This article focuses on the importance of following an evidenced approach to dry eye disease (DED) assessment and the importance of patient communication as DED is a common and all too often chronic condition whereby once the patient begins to feel better, they stop treatment, which can lead to reduced quality of life and depression.
The Aston dry eye study1 showed approximately one-third of the adult UK population have dry eye disease (DED). A slightly higher prevalence was recorded in a survey of UK primary care optometrists involved in management of DED who estimated 47% of their adult patients had DED2 with results suggesting that in some patients DED is unmanaged. Increasing age, female gender, Asian ethnicity, autoimmune disease, environment, and medication are all some of the suggested risk factors.3,4

The refined TFOS DEWS II global dry eye definition3 recognised that dry eye is a complex multifactorial disease as it has several contributing aetiologies. Tear osmolarity (a measure of salt concentration in the tears) is a key component in the development of the two main subtypes of DED, aqueous deficient dry eye (ADDE) where there is a reduced lacrimal secretion, subdivided into Sjögren syndrome dry eye (SSDE) and non-Sjögren syndrome dry eye (NSDE).5 Sjögren syndrome is a chronic autoimmune disorder, autoimmune disorders affect around one in 10 individuals in the UK.6
NSDE may be caused by ablation of the lacrimal gland, severing of the ducts, congenital alacrima, or Triple A-syndrome also, age-related NSDE.5 Evaporative dry eye (EDE) has normal lacrimal secretion but excessive water loss (evaporation), due to meibomian gland dysfunction causing a deficient lipid layer, mucin dysfunction or blink abnormalities affecting stability of the tear film. A retrospective study showed that EDE is three times more common than ADDE7 although, patients can have both ADDE and EDE, which is referred to as mixed dry eye.
Depending upon scope of practice from specialty optometrist (AS/SP/IP), optometrist, contact lens optician and dispensing optician, the very first thing is to actively listen to the patient completing a full history including symptoms, occupation, screen use, working environment, sports/hobbies, if they drive, medication prescribed and any over-the counter medications, including herbal or alternative medicines. Dry eye questionnaires are a good tool, a recently published scoping review highlighted the importance of using questionnaires that were supported by validity and/or reliability studies.8 The OSDI (Ocular Surface Disease Index) was reported as the most widely used in clinical studies for patient reported outcomes. Questionnaires do require patient participation but help clinical decision-making as well as ensuring a patient centred approach.
Evidence-based assessment of DED
Tear film stability
TFOS DEWS II Diagnostic Methodology Report9 suggests a variety of diagnostic tests that can be used for DED assessment starting with the least invasive, firstly tear film stability using non-invasive tear break up time (NIBUT) with <10 seconds as the cut off level indicating dry eye. Fluorescein breakup time (FBUT) also <10 seconds. Paugh et al10 felt that 5.3 to 6 seconds differentiated a dry from a normal eye, also instillation of sodium fluorescein does reduce tear film stability. Sodium fluorescein (NaFL) is a very familiar diagnostic stain for most practitioners and routinely used in contact lens practice whereas the non-invasive measurement is not widely used.
Tear osmolarity
TFOS DEWS II Diagnostic Methodology Report indicates an osmolarity >308mOsm/L and a difference between the eyes of >8mOsm/L is a good indicator of loss of stability in the tear film.9 Measurement of tear film osmolarity is often thought to be a gold standard in diagnosing DED11 and irrespective of aetiology elevated osmolarity is thought to be one of the core mechanisms of symptoms of DED12 although it appears there is growing evidence of mixed results regarding the association of osmolarity and DED,13 results from subjects of the Dream study14 with moderate to severe DED showed no correlation with symptoms and a weak correlation with signs of DED.
