In December 1986, the LA Times published an article highlighting an outbreak of acanthamoeba keratitis.1 The number of cases had been growing for some time and both professional bodies and the media were taking note. So, what led to this unfortunate episode? Well, in part, us.
To combat ocular irritation induced by preservative treated lens solutions, well-intentioned eye care professionals (ECPs) had been advising patients to make their own saline at home instead. This episode illustrates how approaches we adopt and may consider acceptable practice at the time, can look a lot less favourable in the face of evidence. As the old adage goes, hindsight is always 20/20.
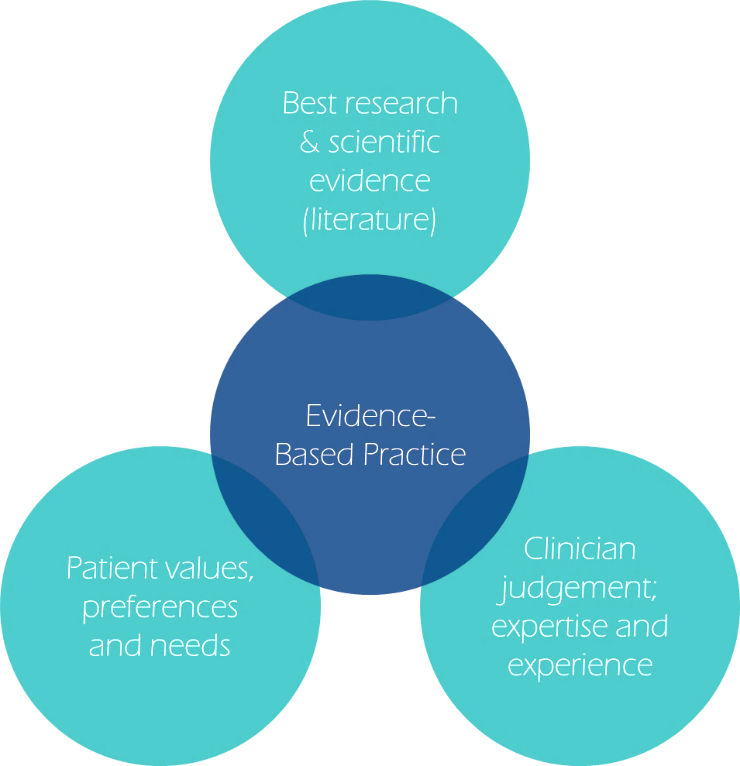
So, which of our current practices is destined for the history books? How can ECPs ensure our current practice is evidence-based? Well, we often hear the phrase evidence-based practice (EBP) and may assume it involves considering scientific evidence alone, but the BCLA CLEAR report on EBP describes how scientific evidence should be integrated with clinical expertise, practice context, and individual patient values (figure 1).2
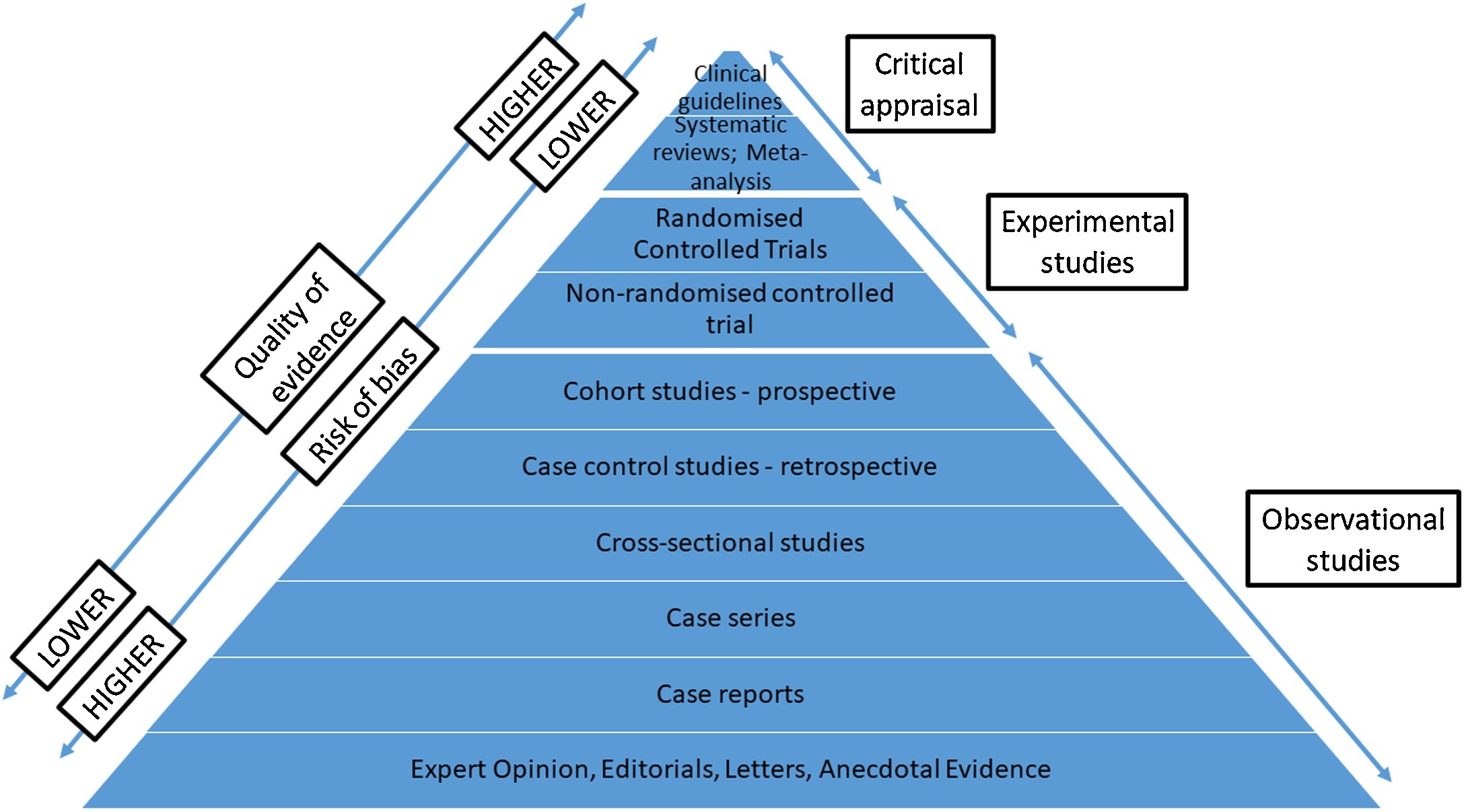
The report also highlights how scientific evidence itself is often stratified into a hierarchy ranging from robust randomised clinical trials through to clinical case reports, but ultimately each approach comes with its own merits and limitations (figure 2). In short, not all science is the same.
Figure 1: Fundamental elements of evidence-based practice (based on Wolffsohn et al 2021)2
The reports also provided an opportunity to address inconsistencies in terminologies and favoured descriptors used by the Federative Committee on Anatomical Terminology (FCAT), such as renaming Bowman’s and Descemet’s membranes, the anterior and posterior limiting lamina respectively. Rigid corneal lens (RCL) was preferred to support differentiation from scleral lenses and ‘planned’ or ‘sporadic’ ‘overnight wear’ were deemed more appropriate representations for clinical use.
Below we discuss some of the highlights of the BCLA CLEAR EBP report, in part 1, with respect to contact lens fitting and consider areas of clinical practice that may warrant an update. Part 2 will focus on subsequent contact lens reviews and aspects that influence retention.
