
Over the past two decades there has been a growing interest in predictive genetic testing and its clinical utility for healthcare practitioners. Unlike standard methods of examining patients for signs of disease being present or absent, such as blood counts or imaging methods (e.g., x-ray, CT scans, OCT or even fundus photography), genetic testing is becoming more popular for the early identification of patients who may be at risk of developing a particular disease prior to any signs. Simply put, predictive genetic testing or the identification of genetic risk factors, suggests to clinicians whether a patient is likely to suffer from a particular condition in the future, in the absence of any current signs and symptoms.
One such example of this type of genetic testing has been used extensively to test patients for their susceptibility to develop breast cancer, allowing for prophylactic treatment of the disease. In the example of breast cancer, two mutations in the BRCA gene (referred to as BRCA1 and BCRA2) have been shown to be strongly associated with the increased risk of developing breast cancer if present. Usually, the general population may have a risk of one in eight chance, but this rises to 50% risk or higher with one of these mutations.
Alongside this growing interest of predicting disease, the general idea of genetic prediction of traits has begun to gain interest. Recent advances in genetic testing techniques have made it much more accessible and affordable to all. As a commercial enterprise, companies such as 23andMe have been able to provide insights into the genetic background of their customers, and researchers and clinicians have been working alongside this to focus on the possible healthcare applications. There have been a growing number of research papers investigating the links between a patient’s genetic makeup, and their predisposition to diseases or traits. This has also included the development of similar genetic prediction models for refractive error and ocular disease, with an example of the increased interest in genetic prediction shown in Figure 1.
Figure 1: Number of peer reviewed articles about predicting refractive error via genetics by year of publication in the PubMed library.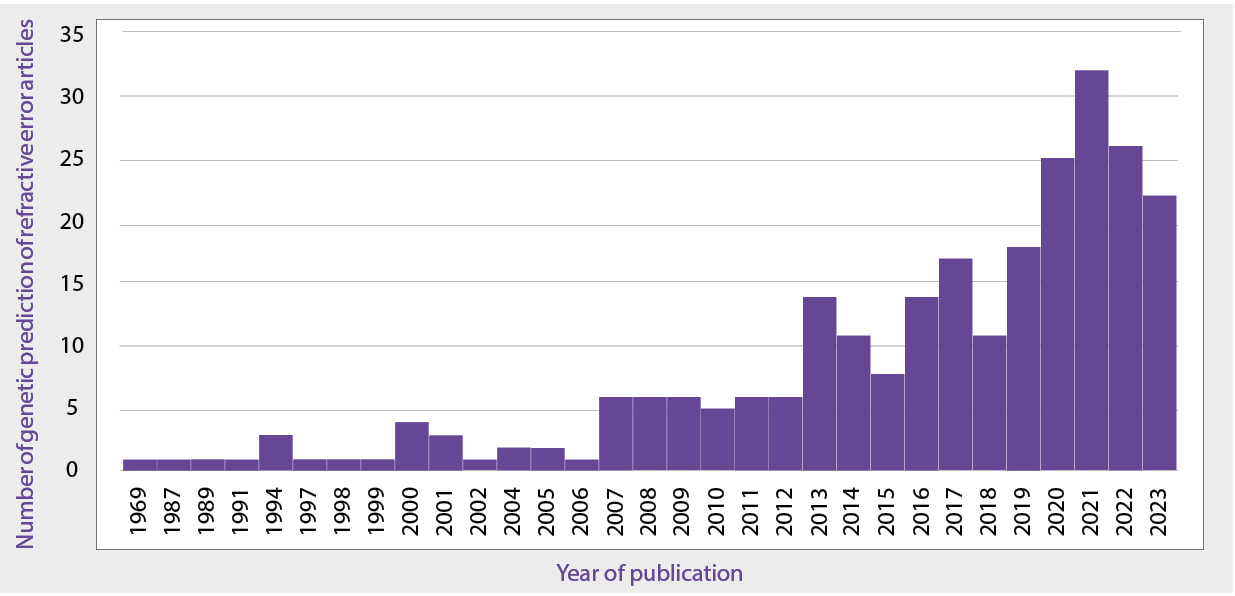
Recent scientific research has found that many diseases previously believed to be caused by environmental factors alone, such as diabetes and obesity, which are primarily associated with diet and lifestyle choices, also have a genetic component, making patients much more susceptible to both the onset and the severity of their condition13. Some examples of relevant ocular diseases include age related macular degeneration (AMD), glaucoma, and visual-developmental conditions such as amblyopia. Given that refractive errors, such as myopia, also come about from differences in ocular growth and development, it is no surprise therefore, that myopia (and more broadly, astigmatism and hyperopia too), have been found to have genetic components.