Tear meniscus height
The tear meniscus contains 75-90% of the tear volume and acts as a reservoir supplying tears to the pre-corneal tear film.15 Using a slit lamp examination of the tear meniscus height can be assessed against the slit lamp beam narrowed to align with the tear menisci but is dependent upon practitioner skill, using a reticule and magnification of 32x improves accuracy and is an accepted method to assess tear volume and less invasive than the Schirmer test. Anterior optical coherence tomography (OCT) can also be used to measure tear meniscus height, this is the most repeatable method followed by slit lamp with reticule (32x magnification) also OCT has been found to measure higher but only marginally from a clinical perspective.16 The TFOS DEWS II report recommends to discriminate normal from abnormal at a cut off of 0.20mm.9
Ocular surface staining
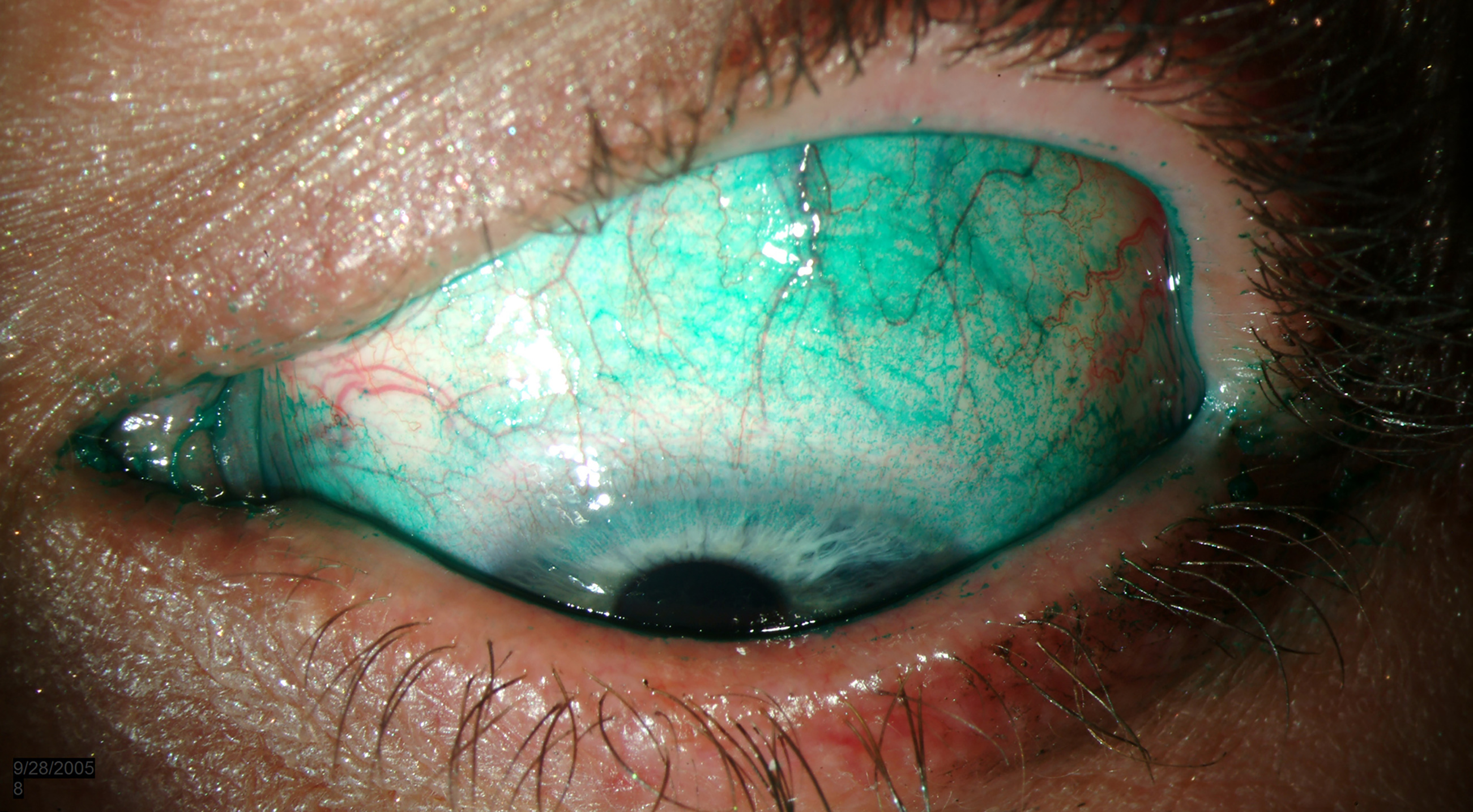
Both corneal and conjunctival staining are markers of DED, sodium fluorescein (NaFl) stains the tear film making it visible as well as damaged epithelial cells a ocular staining score of >5 dots is a positive result.9 Lissamine green stains membrane devitalised cells or dead cells and mucous ocular staining >9 dots is a positive results. Lissamine green is mostly used for assessing the conjunctiva and lid margin and sodium fluorescein for corneal damage (figure 1.) Lid wiper epitheliopathy can be seen using either stain but generally lissamine green is preferred, ≥2mm in length and/or 25% sagittal width (excluding Marx’s line). Lid parallel conjunctival folds (LIPCOF) are conjunctival folds behind the temporal and nasal tear meniscus and often cause tear meniscus height to be underestimated.9
Figure 1: Diffuse Lissamine green staining. Image: eyerounds.org
Unlike conjunctivochalasis are unrelated to increasing age but LIPCOF is a potential indicator for DED.17 Increased tear osmolarity or hyperosmolarity occurs from either reduced lacrimal secretion or tear film break up from evaporation of the tear film lipid layer creating a cascade of inflammatory events in what is known as the ‘vicious cycle’ of dry eye5 (figure 2). The ocular surface when under stress releases cytokines, matrix metalloproteinases (MMPs) and chemokines,18 which activate resident antigen presenting cells (APCs), and mature APCs travel to lymph nodes via lymphatic vessels to activate Th1 and Th17 cells.19 These cells then migrate to the ocular surface and induce damage, epithelial cell apoptosis and goblet cell loss.5 This leads to corneal punctate epitheliopathy, instability of the tear film and back to hyperosmolarity completing the cycle.
