One would think that you would need expensive equipment to carry out extensive investigations to diagnose the type and severity of dry eye presented in practice. In reality, a slit lamp alone is more than sufficient. This article will inform on the methods we can employ to elevate our services.
Alcon Talks in Optician Contact Lens Monthly 2023
- March: Toric contact lenses
- June: Contact lens comfort
- September: Dry eye disease
- December: Multifocal contact lens option
Definition and classification of Dry Eye Disease
Before we begin, it is important to look at the definition of dry eye disease (DED). The TFOS DEWS II report defines DED as ‘a multifactorial disease of the ocular surface characterised by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.’1
Put simply, Dr Travé Huarte explains that in order for the ocular surface to maintain its integrity, we need a balance, otherwise known as homeostasis. When this balance is interrupted, this can translate to ocular surface disease and patient may begin to show symptoms of DED.1
These symptoms could include, but are not limited to, pain, grittiness and discomfort. The instability of the tear film results in tears that are more ‘salty’ or hyperosmolar.2 This may also be accompanied by inflammation of the eye or the lids,1 resulting in damage of the surface of the eye either by friction, loss of cells, dry spots or a combination of these.3
The nerves also play an integral role in this process, with some nerves being overstimulated, other nerves perhaps under-stimulated, and in turn this can lead to neurosensory abnormalities contributing to DED.1,4
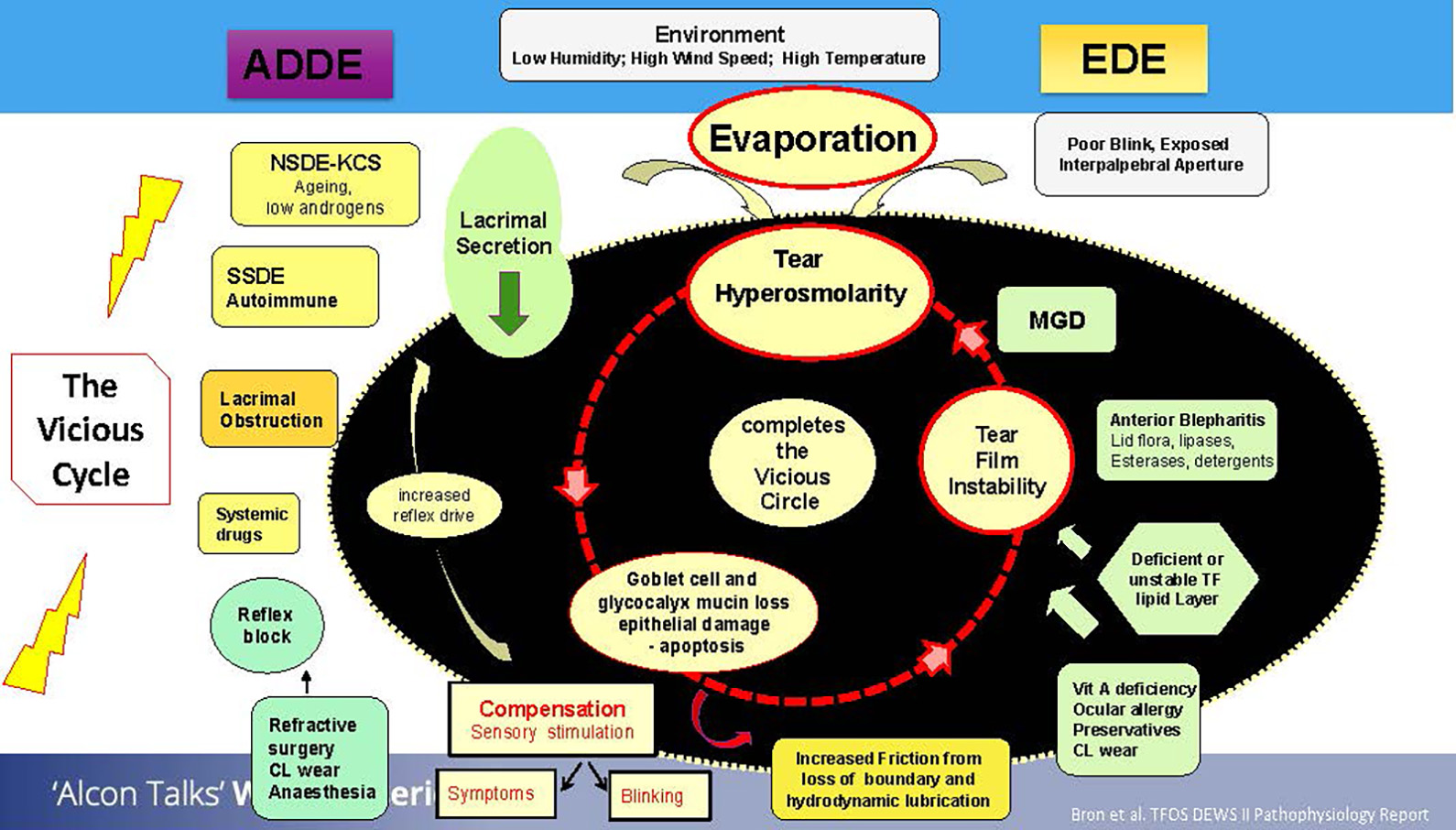
DED can be classified into two subtypes: aqueous deficient dry eye (ADDE) and evaporative dry eye (EDE). It is important not to think of these as completely separate entities, but rather, to consider these as a spectrum and that the patient presenting to you with DED can fall anywhere on this spectrum.1
One thing that is common with both subtypes is hyperosmolarity. When tears evaporate, the remaining tear film becomes increasingly concentrated in salts and electrolytes, which sit on the ocular surface. This can lead to consequential damage to the ocular surface. Evaporation of the tear film could occur due to poor blinking or exposed areas due to impartial blinking.3