Figure 2: Hierarchy of clinical scientific evidence (Wolffsohn et al 2021)2
What is evidence based practice?
- The integration of best research evidence with clinical expertise and patient values to inform decision making by practitioners to provide tailored patient care
History and symptoms
To begin, history and symptom taking can be used to elicit reasons for a patient’s visit, identify any key concerns, and inform management decisions and lens choice. The main motivation for contact lens wear typically stems from a desire to improve cosmesis, for use when playing sports, or to improve optics.
Information gathering can also be conducted by remote virtual methods or using questionnaires ahead of the consultation.
Figure 3 lists some of the major areas covered when history and symptom taking:

Anterior eye exam
Slit lamp biomicroscopy
Although easy to overlook its importance, a comprehensive slit lamp examination provides essential baseline data, which can be useful when monitoring ongoing anterior eye changes. While video slit lamps have been around for decades, more recently smartphone camera mounting systems have also become available and there seems to be a growing possibility that AI powered diagnosis systems will eventually reach our exam rooms.
Less commonly, other imaging equipment can also be used to assess anterior eye health and/or contact lens fitting, see figure 4.
Figure 4: Anterior eye imaging techniques other than slit lamp biomicroscopy

Anterior eye grading scales:
Most ECPs (>80%) use some form of anterior eye grading scale or photography to facilitate recordkeeping and are useful when communicating findings to patients. A grade change of >1 unit is typically considered clinically significant. While, previously, ECPs had been encouraged to grade in increments of 0.1, more recent evidence supports grading to the nearest 0.5 as being as accurate and better than just 1 unit intervals. Grading scales are not without their shortcomings and in practice it is worth remembering that scales are not interchangeable, thus the name of the grading scale used ought to be recorded.