Figure 2: The vicious cycle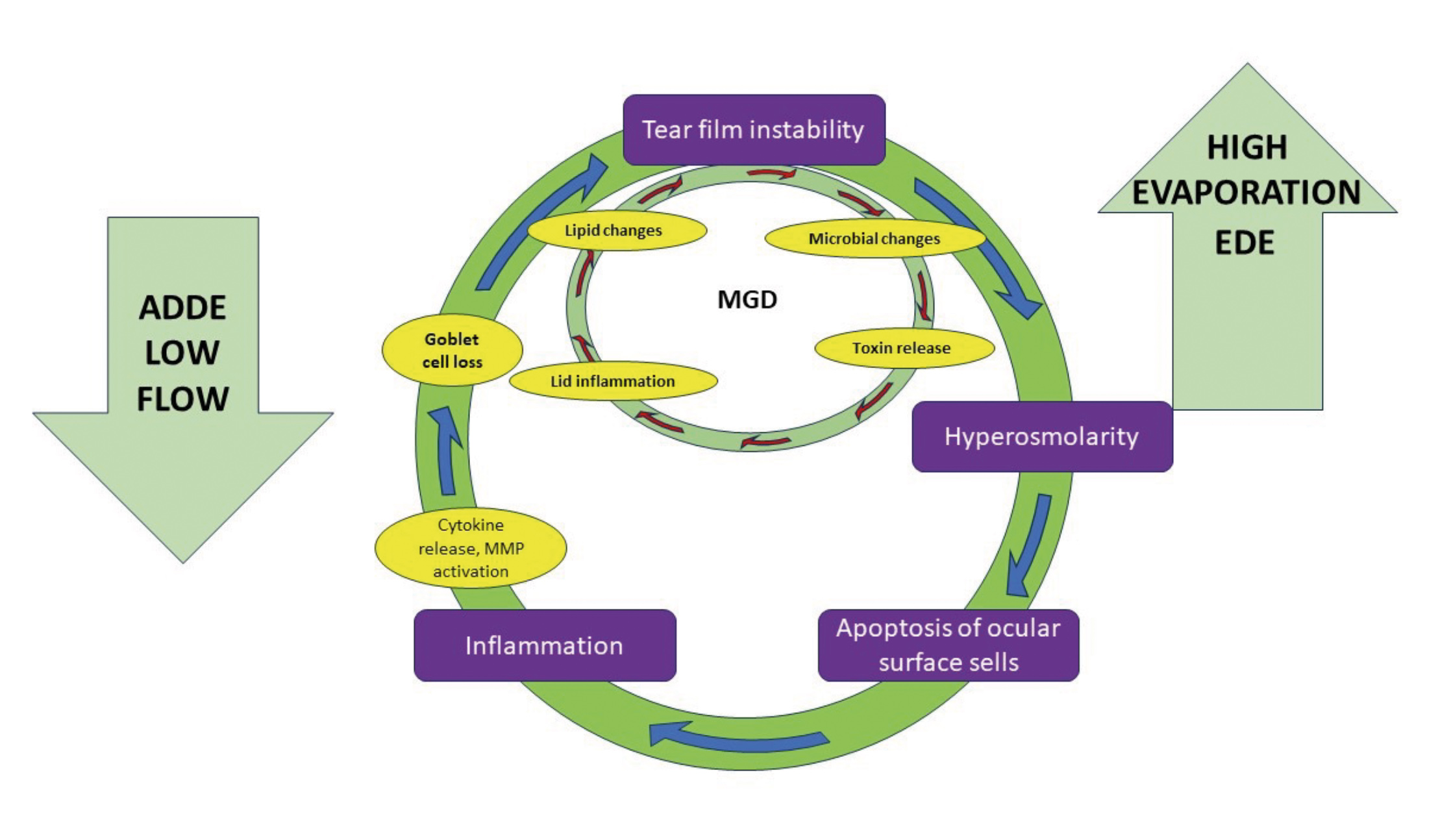
Having considered the assessment of lack of homeostasis of the tear film in the diagnosis of dry eye disease and the two main subtypes, ADDE and EDE, it is important to cover meibomian gland dysfunction as it is the leading cause of EDE.3 A systematic review with meta-analysis found the pooled prevalence of MGD was 35.8%.20 Despite contradictory evidence this review found that the rate of MGD was significantly higher in men than women (47.3% vs 42.8%) and that Asian ethnicity was also a risk factor. Meibomian glands secrete most of the lipids found in the tear film lipid layer (TFLL),21 which is composed of 96% nonpolar lipids that are at the air-tear interface and the amphiphilic polar lipids alongside the aqueous-mucin layer.22 MGD is a chronic condition of the meibomian gland structures whereby duct blockage, drop out, inflammation, lipid deficiencies and microbial increase lead to a decrease in meibum flow.