Meibomian gland dysfunction (MGD) is another reason for increased evaporation rates. Changes to the flora of the eyelids due to the presence of blepharitis could also contribute to increased evaporation rates, initiating inflammation of the lid margin and in turn, ocular irritation.3
Additionally, overwearing of contact lenses, having certain deficiencies or using eye drops containing preservatives could also all contribute to the vicious cycle of dry eye.3
Shifting our focus on the aqueous deficient side of the spectrum, aging is one of the drivers of this type of DED. Autoimmune conditions such as Sjogren’s syndrome or rheumatological conditions where there is an issue with the production of tears and the quality of tears being produced can also be major factors for ADE. Tear production may be impaired due to a physical obstruction in the lacrimal gland, or the decreased production may be linked to the consumption of certain systemic drugs.3
Furthermore, the reflex block may have an impact on the production of tears, and indeed some dryness may be linked directly to ocular surgeries.3
The vicious cycle of dry eye has an overarching element of the environment which can influence this cycle. Low humidities, high temperatures or wind speeds can directly affect the evaporation rates of the tear film, which in turn can negatively impact the stability of the tear film.3
In ADDE, the decreased tear secretion can promote a compensatory mechanism. If there is a lack of tears or the signals suggest there is something in the eye, the sensation feeds back to the brain which is alerted to produce more tears to compensate. These tears however, may lack in quality, ultimately resulting in a trade-off between quality and quantity.3
The vicious cycle linking ADDE and EDE is hyperosmolarity, which leads to tear film instability and results in ocular surface damage. The epithelial damage then leads to further hyperosmolarity and so the vicious cycle continues. Patients can enter this vicious cycle due to multiple reasons, as shown in the figure 1.
Figure 1: The ‘vicious cycle’. Adapted from TFOS DEWS II3