This can be partially why we see that myopia runs in families, and why parental myopia is also used in risk profile indicators like the PreMO formula from Ulster University1.With the recent advancements in genetic testing, it has enabled researchers to successfully identify many common genes associated with myopia. Experts have suggested that this new genetic evidence could be used to predict myopia potentially at birth before it’s onset, and so has the potential to affect clinical practice in the future11.
The basics
Human cells are made up of 23 pairs of chromosomes that are inherited from parental cells, 23 chromosomes from the mother and 23 from the father. Chromosomes are threadlike structures composed of proteins and nucleic acids, in the form of DNA (Deoxyribonucleic Acid) molecules. These structures contain millions of base pairs and thousands of genes and are found within or in the nucleus of all human cells. The fact that we get 23 pairs from each of our parents, means that we have two full copies of our genes. This means that humans are diploid i.e., two chromosome pairs, whereas some plants and animals have a different number or pairs, one such example, is wheat that has six pairs of chromosomes.
Figure 2: Image depicting the nucleus of a human cell. Each chromosome, as shown, is made up of long stands of helical DNA.
DNA is a double helical molecule that carries genetic information and it is made up of small building blocks called nucleotides. Nucleotides are made up of a sugar molecule and a ‘base’ molecule in the form of adenine (A), cytosine (C), guanine (G), thymine (T) or uracil (U).These base molecules can give rise to variation within individuals, as there may be one of two (or more) versions of a DNA sequence. This could be the difference of a single base molecule, or a series of bases at a particular location within the human genome. Each of these different versions at a particular genomic location, is known as an allele.
Alleles are a specific form of a variant that is responsible for common genetic variation and can consist of several different ‘variants’ within a population of people, leading to small genetic differences, such as blue, green, or brown irides. The visible expression of these genes, as in the example of iris colour, is known as the ‘phenotype’ or ‘trait’. The less commonly known term ‘genotype’ refers to the entire collection of genes in an individual.
Occasionally, changes in the DNA sequence can be found called mutations, which can cause an organism to evolve and improve, or which can have detrimental effects to the individual. One such detrimental genetic mutation occurs in people that suffer from retinitis pigmentosa; this is contrary to the mutation that has been observed in populations in sub-Saharan Africa where individuals have high rates of mutated haemoglobin. This mutation, which leads to haemoglobin C, makes affected individuals much less susceptible to malaria, which is more common in sub-Saharan Africa9.
Not all differences within the human genome cause large mutations, there can also be small differences between individuals called ‘polymorphisms’, which are changes to the DNA sequence that may change part of the end phenotype or trait in a small way. The most commonly investigated type of polymorphism is the change of a single nucleotide polymorphism (SNP). As humans have two copies of the same chromosomes, they may have different versions of the same gene through differences in their SNPs. This can lead to individual variations, including differences between each parent and between children and siblings.
Most diseases or traits are not found to be caused by a single gene (i.e. monogenic) but are more likely to be caused by a combination of changes within the genes, with each variant making a small difference in the overall trait. These small changes have an additive effect, and together they influence the outcome, in what is called a complex or polygenic trait.
A person’s height is an example of this, and several hundreds of genes have been linked to this physical characteristic. Meaning, in this case all SNPs involved individually have a small effect on the resultant height of the individual. This is why siblings’ heights can vary, depending on the number of different variants in genes they’ve inherited from each parent. In a similar fashion, axial length and refractive error that also tends to run in families, has a complex inheritance, with many genes making small differences in the likely end result of refractive error12.
Current genetic research in refractive error
Refractive error, when looking at non-syndromic or pathological types usually results from the complex interaction between genetic and lifestyle factors. This means, that to different extents, the resultant refraction that a person has, along with their individual lifestyle differences can be linked to the predisposing genes they may have and is not necessarily because of genetics alone.
A regularly used method in which complex traits, such as refractive error are analysed genetically, is through what is known as ‘Genome Wide Association Studies’ (GWAS)12. This is a type of analysis that examines millions of locations within the genome at the same time, to determine if specific alleles associated with a trait or condition are present, by comparing variants that occur most often in individuals with that particular trait (for example high myopia) against people who don’t have the trait (i.e., those who do not have myopia).