TFOS DEWS II Pathophysiology Report5 explains the etiology of the two forms of MGD, which cause low delivery of meibomian oil: cicatricial and non-cicatricial MGD. Increasing age causes an increase in meibomian gland dropout, particularly over the age of 50 years. Reduced androgen levels may be a contributing factor for MGD and skin conditions like acne rosacea, atopic dermatitis, seborrhea sicca, and psoriasis are also associated with non-cicatricial MGD, while cicatricial conjunctival diseases like trachoma, erythema multiform and pemphigoid produce cicatricial MGD.
Non-cicatricial MGD
Ductal hyperkeratinisation causes duct obstruction and atrophy of the glands, which can lead to obliteration of the orifices.5 Increased meibum viscosity also adds to the obstruction and inflammatory signs are commonly found at the lid margins. The gland orifices position is unchanged but as age increase and the muco-cutaneous junction (MCJ) moves forward they can become located posterior to the MCJ. If the function of the gland can be restored the gland orifices will remain in a position to deliver oil.5
Diagnosis is made on changes to the meibomian orifices in the early stages expression reveals a cloudy substance and in severe disease telangiectasia and distortion of the orifices at the lid margin can be seen.24 Grading can be undertaken by scoring gland drop out, the amount of oil in the lid margin reservoir, and assessment of the TFLL.5
Cicatricial MGD
Primary cicatricial MGD from scarring of the conjunctival submucosa stretches and narrows the meibomian gland orifices and pulls the meibomian ducts posteriorly into the marginal conjunctival mucosa meaning they can no longer deliver oil effectively.5
Secondary MGD is from conjunctival scarring in cicatricial conjunctival diseases, which tends to be more extensive than in primary cicatricial MGD where the ducts are pulled into the tarsal mucosa and even absorbed into the scar tissue, again delivery of oil is compromised. TFOS DEWS II Pathophysiology Report highlights the need for further research into this condition. This low and changed oil delivery feeds into tear instability, increased evaporation and EDE. High delivery of oil, meibomian seborrhea the gland releases an ‘abundance’ of meibum leading to hypersecretion in this condition there are less gland dropouts but clear changes in the composition of the meibum.5
Blinking
Blinking is essential not only for clarity of vision, sweeping the tear across the ocular surface, aiding expression of lipids from the meibomian glands and drainage of tears. A reduced blink rate and computer/digital screen use is widely reported. The role of blinking was highlighted by Talens-Estarelles et al25 as a reduced blink rate and incomplete blink associated with uses of digital screen may lead to an increase in MGD. Gaze angle being a particular aspect for consideration as desktop computers are often replaced by multiple display workstations.
Case Study – Patient A
An 84-year-old lady recently moved into a care home from independent living accommodation as she was now at the stage where she required more support with day-to-day living and to be nearer to family.
History: Age-related macular degeneration (AMD) diagnosed 16 years ago; Right eye intravitreal injections; Left eye stable. Long standing dry eye disease, also dry mouth and suffers with anxiety. Osteoarthritis in feet, ankles and hands.
Medication: Sertraline, Lanzoprazole, paracetamol, nefopam.
Patient A having moved to a new area attended for her intravitreal injection to a new and unfamiliar clinic. The clinic was very busy and running late and Patient A felt very rushed and on returning to the care home complained of a painful right eye with numerous floaters. She had previously experienced increased floaters so was not unduly alarmed, but the eye felt incredibly bruised and painful unlike anything she had ever experienced before.