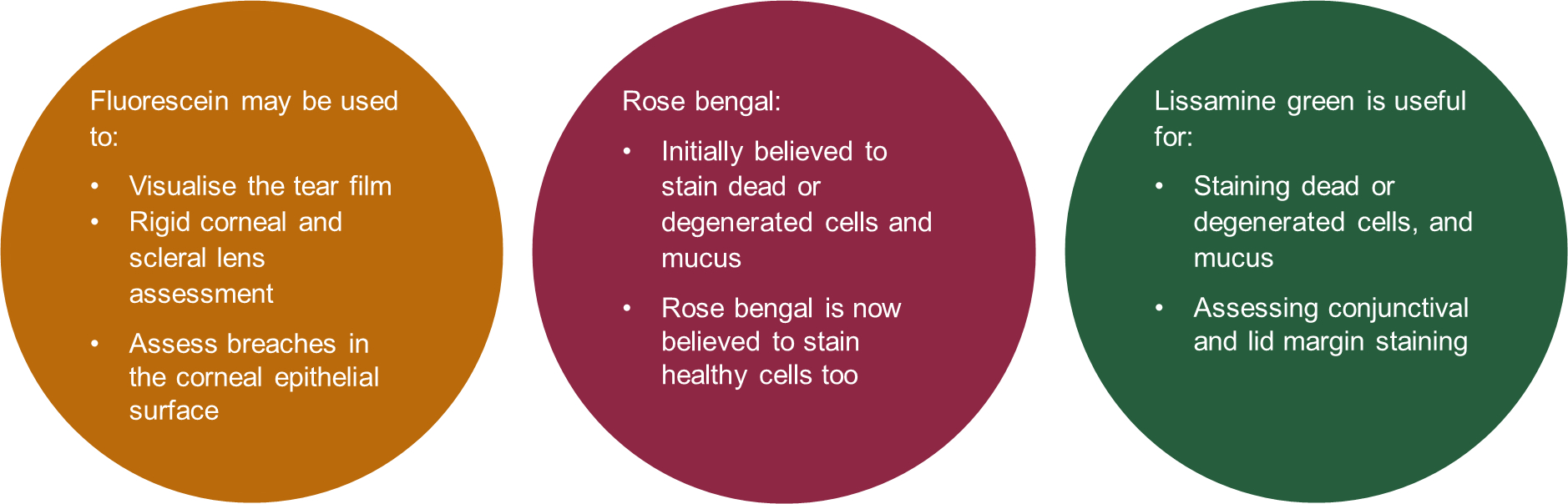
Figure 5 Applications of diagnostic dyes
Ophthalmic dyes
Sodium fluorescein
Since excess fluorescein can impede the amount of fluorescence, the recommended approach is to use a drop of saline on the paper strip and then remove the excess liquid by shaking. Ideally, assessment ought to be undertaken 1-3 mins after instilling the dye. Fluorescence can be observed using a blue light, with a peak of around 495nm, and enhanced using a yellow filter, with a sharp cut-off at 500nm.
Lissamine green
Lissamine green had largely superseded the use of Rose bengal. For lissamine green ophthalmic strips, it recommended that the saline drop be left on the strip a minimum of five seconds. Assessment may begin 1-4 mins following instillation. The ocular surface can be assessed under white light, and visualisation of the dye enhanced using a red-free filter.
Clinical learnings:
- Anterior eye grading at least the nearest 0.5 units
- Fluorescein sodium for corneal assessment. Lissamine green for conjunctival and lid margin assessment
Ocular surface measurements and lens selection
A range of ocular surface measurements are typically taken prior to lens fitting. While most measurements can be reasoned as essential for determining first choice lens parameters, their value may be called into question where the eventual lens is mass produced and thus most lens parameters fixed for example, BOZD for a typical soft lens. Nevertheless, baseline measures can facilitate the future monitoring of ocular surface changes.
The main measurements for lens parameter selection are listed below, additional considerations relating to lens modality, lens material and lens type will also influence eventual lens selection.
Horizontal visible iris diameter (HVID)
HVID refers to the horizontal distance between nasal and temporal limbi. Conventionally measured using a ruler or slit lamp graticule, ECPs with access to equipment such as biometers and corneal topographers will have noticed that many of these machines allow objective measures of HVID. It should, however, be noted that HVID values can differ significantly depending on the method of measurement.
Typical adult values range between 10-14mm, with an average of 11.8±0.5mm.
What the science says: The BCLA CLEAR EBP report acknowledges that there is little published evidence to inform soft lens diameter choice. It is commonly accepted that interactions between the lens edge and limbal area be avoided.
For rigid corneal lenses neophytes, changing the lens diameter is not found to confer any benefits during the initial adaption process.
Vertical palpebral aperture (VPA)
Defined as the vertical fissure between superior and inferior lid margins with the eyes in primary gaze, VPA typically measures 9.7±1.2 mm with a range of 9.1-10.8 mm.
What the science says: A surprising finding of the BCLA CLEAR EBP report was the lack of published evidence describing the relevance of VPA to lens fitting.
Pupil size
Pupil size is typically measured under room and mesopic lighting conditions, often with a ruler although pupillometers and other objective approaches are available. Average values in adults range from 2.0 to 7.0mm in bright light to 4.0 to 8.5mm in the dark. Numerous factors can influence pupil size including luminance, accommodation, convergence, age, and refractive status.
What the science says: Pupil size may be important for bifocal and multifocal lens performance. Yet, some studies have shown how patient performance and preference for different multifocal lenses was unaffected by pupil size.