Figure 3: Example chromosomes, Chromosome 1 with base T, while chromosome 2 has base G at the same location, these are examples of two alleles.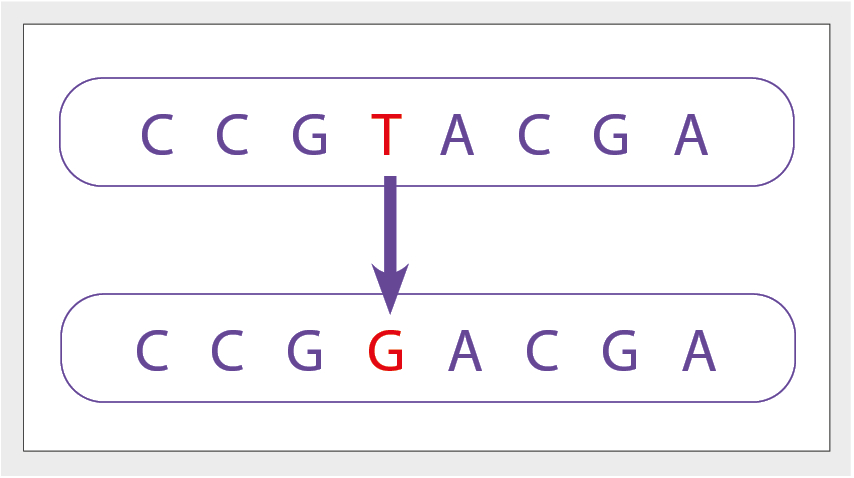
The variants that are associated with the condition are then assumed to be additive; in other words, all the small effects each gene has on the refractive error can be added together and combined into a total score, and this can give a total genetic risk outcome. Performing this for a single individual is a way of calculating a genetic risk score for that person. These polygenic (i.e., multiple gene/variant) risk scores are calculated through the statistical analysis of the total number of genetic variants that individual has, and the average effect of each of those genetic variants.
This gives an estimate to researchers as to whether or not the individual in question is likely to inherit a certain condition, or what their phenotype is likely to be e.g., refractive error in diopters, providing an estimated outcome. This, in the future, may be used to give eye care practitioners an idea of the likelihood that their patient may or may not go on to develop significant refractive error. Despite the suggestion that myopia prevalence is largely related to changes in human lifestyle and environmental influences, studies have found over 500 locations within the human genome that are associated in some way with refractive error to a level much greater than expected by chance (i.e., a genome wide significance, meaning a statistical P value of 5x10-8 or smaller)12.
Therefore, although environmental changes, which we still don’t fully understand7, may be the direct factors behind the recent increase in myopia, the relative influence of genetic factors for each person, and the presence of gene-environment interactions means that genetics will likely have had some contribution to the end result of most refractive errors of the majority of the population. Nevertheless, despite the 500+ locations in the genome associated with myopia, there are likely even more areas of interest within the human genome that are linked to refractive error and myopia development, but we currently haven’t been able to detect them as they may be relatively rarer within the population, or may be locations of reduced genetic variation that have yet to be investigated.
How can we use this information?
In the case of myopia, which is more likely to develop in late childhood and teenage years, it would benefit practitioners to know which patients should be more closely monitored or have more frequent sight tests. Or even hypothetically, be prophylactically managed at the pre-myopia stage to prevent high myopia developing. One such example of this type of prophylactic treatment is that of pre-myopic patients in Asia who have been treated with low dose atropine or with repeated low-level red-light therapy5. The theory being that by delaying the onset, or preventing high myopia developing in the first instance, we will also be reducing the risk of myopia associated disorders, such as myopic macular degeneration, and retinal detachment.
In the case of hyperopia, we could potentially use genetic data collected at birth to determine which children are at a higher risk of developing high hyperopic prescriptions or may fail to emmetropise in childhood. Research has shown that the higher the level of hyperopia, the higher the risk of amblyopia and strabismus8. So, if eye care professionals can find out about the chance of a child’s increased risk in advance, they could possibly initiate closer and more frequent sight tests, to provide any necessary treatment as soon as needed within the critical period of ocular development. This could potentially prevent the loss of stereoacuity and the reduction of visual acuity in one or both eyes.
Currently, the use of genetic testing to help with day-to-day clinical decision making in optometry is still hypothetical and only currently used in research settings. This is because the accuracy of genetic risk scores for predicting the specific refraction of an individual has been found to be of restricted clinical benefit and no more accurate than the current gold standard practice of performing a cycloplegic refraction on a young child. The reason is because of refractive error being a complex trait, meaning that although we can create and use genetic risk scores to identify an individuals’ relative risk of developing myopia, it is hard to determine, with sufficient accuracy, the exact risk with genetics alone, as we do not capture the impact of their day-to-day lifestyle.