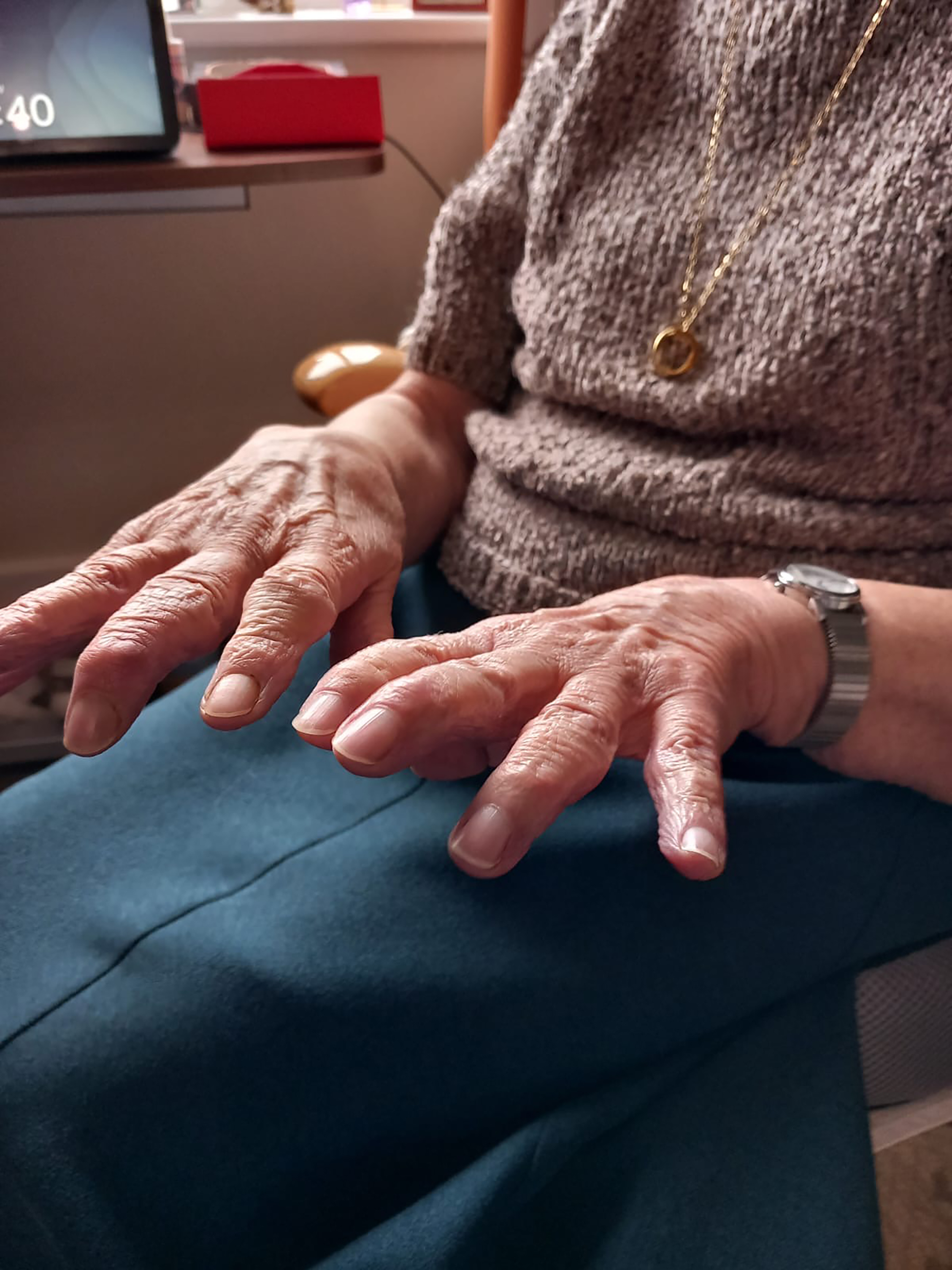
FIGURE 3: Patient A has osteoarthritis of the hands
That evening care home staff noted her eye was bloodshot. The pain did not subside, so she was seen back in clinic two days later. Her visual acuity was R 6/12-2 L6/36, consistent with last recording, OCT scan unremarkable but the hospital doctor noted severe dry eye disease. Patient A said she regularly used dry eye drops. The hospital doctor being helpful said: ‘You shouldn’t be buying drops I will prescribe some for you and some gel for nighttime.’
No serious complications were found but an explanation for a subconjunctival haemorrhage was given, and she was told that this would resolve in 7-10 days and reassured her not to worry. Patient A informed the doctor she felt the staff were busy on the day and a little ‘rough’ but had not wanted to complain at the time. Patient A found she was unable to use the new eye drops due to her osteoarthritis, so the care home took responsibility for administering the drops.
Unfortunately, the care home staff were busy due to holidays and sickness, so Patient A did not receive her drops the prescribed 4x per day and her nighttime gel was administered sometimes as early as 6.30pm, when the appropriately trained member of staff went home, which left Patient A with blurred vision and unable to see her TV, so she would just go to bed. The DED symptoms got worse, and she became more depressed. After two weeks the subconjunctival haemorrhage resolved but Patient A was becoming very withdrawn.