Symptomatic vs Asymptomatic patients
Now, we have studied the subtypes of DED and the vicious cycle linking the subtypes, we will go on to study the patients presenting to our practice. Though patients can present at any point on the vicious cycle, they can be categorised into two main groups; those presenting with symptoms, and those presenting without symptoms.1
Referring to the classification flowchart as illustrated by the TFOS DEWS II report (figure 2), the asymptomatic patient presenting to practice with signs of ocular surface disease may suggest a predisposition to DED and so it would be important to educate the patient and proactively manage them to prevent the transition to a symptomatic problem.1
Figure 2: Classification of dry eye disease. Adapted from TFOS DEWS II1
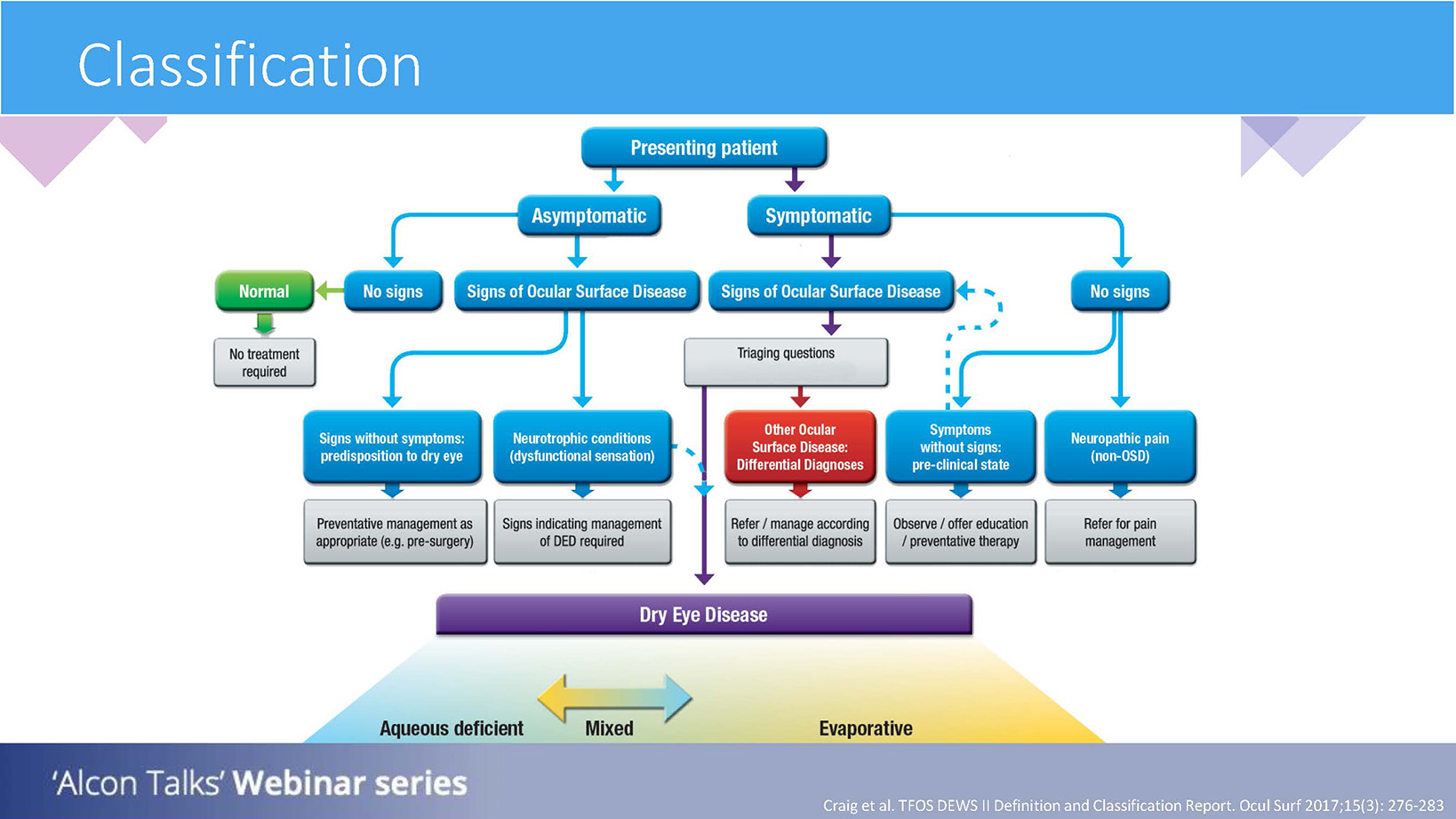
There may also be a need to refer in cases where there are obvious ocular surface disease (OSD) signs, but dysfunctional sensations. In such a case, the patient may not experience sensations caused by OSD, indicating a neurotrophic condition. Although such cases may not be common in practice, it is important to have an oversight of this.1
If a patient presents as symptomatic, and shows signs of OSD, then the path of triaging questions and risk factors is indicated to establish what type of DED is present and which management pathway is necessary.1
If a patient has symptoms but no obvious signs, there are two pathways which may be followed. One of those may be that the patient has neurotrophic pain, where there is an overstimulation of pain being signalled to the brain, but we cannot see this as signs. In other words, ‘pain without stain’. In these cases, a referral will be needed for pain management as the root cause may be unrelated to the surface of the eye.4
The alternative may be a patient who is symptomatic but without signs, and they may be in a pre-clinical state to DED. We can therefore offer preventative therapy.1
TFOS DEWS II Diagnostic Criteria
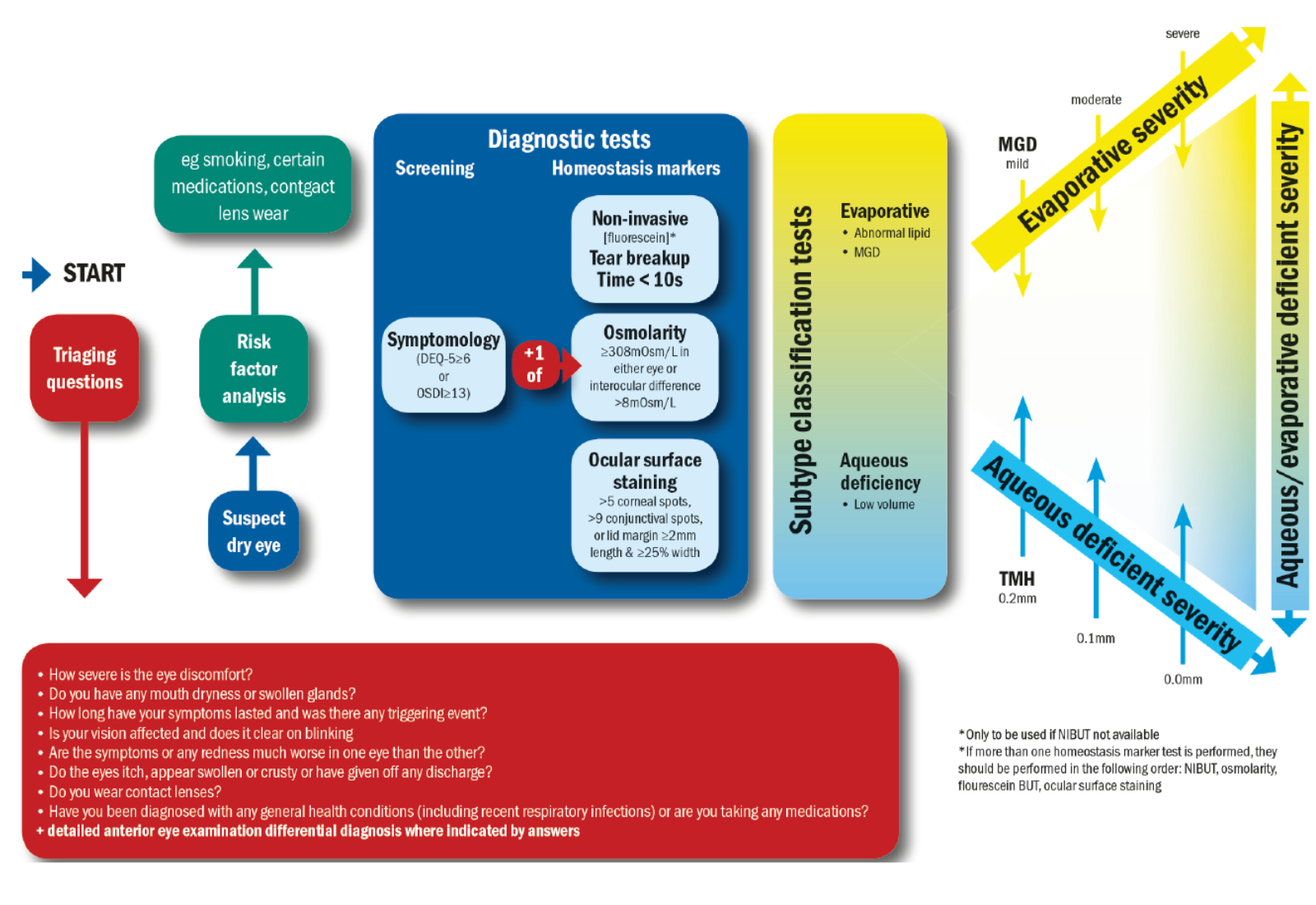
The TFOS DEWS II report was published in 2017 and it illustrates a stepwise approach to the diagnosis of DED (see figure 3). The first thing in this flowchart is to ask triaging questions, to understand whether there are intrinsic factors, extrinsic factors or a combination of the two, which could be contributing to the DED.