Clinicians are often asked about how much of an increased risk having one or both myopic parents can have on a child, but this approach can be limited for several reasons. Firstly, there may be a lack of knowledge (some parents are unsure of their own or the other parent’s refractive status), or the self-reported nature may provide erroneous answers due to reporting on current vision difficulties despite prior relevant history such as laser eye surgery or cataract surgery.
Secondly, counting the number of myopic parents can be a rather blunt measure i.e. two parents with high myopia (more than -6D) may score the same risk as two myopic parents with less than -1D of myopia, which can reduce some of the sensitivity of measure. And additionally, there are conflicting relative risk estimates by experts on the amount of risk posed by having one or both myopic parents2, depending on the age of the child, ethnicity, and the study assessment. For example, a study from Australia found that having one or two myopic parents provided the same relative risk of myopia14, but other studies have found an increased risk with two parents compared to one, and the exact increases varies depending on the study.
For these reasons, it has been suggested that genetic testing carried out at birth, can provide clinicians and parents with a better idea of their child’s general risk and stratify them more sensitively regarding the chances of them developing myopia, and the general likelihood of developing other refractive errors, which could enable treatment long before other clinical signs.
One of the greatest limitations of current large genetic studies that have been performed in the UK, Europe, and the US, is the lack of diversity in the ethnicities of the populations studied. Most of these studies have only been performed in those with European or Caucasian ancestry, meaning that there is less applicable data available for prospective patients from other ethnic backgrounds, and the data cannot be as accurate for prediction in a diverse population3. Nevertheless, the number of these studies has started to increase, and the use of genetic risk scores has been applied to other ethnicities and shown some viability in application3.
Future of myopia genetics
The future of research in myopia-genetics is likely to focus more on looking at how to apply the knowledge we have for genetics, for new applications, and gain a more detailed understanding of the influences that are causing myopic development. Currently, as most large scale genetic studies have only looked at caucasian populations, there has been a movement towards multi-ethnic GWAS studies and reports.
This has led to an increase in the reporting of the shared genetic influences of myopia development and axial elongation5. Other studies are aiming to utilise genetics to understand associations to dietary differences and myopia6, as well as aiming to explore the interactions between genes and the environment, one such example of this, is how formal education delivery is having different levels of effect and influence on some children more than others10.
Worldwide, it has been estimated that myopia will affect up to 50% of the population by 20504 and as is already evident, myopic prevalence has increased significantly, particularly in children living within the UK1. Understanding the genetic influences of myopia, identifying those that may be at a greater predisposition of developing high myopia, and learning about how those genetic factors influence the development of myopia, is important to help us tackle the ‘myopia boom’ we’re experiencing.
References
- Fulton, J. M., T. W. Leung, S. McCullough, L. Doyle, N. S. Logan, C. S. Lam & K. Saunders (2023) The PreMO (Predicting Myopia Onset and progression) risk indicator reliably predicts future myopia in East Asian and UK children. Investigative Ophthalmology & Visual Science, 64, 4957-4957.
- Ghorbani Mojarrad, N., C. Williams & J. A. Guggenheim (2018) A genetic risk score and number of myopic parents independently predict myopia. Ophthalmic Physiol Opt, 38, 492-502.
- Ghorbani Mojarrad, N., D. Plotnikov, C. Williams & J. Guggenheim (2022) Genetic prediction of myopia in different ethnic ancestries.
- Holden, B. A., T. R. Fricke, D. A. Wilson, M. Jong, K. S. Naidoo, P. Sankaridurg, T. Y. Wong, T. J. Naduvilath & S. Resnikoff (2016) Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology, 123, 1036-1042.
- Jiang, Y., Z. Zhu, X. Tan, X. Kong, H. Zhong, J. Zhang, R. Xiong, Y. Yuan, J. Zeng & I. G. Morgan (2022) Effect of repeated low-level red-light therapy for myopia control in children: a multicenter randomized controlled trial. Ophthalmology, 129, 509-519.
- Li, F.-F., M.-C. Zhu, Y.-L. Shao, F. Lu, Q.-Y. Yi & X.-F. Huang (2023) Causal Relationships Between Glycemic Traits and Myopia. Investigative Ophthalmology & Visual Science, 64, 7-7.