FIGURE 4: Patient A’s subconjunctival haemorrhage
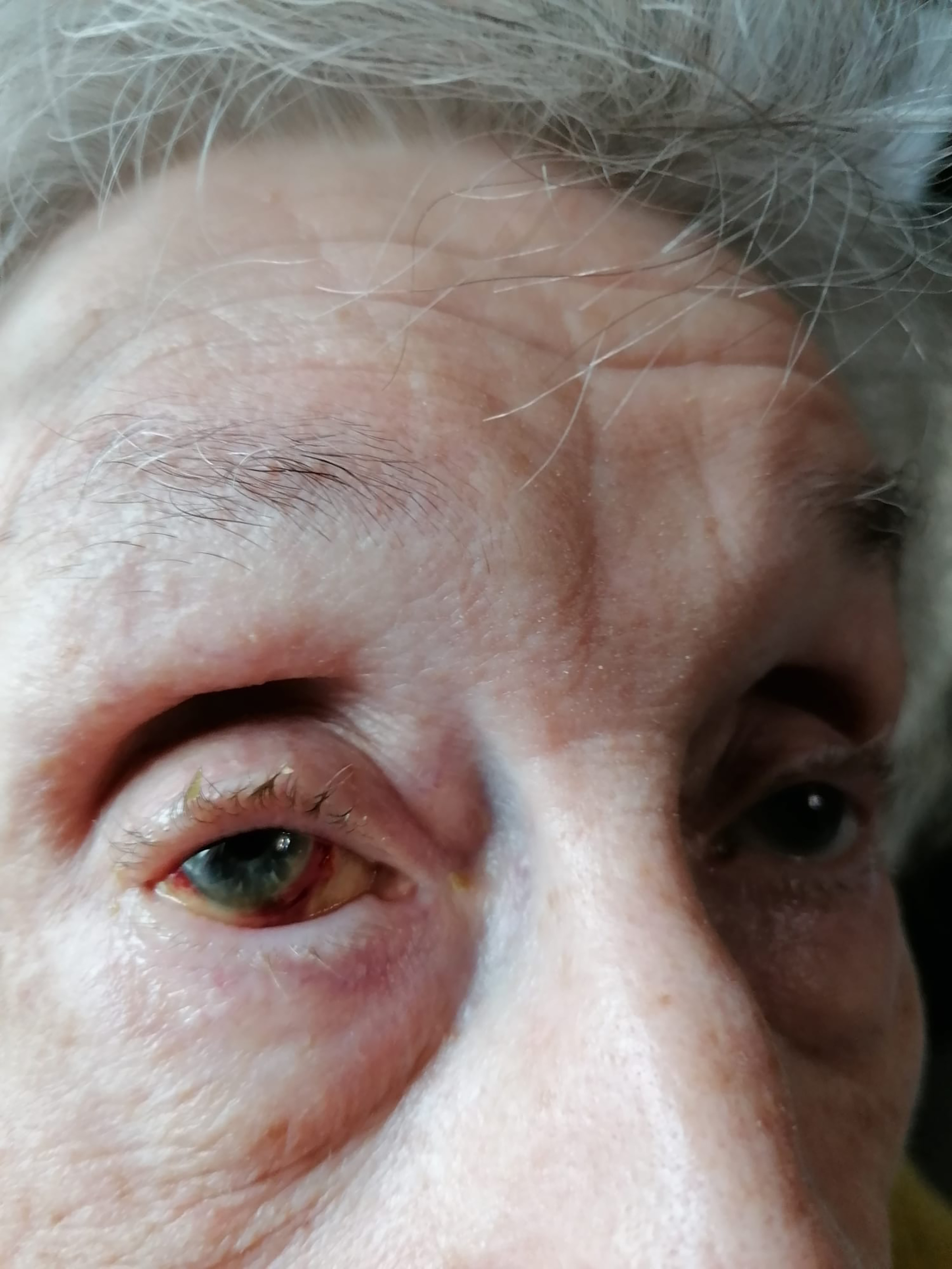
Patient A’s former optician had prescribed Patient A preservative-free eye drop with hyaluronic acid, which had proved successful, particular care had been given to the osteoarthritis in her hands ensuring she could administer the eye drops herself as she lived alone. After many demonstrations and extensive education, Patient A had a clear understanding of how and when to use the drops (at least 4x per day) and all instructions were given both verbally and written.
Nighttime gel had been tried but Patient A found administering from a tube too difficult with her arthritic disability and did not like the blurred vision as she felt vulnerable living alone, so the daytime drops were also used at night, which meant her DED was managed. There had also been a referral to the GP to review medication and consider referral for Sjögren’s syndrome.
Key points
Patient education; be aware of patient circumstances when eye drops are prescribed, especially for patients living in care homes and can the patient administer the drops? Sometimes, just time and patience can make such a huge difference to patients’ lives.
TFOS DEWS II Management and Therapy Report26 advocates a staged management approach according to severity of the disease and will be covered in detail in a following article. Although, within Step 1 of these recommendations is ‘education’; education of the condition, management, treatment and prognosis and, in the author’s opinion, this is the foundation on which all patient discussions should be based.
DED is a chronic condition requiring ongoing management and patients should be educated accordingly using both verbal communication and written literature in the form of leaflets explaining their condition and visual guides on management covering instillation drops, MGD management and lid management. As seen from the case study of Patient A, the individual patient circumstances should be fully considered as dexterity for older patients can be a major concern. Demonstration and adaption of management techniques is essential as if it is beyond the physical ability of the patient to instil a particular eye drop the patient will be left feeling demoralised with a worsening condition.