Next, there is the need for a risk factor analysis. This entails finding out about the lifestyle of our patients. Then, it would be the diagnostic tests needed to establish the presence of DED, and further tests which could help with the sub-classification.5
Figure 3: DED Diagnostic test battery. Once dry eye disease is suspected the core diagnostic tests should be carried out;
symptomology, tear break up time (TBUT) osmolarity and ocular surface staining. Adapted from TFOS DEWS II5
Triaging questions
Starting at the beginning of this flowchart then, the TFOS DEWS II report proposed the following triaging questions to investigate things further:5
- How severe is the eye discomfort?
Some patients may feel a lot of pain, others might feel very little.
- Do you have any mouth dryness or enlarged glands?
This could be indicative of an underlying condition.
- How long have your symptoms lasted and was there any triggering event?
Perhaps the patient has had ocular surgery which precipitated the problem, or maybe they work in dusty conditions which could be triggering the symptoms.
- Is your vision affected and does it clear on blinking?
If the vision does not clear on blinking, it could be indicative of a refractive issue. If the vision does clear on blinking, it would suggest that there is a problem with the spreading of the tear film.
- Are the symptoms or any redness much worse in one eye than the other?
This could help to narrow down the causative factor.
- Do the eyes itch, are they swollen, crusty or is there any discharge?
These symptoms could be indicative of infections, inflammation or allergies.
- Do you wear contact lenses?
It is important to establish what modality of contact lenses the patient is wearing, what their care regime looks like, assess the compliance, since all of these factors could contribute to symptoms.
- Have you been diagnosed with any general health conditions (including recent respiratory infections) or are you taking any medications?
Certain systemic conditions will influence the tear film and can increase the risks of DED, as can certain classes of medication.