Corneal shape
The sagittal height of the cornea and lens inform lens fitting, but in the absence of these measurements, we have widely relied upon corneal curvature to estimate lens base curve. Traditional manual topographers typically only measure the central 3mm, modern topographers and computer software programmes can now model the full eye shape profile.
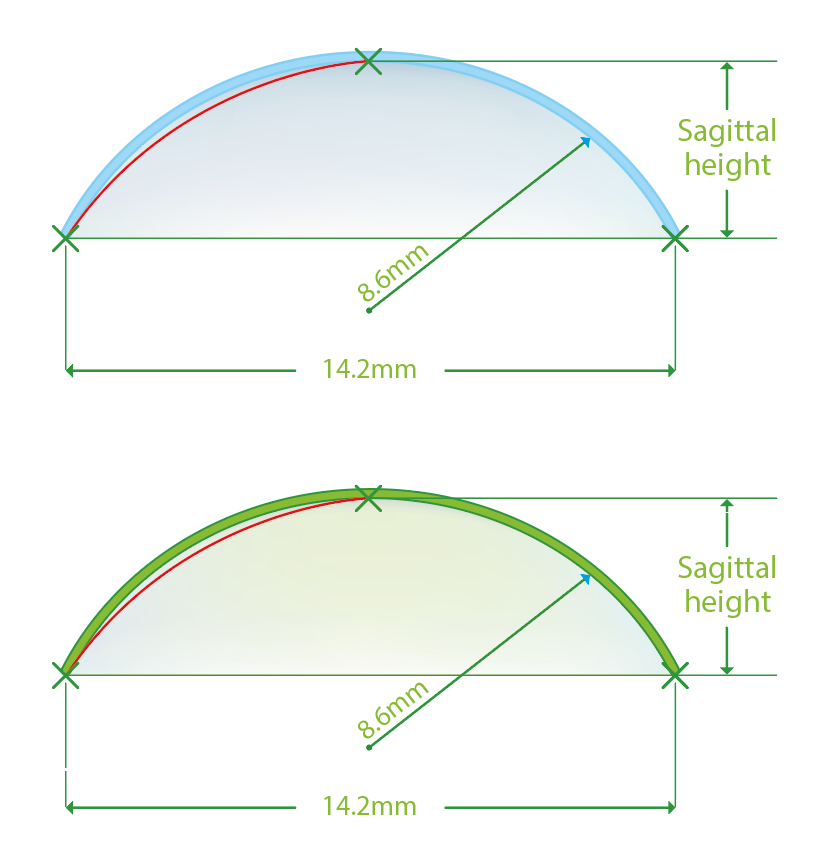
Sagittal height refers to the distance that the contact lens ‘stands off’ the eye, such as the perpendicular space between the lens back surface and the cornea (figure 6). The larger the sagittal depth, the tighter the lens. Altering the lens diameter and/or base curve can influence this and make the contact lens looser or tighter.
Characterising corneal shape at baseline can help monitor changes over time and, although of less relevance to soft lenses, can identify the presence of corneal astigmatism.
What the science says: The limited number of base curves available for mass produced soft contact lenses can be used successfully for approximately 75-90% of eyes, the remainder may require a custom designed lens.
For RCL fitting, corneal topography is typically used for BOZR selection and can be used to show the effects of a decentred lens. The eccentricity values produced from video keratography are a better predictor of base curve to corneal relationship than central keratometry readings alone.
Figure 6 Illustration of sagittal height
Lens power
The BCLA CLEAR report reminds us that trial frame/phoropter mounted prescriptions require conversion to the corneal plane equivalent. For prescriptions under 4D the difference in power is likely small and so spectacle power may be used as a starting point. For spherical lenses, mean spherical power can be used but, where required, toric corrections can provide improvements in visual quality.
Rigid corneal lenses
Computerised software can support lens selection, making the process simple and can also be used to generate expected fitting pattern profiles and predict the lens fitting behaviours (figures 7 and 8).