Many patients are compliant, but as soon as their symptoms improve, they stop treatment. At each appointment a discussion should be undertaken with motivation and encouragement a central part also, meticulous clear, legible and contemporaneous patient records should be kept so that any member of the clinical team that follows has a detailed account of what has been discussed, advised and prescribed. The whole practice team requires training on DED, with front of house staff aware of the requirement for patients to have a full eye examination or dry eye assessment to rule out conditions that can mimic dry eye symptoms and require referral. We are an evidence-based profession keeping up to date with new research is essential as this enables us to provide the best care for our patients.
Finally, artificial tears (ATs), which patients can purchase as an over-the-counter product, are the most used treatment option for DED. They aim to support the tear film by increasing the volume of tears and improve lubrication. A point that should be clearly explained to patients using ATs is that most research recommends a minimum use of 4x per day, and use over a period of a month to decide if a particular eye drop proves successful but signs of DED can take longer to show improvement, often up to four months.27
In the author’s experience, most practitioners select ATs on a basis of ‘this one usually works for me’. When asked what was different about that particular formulation, the reply has been ‘I don’t know, it just works.’ As written earlier we are an evidence-based profession and as such a more systematic approach should always be adopted.
A comprehensive review by Laboulle et al28 explains the types and roles of ingredients used in ATs outlining the category of tear substitute against the targeted dry eye signs. Unfortunately, selecting the most effective dry eye drop can be a challenge as there is no evidence comparing different ATs to support which are the most effective. The complex nature of DED requires consideration of each patient’s unique circumstances, environment, work, medical history including any underline systemic disease, diet, eyelid anatomy and finally quantity and quality of tears.
With dry eye disease affecting around half the population many of whom get little or no relief, and with a host of effective solutions available to alleviate symptoms and treat causes, DED represents a great opportunity to help patients and improve professional practice.
- Tina Arbon Black, BSc (Hons) FBDO CL, is an experienced CPD author and presenter and is one of the clinical editors for Optician Magazine as well as a director of OrbitaBlack Ltd, an approved CPD provider that works with customers to produce CPD programmes. She is an ABDO College tutor, theory script marker and former ABDO practical examiner and frequently authors and delivers CPD for ABDO.
References
- M vidal-Rohr, JP Craig, LN Davies and JS Wolffsohn, “The epidemiology of dry eye disease in the UK: The Aston dry eye study,” Contact Lens and Anterior Eye, vol. 46, no. 3, p. 101837, June 2023.
- RK Casemore, JS Wolffsohn and D Dutta, “Dry eye clinical practice patterns of UK optometrists,” Contact Lens and Anterior Eye, vol. 46, no. 5, p. 101889, October 2023.
- JP Craig, KK Nichols, EK Akpek, BC Caffery and et al, “TFOS DEWS II Definition and Classification Report,” The Ocular surface, vol. 15, no. 3, pp. 276-283, July 2017.
- KF Farrand, M Fridman, IO Stillman and DA Schaumberg, “Prevalence of Diagnosed Dry Eye Disease in the United States Among Adults Aged 18 Years and Older,” American journal of Ophthalmology, vol. 182, pp. 90-98, October 2017.
- AJ Bron, CS De Paiva, SK Chauhan, SB Bonini and et al, “TFOS DEWS II Pathophysiology Report,” The Ocular Surface, vol. 15, no. 3, pp. 438-510, 2017.
- N Conrad, S Misra, J Verbakel, G Verbeke and et al, “Incidence, prevalence and co-occurence of auto immune disorders over time and by age, sex, and socioeconomic status: a population -based cohort study of 22 million indiviual in the UK,” The Lancet, vol. 401, no. 10391, pp. 1878-1890, 2023.
- MA Lemp, L Crews , L Bron and G Foulks, “Distribution of aqueous-deficient and evaporative dry eye in a clinic based patient cohort,” Cornea, vol. 31, no. 5, pp. 472-478, 2012.
- M Sanchez-Brau, M Sequi-Crespo, N Canto-Sancho, A Tauste and et al, “What are the dry eye questionnaires available in the scientific literature used for? A scoping review,” American Journal of Ophthalmology, vol. 246, pp. 174-191, February 2023.
- JS Wolffsohn, R Arita, R Chalmers, A Djalilan and et al, “TFOS DEWS II Diagnostic Methodology Report,” The Ocular Surface, vol. 15, no. 3, pp. 539-574, 2017.
- JR paugh, J Tse, T Nguyen, A Sasai and et al, “Efficacy of the Fluorescein Tear Breakup Time (TBUT) Test in Dry Eye,” Cornea, vol. 39, no. 1, pp. 92-98, Janaury 2020.