Risk factor analysis
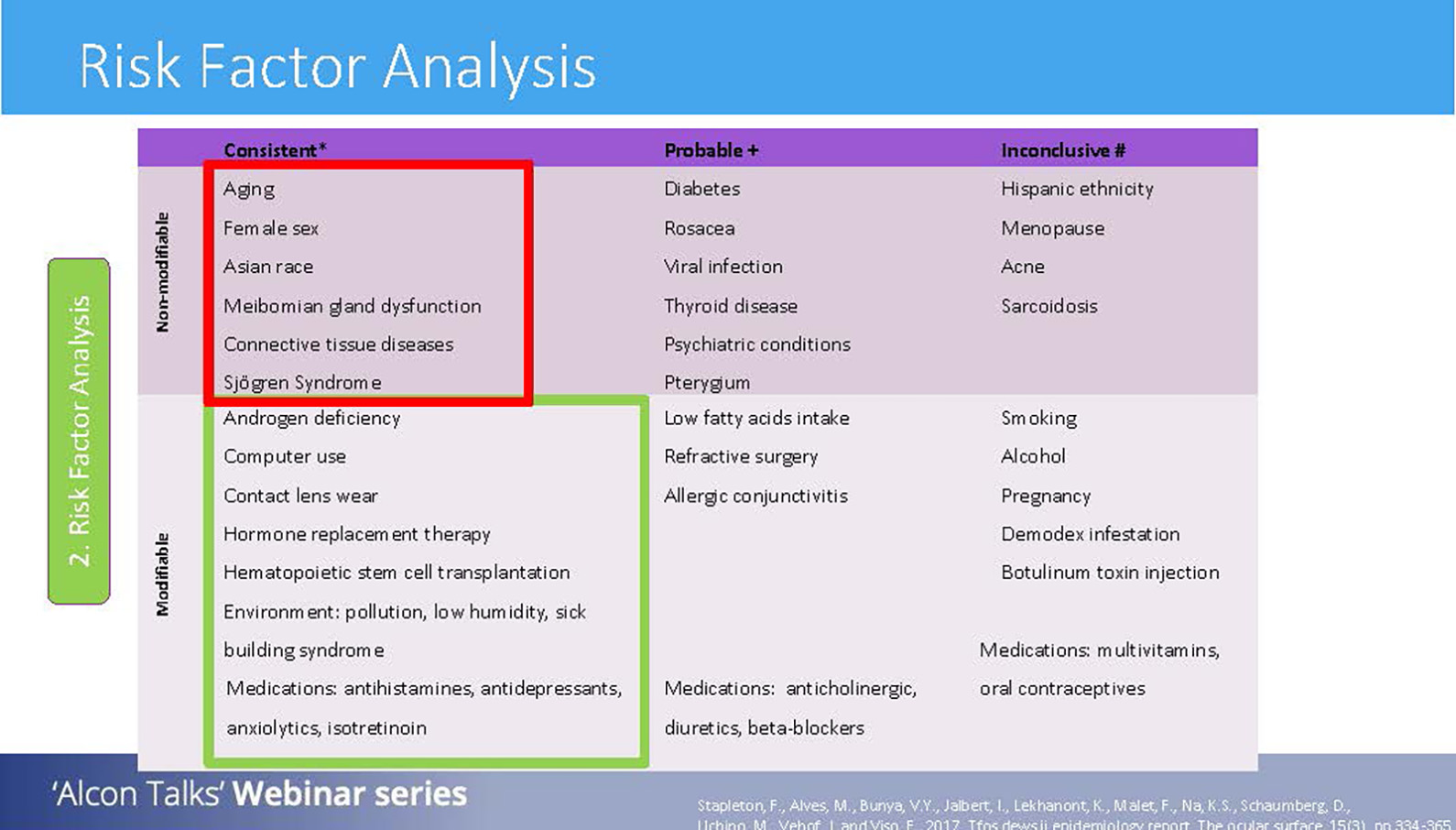
After the triaging questions have been completed, the next step to carry out is a risk factor analysis. Risk factors of DED can be classified into two groups: non-modifiable (which means nothing can be done to alter these) and modifiable (where interventions can be made to these risks). These can then be divided further into consistent, probably and inconclusive risks depending on how much research is available to support such risks as contributing to DED.6
Under non-modifiable factors, we have aging, female sex, Asian race, MGD, connective tissue disorders and Sjögren’s syndrome. With the research evidence we have, these factors have been proven to be consistent risk factors of DED.6
In terms of modifiable and consistent risk factors, things such as computer use, contact lens use, environmental conditions and medications are some examples. These factors may be changed or addressed to help reduce the risk of DED.6
Recently, TFOS released a series of new reports specifically focusing on the lifestyle of patients, discussing how such lifestyle factors could impact the surface of the eye.
These reports are outlined below:
1. Impact of contact lenses on the ocular surface7
This outlines how factors such as wear, the material and contact lens attributes affect the ocular surface, as well as investigating how lifestyle choices could impact successful contact lens wear.
2. Impact of elective medications and procedures on the ocular surface8
This report looks at the evidence of elective or non-urgent medical and surgical interventions which may impact the ocular surface.
3. Impact of nutrition on the ocular surface9
How do micro and macronutrients affect the microbiome, and how do nutritional lifestyle factors contribute to OSD?
4. Impact of the digital environment on the ocular surface10
Digital devices have become a part of most people’s day-to-day life. This report examines the prevalence of digital eye strain, and how factors such as type of device, intensity and activity of use impact the ocular surface.
5. Impact of cosmetics on the ocular surface11
There are an array of cosmetics and cosmetic procedures available to us. The ingredients in such cosmetics may be toxic to the ocular surface and cosmetic procedures may produce adverse events, both areas which are explored in detail in this report.
6. Impact of societal challenges on the ocular surface12
This report delves into detail around how societal influences in terms of education, access to, and uptake of services can impact the presentation and management of OSD.
7. Impact of environmental conditions on the ocular surface13
Environmental factors encompass everything from humidity, temperature, sunlight to air-condition and indoor heating, and this report investigates how such factors affect the ocular surface.
8. Impact of lifestyle challenges on the ocular surface14
Mental, physical and social health can all influence the body as well as the eyes, and this report specifically explores these areas.
9. Evidence quality report: Advancing the evaluation and synthesis of research evidence15
Looking at the science behind the evidence used in the lifestyle reports, to ensure that high quality and reliable data is used, and identifying where there are still gaps and require further research.