Figure 7 & 8: (top) Lens selection and predicted fitting pattern using computerised software; (bottom) Predicted fitting pattern appearances on the same eye with 0.3 adjustments to the radius, showing a flat and steep fit respectively
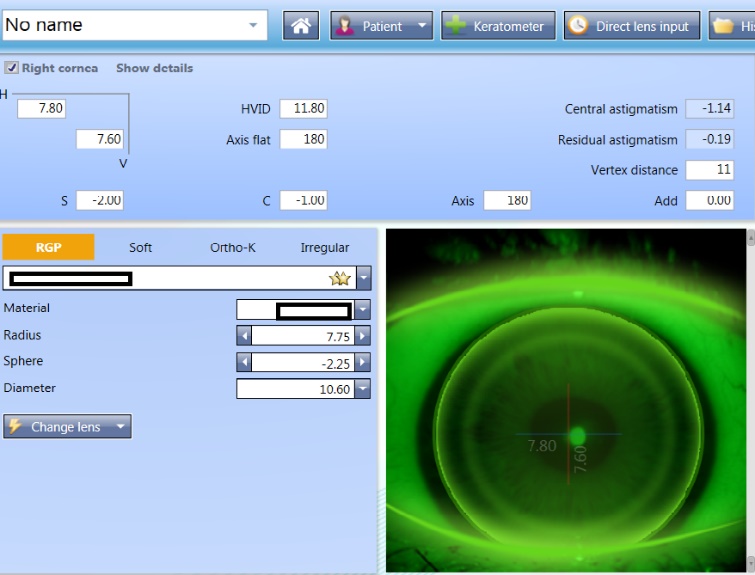
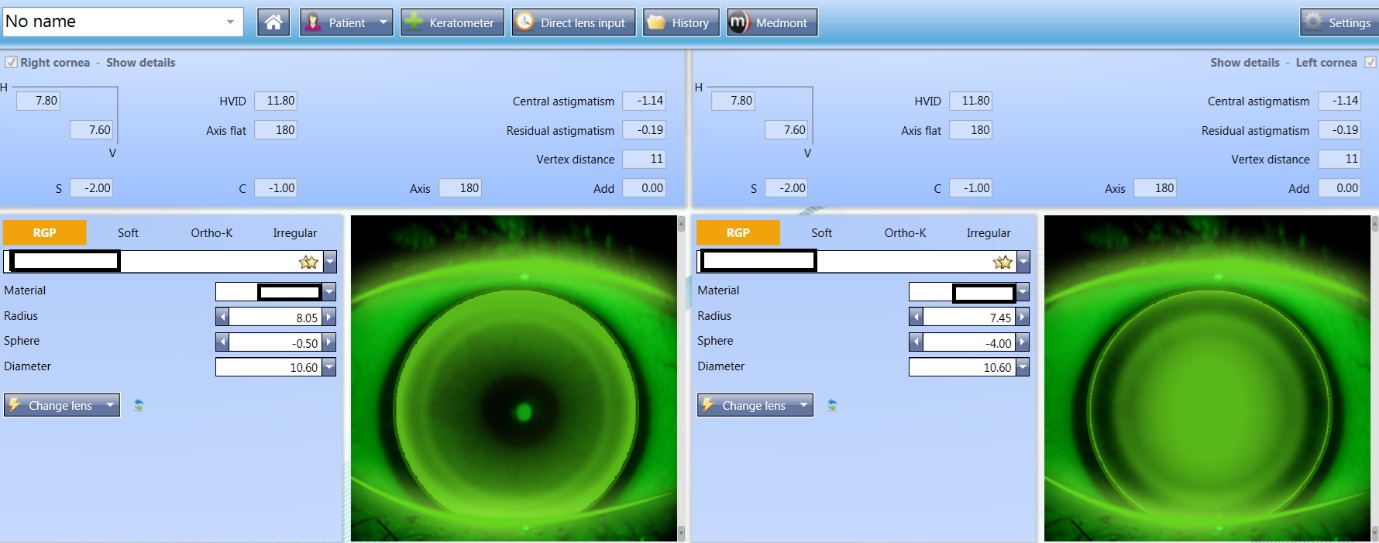
Lens material, design and modality
The majority of new lens fits are soft lenses, possibly reflective of the short adaptation and fitting processes, wide availability, and strong performance they can offer. In 1998, it was predicted that by 2010 we would see the demise of rigid lenses.3,4 Given rigid corneal lenses can be more effective for those with ocular pathology, high corneal astigmatism, are associated with lower levels of some contact lens complications, used for orthokeratology and show better tolerance in dry eye and papillary conjunctivitis patients compared to soft lenses, rigid corneal lenses are still very much available and in use.
There has also been a recent surge in scleral/mini-scleral prescribing especially for medical/therapeutic use, those with poor acceptance to other lens types and dry eye sufferers. More details on these were covered earlier in the series.
Contact lens wear with good compliance carries a low risk of serious complications.5 For soft lenses, daily disposables may be the preferred choice for individuals with higher risk of corneal infiltrative events or those who favour convenience.
The influence soft lens material is unclear except for when high oxygen transmissibility is the goal (for overnight wear, or for those considered to have a high swelling response to hypoxic conditions, high prescriptions or potentially for therapeutic use).
Evaluating the fitting and assessing visual performance
Soft lens fit
Assess the contact lens fit approximately 10 mins after application following the guidance in figure 9.
- Observe contact lens surface, wettability and quality
- Use a fitting cross to denote any decentration relative to the corneal centre
- Assess post-blink movement in upgaze – ideal values are 0.25 to 0.50mm
- Horizontal lag: assess change in overlap of lens onto limbus – ideally 0.5 to 1.0mm
- Push up recovery speed: ideally 2-4mm/s or non-sluggish, visible recovery
- A comfort score from 0 (poor comfort) to 10 (can’t feel) can also be used during lens assessment
- For toric lenses, assess the position of orientation markings, compensating for any rotation using the CAAS rule (Clockwise Add, Anticlockwise Subtract) or LARS rule (Left Add, Right Subtract). Assessments of the marker in both the primary position and during different directions of gaze is useful. Physically rotating the lens can also help ascertain rotational stability to judge the recovery of the toric marker back to the starting position.