- U. Stahl, M Willcox and F Stapleton, “Osmolarity and tear film dynamics,” Clinical and Experimental Optometry, vol. 95, no. 1, pp. 3-11, October 2011.
- “The Definition and Classification of Dry Eye Disease: Report of the Definition and Classification Subcommittee of the International Dry Eye Workshop (2007),” The Ocular surface, vol. 5, no. 2, pp. 75-92, January 2007.
- F Amparo, Y Jin, P Hamrah, D Schaumberg and R Dana, “What is the value of incorporating tear osmolarity measurement in assessing patient response to therapy in dry eye disease?,” Am J Ophthalmol, vol. 157, no. 1, pp. 69-77, January 2014.
- JV Greiner, G Shuang, M Pistilli, M Maguire and et al, “Association of Tear Osmolarity with signs and symptoms of dry eye disease in the dry eye assessment and management (DREAM) study,” Investigative Ophthalmology & Visual Science, vol. 64, no. 5, January 2023.
- FJ Holly, “Physical chemistry of the Normal disordered tear film,” Transactions of the Ophthalmological Societies of the United Kingdom, vol. 104, no. Part 4, pp. 374-380, 1985.
- B Niedernolte, JS Wolffsohn, H Pult and S Bandlitz, “Evaluation of tear meniscus height using different clinical methods,” Clinical and experimental Optometry, vol. 104, no. 5, pp. 583-588, 2020.
- J Nemeth, e Fodor, Z Lang, K Kosina-Hagyo and et al, “Lid Parallel conjunctival Folds (LIPCOF) and dry eye: a multicentre study,” British journal of Ophthalmology, vol. 96, no. 11, 2012.
- KR Vandermeid, SP Su, KL Krenzer , KW Ward and et al, “A method to extract cytokines and matrix metalloproteinases from Schirmer strips and analyze using Luminex,” Molecular Vision, vol. 17, pp. 1056-1063, April 2011.
- ME Stern, CS Schaumburg and SC Pflugfelder, “Dry eye as a Mucosal Autoimmune Disease,” International Reviews of Immunology, vol. 32, no. 1, pp. 19-41, 2013.
- S Hassanzadeh, M Varmaghani, S Zarei-Ghanavati, JH Shandiz and et al, “Global Prevalence of Meibomian Gland Dysfunction: A systematic Review and Meta-Analysis,” Ocu;ar Immunology and Inflammation, vol. 29, no. 1, pp. 66-75, 2021.
- R Khannna, S Catanese, EP Pharm, P Corcia and et al, “Metabolics and lipidomics approaches in human tears: A systematic Review,” Survey of Ophthalmology, vol. 67, no. 4, pp. 1229-1243, 2022.
- J Sheppard and K Nichols, “Dry Eye Disease Associated with Meibomian Gland dysfunction: focus on Tear Film Characteristics and the Therapeutic Landscape,” Ophthalmology and therapy, vol. 12, pp. 1397-1418, Marvh 2023.
- E Knop , N Knop, T Millar, H Obata and D Sullivan, “The International workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland,” Invest Ophthalmol Vis Sci, vol. 52, pp. 2938-1978, 2011.
- H Obata, “Anatomy and histopathy of human meibomian gland,” Cornea, vol. 21, pp. 70-74, 2002.
- C Talens-Estalles, J Garcia-Margues, A Cervino and S. Garcia-Lazaro, “Use of digital displays and ocular surface alterations: A review,” The cular surface, vol. 19, pp. 252-265, 2021.
- L Jones, LE Downie, D Korb, JM Bernitez-del-Castillo and et al, “TFOS DEWS II Management and therapy Report,” The Ocular Surface, vol. 15, no. 3, pp. 575-628, 2017.
- P Asbell, AJ Vingrys, J Tan, A Ogundele and et al, “Clinical Outcomes of Fixed Versus As-needed Use of Artifical Tears in Dry Eye Disease: a 6-week observer-Masked Phase 4 Clinical Trial,” Clinical and Epidemiological Research, vol. 59, pp. 2275-2280, 2018.
- M labetoulle, JM Benitez-del-Castillo, S Barabino, RH Vanrell and et al, “Artificial Tears: Biological role of Their Ingredients in the Management of Dry Eye Disease,” International Journal of Molecular Sciences, vol. 23, no. 5, p. 2434, 2022.
- JC Pinto-Bonilla, A Del Olmo-Jimeno, F Llovet-Osuna and E Hernandez-Gallilea, “A randomised crossover study comparing trehalose / hyaluronate eye drops ans standard treatment: patient satisfaction in the treatment of dry eye synsdrome,” therapeutics and Clinical Risk Management, vol. 11, pp. 595-603, 2015.
- DP Wilcox, P Argueso, GA Georgiev, JM Holopainen and et al, “TFOS DEWS II Tear Film Report,” The Ocular surface, vol. 15, no. 3, pp. 366-403, 2017.