Dr Travé Huarte encourages practitioners to read some or all of these reports, as to better inform and educate patients where lifestyle changes could positively influence the ocular surface.
Implementing diagnostic tests into the routine practice
Now that triaging has been done, a risk factor analysis carried out, where then can you squeeze in all of the necessary tests with the normal eye test?
Following the TFOS DEWS II diagnostic flowchart, symptomatology is the next step in the patient journey. Dr Travé Huarte suggests that the easiest way to assess symptoms is through the use of questionnaires. To save time in the actual appointment, these could be done over the phone when booking the patient in, while the patient is waiting to be seen, or given to the patient to fill in prior to their appointment.
It is recommended that the Dry Eye Questionnaire DEQ-516 is used, or alternatively the Ocular Surface Disease Index, OSDI,17 is used. Both of these are well published and validated. Both require scores over a certain threshold to be classed as a case of dry eye.
Following on from the assessment of symptoms, it is important to explore the signs. The TFOS DEWS II criteria proposes three homeostasis markers which should be investigated. One of these is assessment of the tear film through tear break up time (TBUT) testing. Ideally, this would be best performed using non-invasive procedures such as through the use of a keratometer or Easytear View to view the disintegration of mires, without interference from the addition of dyes.
However, where such methods are unavailable, it is acceptable to use fluorescein to help visualise the TBUT. If the TBUT is less than 10 seconds, it can be indicative of an ocular surface problem.5, 18
It is common to start with the least invasive diagnostic tests, and work to the more invasive tests. After non-invasive TBUT then, measurement of the osmolarity of the tear film can be made. If TBUT assessment is made using fluorescein, it is recommended that osmolarity is tested first as to not to disrupt the tear film.
Dr Travé Huarte compares osmolarity to the concentration of solutes in seawater. If the water evaporates, you still have the same amount of solutes in less water, making the solution more concentrated and hence hyperosmolar. If the osmolarity of the tears is 308mOsm/L or more,18 or there is an inter-eye difference of 8mOsm/L or more,19 this could be suggestive of DED.5
The third recommended test is ocular surface staining. Surface staining can be further divided up into corneal staining and conjunctival staining. Corneal staining is conducted using fluorescein and the number of ‘dry’ spots assessed using grading scales. There are many available grading scales to help with this assessment, and Dr Travé Huarte tells us that it does not matter which scale you prefer to use, as long as the use is consistent. The TFOS DEWS II flowchart suggests that more than five corneal spots are indicative of OSD.5, 20
For conjunctival staining, Lissamine Green is the recommended dye to aid visualisation of dry spots. It is important to differentiate genuine dry spots to general pooling caused by folds in the conjunctiva. A positive marker is the presence of nine or more conjunctival spots.5, 20
It is also possible to assess ocular surface staining by assessing the lid margin for lid wiper epitheliopathy. While Marx’s line is a normal observed featured, increasing friction between the eye and the lid can cause staining on the margin, which may increase in length and width according to the severity.21
DED is a subset of OSD; if signs are present without symptoms, then it will be classed as OSD, if symptoms are present with any of the above signs, this translates to DED.5 Once it has been established that there is a positive indication of DED, it is possible to investigate the subtype of DED.
Dr Travé Huarte goes on to explain that her investigations start as soon as the patient presents in front of her. While conversing, it is helpful to note their blinking pattern, are they regular, full blinks or is there some impartial blinking present? This could already tell you a story from the start.
When assessing under the slit lamp, beginning the routine with the patients eyes closed is useful to allow the assessment of the lids and lashes. This nicely leads into lid margin and gland assessment, allowing a general overview.
For ADDE, the focus is on the volume of tears. A simple way to integrate this into the normal routine is to assess tear meniscus height. Anything under 0.2mm could suggest there is some dysfunction in tear production.5, 22
For EDE, looking at the lids and examining for blepharitis and assessing the glands and oil production is vital. Infrared imaging is useful to help with gland assessment if available. The oils being released by the glands can be assessed for their quality using the 1st Purkinje image using the slit lamp, by viewing the lipid layer fringing or movement on blink, which can tell you if you have a thin lipid layer which may cause problems, or perhaps a thick lipid layer which isn’t spreading well across the eye, which could also be troublesome.5
Next, Dr Travé Huarte discusses the need to check for the presence of demodex. When it is difficult to differentiate blepharitis from Demodex, she utilises very fine tweezers to slide down the collarettes, and gently pulls the lash down and to the right to form an ‘L’ pattern, which in the presence of Demodex, disturbs them within the follicle so they ‘wiggle’ out.23
Dr Travé Huarte goes on to emphasise that looking at the patient as a whole can also give you many clues about their potential ocular status. For instance, looking at the nose of a patient and observing rosacea could indicate DED, as it is a probable risk factor.6
Cosmetics such as growth serums, make up, eye lash extensions, glue to attach the fake lashes, could all create a toxic environment for the ocular surface.11
Delving a bit deeper into the assessment of the glands, Dr Travé Huarte recommends upskilling in this area to really benefit from lid margin assessment. Dr Travé Huarte everts both the upper and lower lids, ensuring that all details are seen including the puncta. An ideal way to assess the glands is through meibography, to examine the shape and size of the glands.5
The Meiboscale is the preferred grading scale used by Dr Travé Huarte to assess the meibography results, classifying the upper and lower glands on a scale from zero to four, which corresponds to the area of gland loss.24 Dr Travé Huarte emphasises that we must not just base our judgement on meibography alone. One must assess gland dropout, gland secretion and changes in lid morphology.