Figure 9 Recording of a soft lens fit
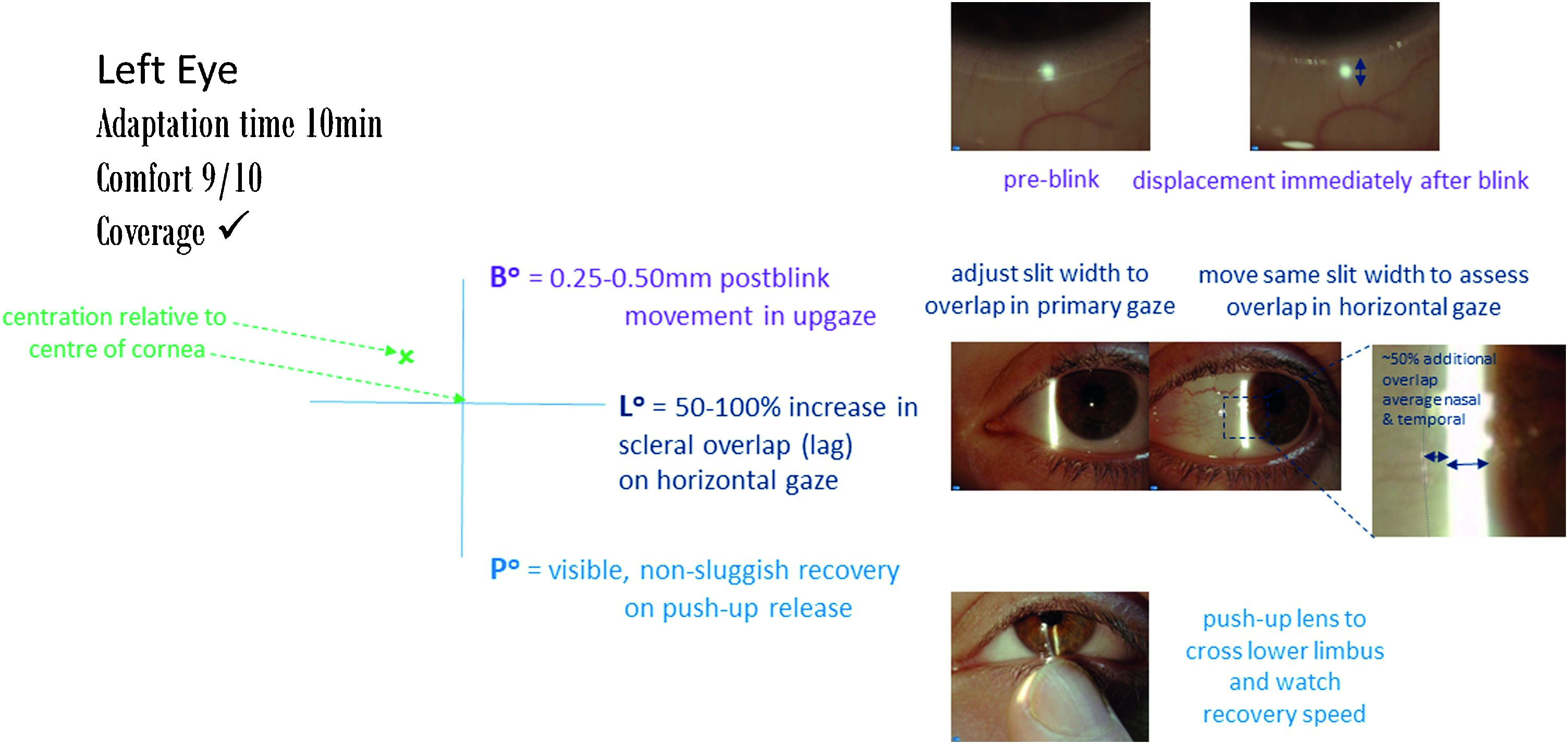
Visual performance
- Even a cyl of 0.75D can impact visual performance, thus the difference between a toric and mean spherical equivalent ought to be demonstrated to the patient.
- For multifocals, assessing sensory dominance (determined by which eye is most adversely affected by a +1.50D add during binocular distance viewing) is recommended
- For complex optical design lenses, real world tasks may provide a better assessment of visual experience than clinical objective measures
Rigid corneal lens fit
Rigid corneal lenses should be fitted to create an alignment fit in at least one meridian, which distributes the weight of the lens over a larger area of the cornea and forms a tear fluid layer of 10-25μm between the back surface of the lens and the anterior surface of the cornea.
Compared to soft lenses, the fit is dynamic with greater lens movement and tear exchange across the ocular surface. The refractive properties of the post lens tear film typically become part of the optical correction and the post lens tear layer profile is easily visualised with fluorescein.