- Morgan, I. G., P.-C. Wu, L. A. Ostrin, J. W. L. Tideman, J. C. Yam, W. Lan, R. C. Baraas, X. He, P. Sankaridurg & S.-M. Saw (2021) IMI risk factors for myopia. Investigative ophthalmology & visual science, 62, 3-3.
- Pascual, M., J. Huang, M. G. Maguire, M. T. Kulp, G. E. Quinn, E. Ciner, L. A. Cyert, D. Orel-Bixler, B. Moore & G.-s. Ying (2014) Risk factors for amblyopia in the vision in preschoolers study. Ophthalmology, 121, 622-629. e1.
- Piel, F. B., R. E. Howes, A. P. Patil, O. A. Nyangiri, P. W. Gething, S. Bhatt, T. N. Williams, D. J. Weatherall & S. I. Hay (2013) The distribution of haemoglobin C and its prevalence in newborns in Africa. Scientific reports, 3, 1671.
- Plotnikov, D., C. Williams, D. Atan, N. M. Davies, N. G. Mojarrad & J. A. Guggenheim (2020) Effect of education on myopia: evidence from the United Kingdom ROSLA 1972 reform. Investigative ophthalmology & visual science, 61, 7-7.
- Tedja, M. S., A. E. Haarman, M. A. Meester-Smoor, J. Kaprio, D. A. Mackey, J. A. Guggenheim, C. J. Hammond, V. J. Verhoeven, C. C. Klaver & C. Consortium (2019) IMI–myopia genetics report. Investigative ophthalmology & visual science, 60, M89-M105.
- Tedja, M. S., R. Wojciechowski, P. G. Hysi, N. Eriksson, N. A. Furlotte, V. J. M. Verhoeven, A. I. Iglesias, M. A. Meester-Smoor, S. W. Tompson, Q. Fan, A. P. Khawaja, C. Y. Cheng, R. Höhn, K. Yamashiro, A. Wenocur, C. Grazal, T. Haller, A. Metspalu, J. Wedenoja, J. B. Jonas, Y. X. Wang, J. Xie, P. Mitchell, P. J. Foster, B. E. K. Klein, R. Klein, A. D. Paterson, S. M. Hosseini, R. L. Shah, C. Williams, Y. Y. Teo, Y. C. Tham, P. Gupta, W. Zhao, Y. Shi, W. Y. Saw, E. S. Tai, X. L. Sim, J. E. Huffman, O. Polašek, C. Hayward, G. Bencic, I. Rudan, J. F. Wilson, P. K. Joshi, A. Tsujikawa, F. Matsuda, K. N. Whisenhunt, T. Zeller, P. J. van der Spek, R. Haak, H. Meijers-Heijboer, E. M. van Leeuwen, S. K. Iyengar, J. H. Lass, A. Hofman, F. Rivadeneira, A. G. Uitterlinden, J. R. Vingerling, T. Lehtimäki, O. T. Raitakari, G. Biino, M. P. Concas, T. H. Schwantes-An, R. P. Igo, Jr., G. Cuellar-Partida, N. G. Martin, J. E. Craig, P. Gharahkhani, K. M. Williams, A. Nag, J. S. Rahi, P. M. Cumberland, C. Delcourt, C. Bellenguez, J. S. Ried, A. A. Bergen, T. Meitinger, C. Gieger, T. Y. Wong, A. W. Hewitt, D. A. Mackey, C. L. Simpson, N. Pfeiffer, O. Pärssinen, P. N. Baird, V. Vitart, N. Amin, C. M. van Duijn, J. E. Bailey-Wilson, T. L. Young, S. M. Saw, D. Stambolian, S. MacGregor, J. A. Guggenheim, J. Y. Tung, C. J. Hammond & C. C. W. Klaver (2018) Genome-wide association meta-analysis highlights light-induced signaling as a driver for refractive error. Nat Genet, 50, 834-848.
- Vassy, J. L. & J. B. Meigs (2012) Is genetic testing useful to predict type 2 diabetes? Best Practice & Research Clinical Endocrinology & Metabolism, 26, 189-201.
- French AN, Morgan IG, Mitchell P, Rose KA. Risk factors for incident myopia in Australian schoolchildren: the Sydney adolescent vascular and eye study. Ophthalmology. 2013 Oct;120(10):2100-8. doi: 10.1016/j.ophtha.2013.02.035. Epub 2013 May 11. PMID: 23672971.