To look at the secretions from the glands, there is a device called a Meibomian Gland Evaluator to assist with this. However, Dr Travé Huarte explains that where a practitioner does not have this available, it is possible to use your thumb. Pressing along the lid margin to express the oils from the glands, it is possible to evaluate the quality of these expressions. Assessing three sections at a time (that encompass ~five glands a section), grading can be made on the following scale:
- 3 (clear liquid secretion)
- 2 (cloudy liquid secretion)
- 1 (inspissated/toothpaste consistency)
- 0 (no secretion)
A total value can then be assigned by summing the grades of all 15 glands, allowing a value that can be monitored from a scale of 0 to 45.25, 26
Figure 4: Incorporating a Dry Eye assessment into your routine. Adapted from TFOS DEWS II6
Dr Travé Huarte’s top tips for routine dry eye assessment
- Listen to clues in history and symptoms or employ the use of a standardised questionnaire to understand the symptoms a bit better.
- Use fluorescein where possible to allow for a more thorough anterior eye assessment; this will allow identification of issues which may otherwise be missed, eg staining from incomplete blinking.
- Dry eye testing can take time, so if it is needed, do not be afraid to book another appointment just for dry eye tests, especially if you are managing a patient and need to reassess the ocular surface to check if the intervention is helping.
- Ask patients to bring things written down before the appointment. This includes questions they might have, as well as the specifics of their health conditions and any medication they are taking. Far too often, patients forget the medication they are taking, which could be affecting their ocular surface. Details are important for us to decipher what the problem is.
- Effective listening can go a long way. Patients may be frustrated and tired of having tried all different solutions and nothing has worked. Listening to their concerns may lessen their stress and allow better management going forward.
- Back up your advice with leaflets and written information, as it is very easy to forget the verbal communication and advice given in the test room. This applies to the diagnosis too; as well as explaining the condition in the test room, supply written information that the patient can refer to.
- Demonstrate how to insert drops or use the prescribed treatment. Many patients are not familiar with inserting drops into their eyes and the treatment will not be effective if it is not reaching the desired destination.
- If treatment does not work after consistent use for one month, it is acceptable to move on to another treatment plan. This will help with patient compliance and chair time, as well as allowing for a quicker turnaround.
- Dr Sònia Travé Huarte is a postdoctoral researcher and optometrist at Aston University. She specialises in dry eye diagnostics and treatments, anterior ocular surface disease management, specialty contact lenses, Meibomian gland dysfunction and corneal pain. She is also a clinical supervisor on the optometry programme at Aston University.
She is an associate fellow of the higher education academy, member of the international Association of Contact Lens Educators and the fellowship lead for the British Contact Lens Association. Sònia is inspired daily by new treatment innovations, research, and best evidence-base treatment care for her patients.
References
- Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, Liu Z, Nelson JD, Nichols JJ, Tsubota K, Stapleton F. TFOS DEWS II Definition and Classification Report. Ocul Surf. 2017 Jul;15(3):276-283.
- Stahl, U, Willcox, M and Stapleton, F, 2012. Osmolality and tear film dynamics. Clinical and Experimental Optometry, 95(1), pp.3-11.
- Bron, AJ, de Paiva, CS, Chauhan, SK, Bonini, S, Gabison, EE, Jain, S, Knop, E, Markoulli, M, Ogawa, Y, Perez, V and Uchino, Y, 2017. Tfos dews ii pathophysiology report. The Ocular Surface, 15(3), pp.438-510.
- Belmonte, C, Nichols, JJ, Cox, SM, Brock, JA, Begley, CG, Bereiter, DA, Dartt, DA, Galor, A, Hamrah, P, Ivanusic, JJ and Jacobs, DS, 2017. TFOS DEWS II pain and sensation report. The Ocular Surface, 15(3), pp.404-437.
- Wolffsohn, JS, Arita, R, Chalmers, R, Djalilian, A, Dogru, M, Dumbleton, K, Gupta, PK, Karpecki, P, Lazreg, S, Pult, H and Sullivan, B.D, 2017. TFOS DEWS II diagnostic methodology report. The Ocular Surface, 15(3), pp.539-574.
- Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, Na KS, Schaumberg D, Uchino M, Vehof J, Viso E, Vitale S, Jones L. TFOS DEWS II Epidemiology Report. Ocul Surf. 2017 Jul;15(3):334-365
- Jones, L, Efron, N, Bandamwar, K, Barnett, M, Jacobs, DS, Jalbert, I, Pult, H, Rhee, MK, Sheardown, H, Shovlin, JP and Stahl, U, 2023. TFOS Lifestyle: impact of contact lenses on the ocular surface. The Ocular Surface, 29, pp.175-219.
