partial pressure of oxygen, a value of 155mmHg equates to standard atmospheric conditions at sea level. This value reduces with altitude and, within passenger aircraft, the equivalent value for 8,000 feet cabin setting is 15.4% in the ‘closed eye’ condition, while the partial pressure at the lens surface tear film is typically taken as 61.5mmHg, based on contact with the palpebral conjunctiva.21
All elements of the physical cornea (endothelium, stroma and epithelium) consume oxygen through specific cellular activity. Nutrients for cell metabolism within the corneal layers is provided by the aqueous humour, which is infused on a continual basis into the anterior chamber.22
Additional Considerations
While such models have been primarily used for the prediction of oxygen levels with contact lens wear, there is scope for wider consideration of oxygen perfusion in ophthalmology. Seller et al outline, for example, the effect of corneal cross-linking with and without supplementary oxygen.23
It may also be appropriate to consider the role that supplementary oxygen delivery to the corneal epithelium may have following surgical procedures of various types, where the operative eye can be subject to extensive periods of ‘closed eye’ condition.
- Dr Douglas Clarkson is a research fellow at the Department of Clinical Physics and Bioengineering, Coventry and Warwickshire University Hospital Trust.
References
- Holden BA, Sweeney DF, Sanderson G. The minimum precorneal oxygen tension to avoid corneal edema. Investigative Ophthalmology & Vision Science, 1984 Apr;25(4):476-80
- Lamb J, Bowden T. Contact Lenses. Editors Anthony John Phillips & Lynne Speedwell. Chapter 2, History of Contact lenses. Elsevier Health, 2018.
- Kate A, Doctor MB, Shanbhag SS. Management of symblepharon prior to keratoprosthesis in chronic ocular burns: A sequential approach. Cureus. 2022 Apr 29;14(4):e24611
- Fick AE. A contact-lens. 1888 (translation). Archives of Ophthalmology, 1988 Oct;106(10):1373-7
- Nissel, G. The Müllers of Wiesbaden, Optician, 1965, 150, 591-594
- Dallos, J. Contact Glasses, The ‘Invisible’ Spectacles. Archives of Ophthalmology, 1936;15(4):617-623
- Fadel D, Ezekiel DF. Fenestrated scleral lenses: Back to the origins? Review of their benefits and fitting techniques. Optometry & Vision Science, 2020 Sep;97(9):807-820
- Fisher D, Collins MJ, Vincent SJ. Corneal oedema during open-eye fenestrated scleral lens wear. Ophthalmic & Physiological Optics, 2022 Sep;42(5):1038-104 Pearson RM. The 75th anniversary of the Tuohy corneal contact lens: A review. Contact Lens & Anterior Eye, 2023 Feb;46(1):101756.
- Wichterle, O, Lím, D. Hydrophilic gels for biological use. Nature, 185, 1960, 117–118
- Sarver MD, Baggett DA, Harris MG, Louie K. Corneal edema with hydrogel lenses and eye closure: effect of oxygen transmissibility. American Journal of Optometry & Physiological Optics, 1981 May;58(5):386-92
- Efron N, Morgan PB. Hydrogel contact lens dehydration and oxygen transmissibility. CLAO J, 1999 Jul;25(3):148-51
- Benjamin W.J., Cappelli Q.A., Oxygen permeability (Dk) of thirty-seven rigid contact lens materials. Optometry & Vision Science, 2002, 79 (2) pp. 103–111
- Tighe BJ. A decade of silicone hydrogel development: surface properties, mechanical properties, and ocular compatibility. Eye & Contact Lens, 2013 Jan;39(1):4-12
- Musgrave CSA, Fang F. Contact lens materials: A materials science perspective. Materials (Basel), 2019 Jan 14;12(2):261
- Fink B, Hill RM. Corneal oxygen uptake: A review of polarographic techniques, applications, and variables. Contact Lens & Anterior Eye, 2006 Dec;29(5):221-9
- Winterton LC, White JC, Su KC. Coulometric method for measuring oxygen flux and Dk of contact lens materials. International Contact Lens Clinic, 1987, 14 (11) pp. 441–449
- ISO 18369-4:2017 Ophthalmic optics — Contact lenses — Part 4: Physicochemical properties of contact lens materials, ISO, Geneva.
- Monod, J. The growth of bacterial cultures. Annual Review of Microbiology, 1949, 3: 371–394
- Moreno VC, Aguilella-Arzo M, Del Castillo RM, Espinós FJ, Del Castillo LF. A refined model on flow and oxygen consumption in the human cornea depending on the oxygen tension at the interface cornea/post lens tear film during contact lens wear. Journal of Optometry, 2022 Apr-Jun;15(2):160-174
- Aldrette JA, Aldrette LE. Oxygen concentrations in commercial aircraft flights. South Medical Journal, 1983 Jan;76(1):12-4
- Goel M, Picciani RG, Lee RK, Bhattacharya SK. Aqueous humor dynamics: a review. Open Ophthalmology Journal, 2010 Sep 3;4:52-9
- Seiler TG, Komninou MA, Nambiar MH, Schuerch K, Frueh BE, Büchler P. Oxygen kinetics during corneal cross-linking with and without supplementary oxygen. American Journal of Ophthalmology, 2021 Mar;223:368-376