Any dark areas indicate contact or close alignment with the cornea, corresponding to a tear layer <20um, In contrast, as the tear layer volume increases, florescence will be brighter and broader.
For RCLs, assess the fit around 20 minutes after lens application to allow for adaption and for reflex tearing to subside. The following summarises the recording scheme advised in the BCLA CLEAR report (figure 10):
- Observe the contact lens surface, wettability and quality.
- Gain a comfort score rating (0-10)
- Assess the positioning and coverage – how much smaller when compared to the HVID (+2 to -2). Is the lens lid attached?
- Assess the dynamic centration, ideally this stays within the limbus (C), rather than crossing the limbus (L) or the edge encroaching on the pupil in dim light (P).
- Record the movement on inter-blink, scales relate to millimetres. 0.5-2.0mm could be acceptable
- Following white light assessment, the fluorescein pattern underneath the lens should be observed within 30 seconds to three minutes of instillation when the lens is centred in the primary position assess the centre, mid periphery and edge regions.
Figure 10: Represents the recording systems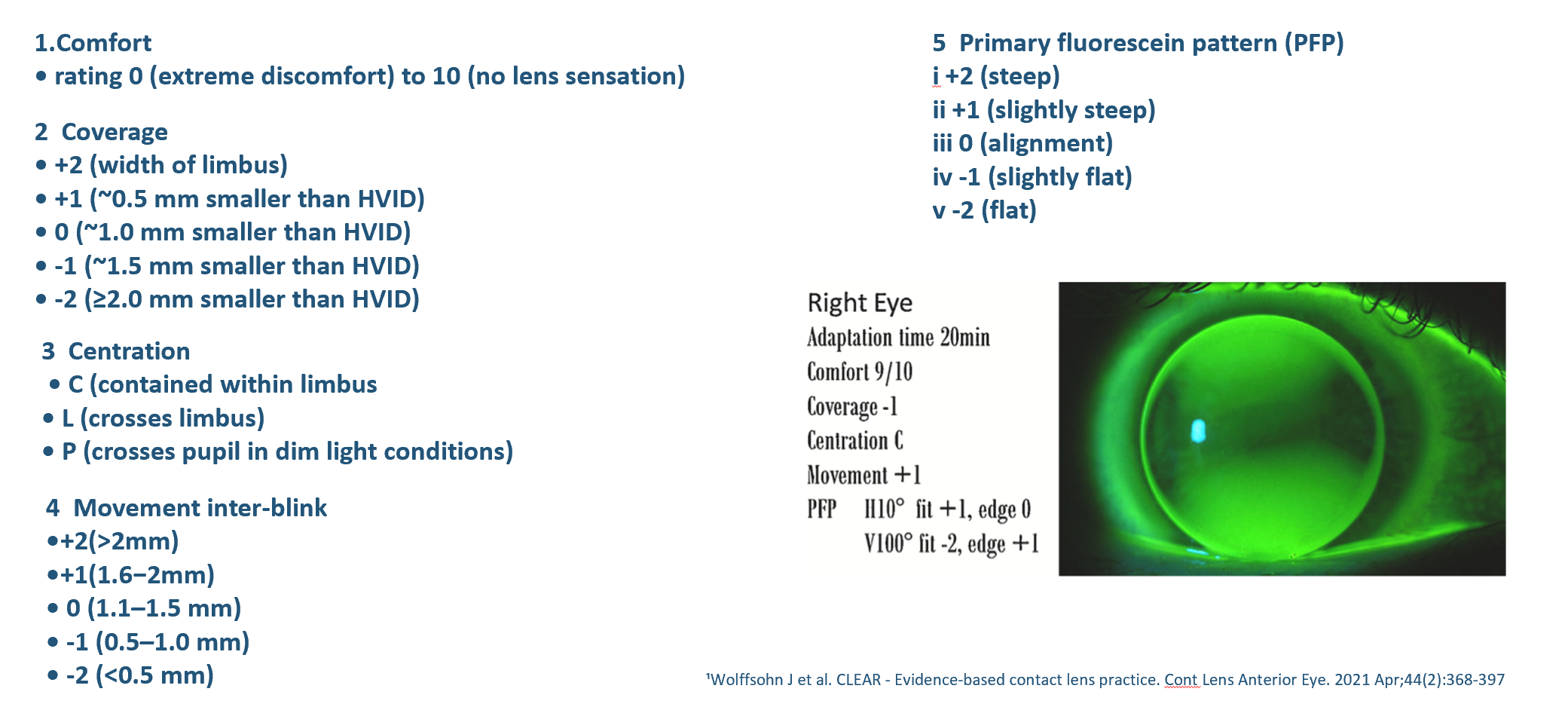
Advanced contact lenses
Previous articles have reviewed in detail the fitting principles of lenses falling beyond the scope of this article, these include:
- Scleral/mini scleral lenses: fitted to vault over the entire cornea, including the limbus, and lands on the conjunctiva overlying the sclera. Broad indications across the entire corneal disease spectrum, ie irregular corneas and ocular surface diseases as well as refractive correction in healthy eyes.6
- Orthokeratology: fitted to lead to a temporary redistribution of the corneal tissue, using reverse geometry rigid corneal lenses when worn overnight, to then be free of any refractive correction during the daytime.7
- Medical contact lenses: fitted for the primary purpose of treating an underlying state or complicated refractive status.8 For keratoconus when using rigid lenses an even distribution is desired, the so called three-point touch approach.