- Gomes, JAP, Azar, DT, Baudouin, C, Bitton, E, Chen, W, Hafezi, F, Hamrah, P, Hogg, RE, Horwath-Winter, J, Kontadakis, GA and Mehta, JS, 2023. TFOS Lifestyle: impact of elective medications and procedures on the ocular surface. The Ocular Surface.
- Markoulli, M, Ahmad, S, Arcot, J, Arita, R, Benitez-del-Castillo, J, Caffery, B., Downie, LE, Edwards, K, Flanagan, J, Labetoulle, M and Misra, SL, 2023. TFOS Lifestyle: impact of nutrition on the ocular surface. The Ocular Surface, 29, pp.226-271.
- Wolffsohn, JS, Lingham, G, Downie, LE, Huntjens, B, Inomata, T, Jivraj, S, Kobia-Acquah, E, Muntz, A, Mohamed-Noriega, K, Plainis, S. and Read, M, 2023. TFOS Lifestyle: impact of the digital environment on the ocular surface. The Ocular Surface, 28, pp.213-252.
- Sullivan, DA, da Costa, AX, Del Duca, E, Doll, T, Grupcheva, CN, Lazreg, S, Liu, SH, McGee, SR, Murthy, R, Narang, P and Ng, A, 2023. TFOS Lifestyle: impact of cosmetics on the ocular surface. The Ocular Surface.
- Stapleton, F, Abad, JC, Barabino, S, Burnett, A, Iyer, G, Lekhanont, K, Li, T, Liu, Y, Navas, A, Obinwanne, CJ and Qureshi, R, 2023. TFOS lifestyle: Impact of societal challenges on the ocular surface. The Ocular Surface.
- Alves, M, Asbell, P, Dogru, M, Giannaccare, G, Grau, A, Gregory, D, Kim, D.H, Marini, MC, Ngo, W, Nowinska, Aand Saldanha, IJ, 2023. TFOS Lifestyle Report: Impact of environmental conditions on the ocular surface. The Ocular Surface.
- Galor, A, Britten-Jones, AC, Feng, Y, Ferrari, G, Goldblum, D, Gupta, PK, Merayo-Lloves, J, Na, KS, Naroo, SA, Nichols, K.K. and Rocha, EM, 2023. TFOS Lifestyle: impact of lifestyle challenges on the ocular surface. The Ocular Surface.
- Downie, LE, Britten-Jones, AC, Hogg, RE, Jalbert, I, Li, T, Lingham, G, Liu, SH, Qureshi, R, Saldanha, IJ, Singh, S and Craig, JP, 2023. TFOS Lifestyle-Evidence quality report: Advancing the evaluation and synthesis of research evidence. The Ocular Surface.
- Chalmers, RL, Begley, CG and Caffery, B, 2010. Validation of the 5-Item Dry Eye Questionnaire (DEQ-5): Discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Contact Lens and Anterior Eye, 33(2), pp.55-60.
- Schiffman, RM, Christianson, MD, Jacobsen, G, Hirsch, JD and Reis, BL, 2000. Reliability and validity of the ocular surface disease index. Archives of ophthalmology, 118(5), pp.615-621.
- Lemp, MA, Bron, AJ, Baudouin, C, Del Castillo, JMB, Geffen, D, Tauber, J, Foulks, GN, Pepose, JS and Sullivan, BD, 2011. Tear osmolarity in the diagnosis and management of dry eye disease. American journal of ophthalmology, 151(5), pp.792-798.
- Sullivan, B, 2014. Challenges in using signs and symptoms to evaluate new biomarkers of dry eye disease. The Ocular Surface, 12(1), pp.2-9.
- Whitcher, JP, Shiboski, CH, Shiboski, SC, Heidenreich, AM, Kitagawa, K, Zhang, S, Hamann, S, Larkin, G, McNamara, NA, Greenspan, JS and Daniels, TE, 2010. A simplified quantitative method for assessing keratoconjunctivitis sicca from the Sjögren’s Syndrome International Registry. American journal of ophthalmology, 149(3), pp.405-415.
- Korb, DR, Herman, JP, Greiner, JV, Scaffidi, RC, Finnemore, VM, Exford, JM, Blackie, CA and Douglass, T, 2005. Lid wiper epitheliopathy and dry eye symptoms. Eye & Contact Lens, 31(1), pp.2-8.
- Nichols, KK, Mitchell, GL and Zadnik, K, 2004. The repeatability of clinical measurements of dry eye. Cornea, 23(3), pp.272-285.
- Muntz, A, Purslow, C, Wolffsohn, JS and Craig, JP, 2020. Improved Demodex diagnosis in the clinical setting using a novel in situ technique. Contact Lens and Anterior Eye, 43(4), pp.345-349.
- https://www.heiko-pult.de/media/files/MEIBOSCALE-2016--Einseiter.pdf accessed on 11/08/2023
- Lane S, et al. 2012. A New System, the LipiFlow, for the Treatment of Meibomian Gland Dysfunction. Cornea. April 2012, Volume 31 (4), p 396–404
- Knop E, Knop N, Millar T, Obata H, Sullivan DA. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011 Mar 30;52(4):1938-78