Figure 11: Five clinical pearls for contact lens fitting

Conclusion
The BCLA CLEAR EBP report highlights several areas where clinical conventions deviate from the science, suggesting a possible need to review and update current practices. It is also important to consider other factors, such as patient values and preferences, alongside the science.
The BCLA CLEAR EBP report serves as a reminder to all practitioners of our obligation to keep abreast of changes in the field and figure 11 provides a summary of clinical pearls for contact lens fitting. One thing is ‘CLEAR’, the clinical norms in force at the start of an ECP’s career are unlikely to resemble those by the end.
Look out for part 2 of the BCLA CLEAR EBP summary that will build on aspects of the contact lens journey after the fitting.
- Dr Manbir Nagra is an optometrist, educator, and researcher. She works as an independent consultant within the optical sector.
- Neil Retallic is an optometrist with experience of working with in practice, education, industry, and head office roles. He currently works for Specsavers and the College of Optometrists and is the Immediate Past President of the British Contact Lens Association.
- The full report and supplementary information can be accessed at https://www.contactlensjournal.com/article/S1367-0484(21)00022-9/fulltext
- The BCLA CLEAR Summary report is a short bite-size evidenced-based practical guide for clinicians, bringing together the key findings from the report. Accessed via CLEAR (bcla.org.uk)
The editors for this series are Neil Retallic and Dr Debarun Dutta
- Listen to the BCLA CLEAR Evidence-based contact lens practice podcast: https://anchor.fm/bcla/episodes/BCLA-CLEAR-Podcast-1-Evidence-based-practice-e179tp5
Acknowledgements
Acknowledgement and recognition to James Wolffsohn, Kathy Dumbleton, Byki Huntjens, Himal Kandel, Shizuka Koh, Carolina Kunnen, Manbir Nagra, Heiko Pult, Anna Sulley, Marta Vianya-Estopa, Karen Walsh, Stephanie Wond and Fiona Stapleton who were the paper’s authors and the educational grants from Alcon and CooperVision.
Original paper: Wolffsohn, J.S.; Dumbleton, K.; Huntjens, B.; Kandel, H.; Koh, S.; Kunnen, C.M.E.; Nagra, M.; Pult, H.; Sulley, A.L.; Vianya-Estopa, M.; et al. CLEAR - Evidence-based contact lens practice. Contact lens & anterior eye : the journal of the British Contact Lens Association 2021, 44, 368-397, doi:10.1016/j.clae.2021.02.008.
References
- Mitchell, Claudia. Warning on Soft Contact Lenses : Cleaning With a Sterile Saline Solution Now Urged. https://www.latimes.com/archives/la-xpm-1986-12-07-vw-1183-story.html. Dec Los Angeles Times, 1986
- Wolffsohn JS, Dumbleton K, Huntjens B, Kandel H, Koh S, Kunnen CM, Nagra M, Pult H, Sulley AL, Vianya-Estopa M, Walsh K. BCLA CLEAR-Evidence-based contact lens practice. Contact Lens and Anterior Eye 2021 ;44(2):368-97.
- Efron N. This expert predicts the demise of RGPs by the year 2010 (Advert). Optician. 1998; 216: 15.
- Efron N. Obituary—Rigid contact lenses. Contact Lens and Anterior Eye. 2010 ;33(5):245-52.
- Stapleton F, Bakkar M, Carnt N, Chalmers R, Vijay AK, Marasini S, Ng A, Tan J, Wagner H, Woods C, Wolffsohn JS. BCLA CLEAR-Contact lens complications. Cont LensAnterior Eye. 2021 ;44(2):330-67.
- Barnett M, Courey C, Fadel D, Lee K, Michaud L, Montani G, van der Worp E, Vincent SJ, Walker M, Bilkhu P, Morgan PB. CLEAR - Scleral lenses. Contact Lens Anterior Eye 44 2021 270-288.
- Vincent SJ, Cho P, Chan KY, Fadel D, Ghorbani-Mojarrad N, González-Méijome JM, et al. BCLA CLEAR-Orthokeratology. Contact Lens and Anterior Eye 2021;44(2):240-69.
- Jacobs DS, Carrasquillo KG, Cottrell PD, Fernández-Velázquez FJ, Gil-Cazorla R, Jalbert I, et al. BCLA CLEAR – Medical use of contact lenses. Contact Lens and Anterior Eye 2021;44:289–329.
