
Worldwide, health systems are being overwhelmed, which is partly due to an ageing population who may suffer from multiple health conditions. Diagnosis of these diseases is complex, and there is a growing need for advanced technologies to improve disease detection and monitoring, thereby enhancing eye care delivery.
Artificial intelligence (AI), which leverages computational power and advanced learning algorithms, offers a promising solution to this problem. A very recent AI development is the Google Brain Deep Learning (DL) algorithm, which is trained to accurately identify the attributes of diabetic retinopathy (DR) in unseen images.1
Besides DR diagnosis, AI-based algorithms also show promise in identifying other ophthalmic diseases, such as age-related macular degeneration (AMD) and glaucoma. Glaucoma is a leading cause of irreversible vision loss in developed nations, with numbers of cases projected to double in the next two decades,2,3 underscoring the importance of early detection.
Traditional diagnostic methods are often manual, expensive and time-consuming. Thus, AI-driven analysis of retinal imaging modalities could assist in faster and more cost-effective early-stage diagnosis.
This article explores state of the art AI techniques in the context of glaucoma and neurodegenerative disease diagnosis using retinal imaging, assessing modalities and features used in existing AI-based systems, and discussing future directions for integrating AI into healthcare delivery.
Clinical relevance and problems
Primary versus secondary neurodegeneration
Primary optic nerve degeneration, as seen in conditions like glaucoma, manifests as a loss of retinal ganglion cells and their axons (retinal fiber layers – RNFL). Loss of retinal ganglion cells may also occur due to other causes such as primary optic atrophies or secondary to ischaemic insult or oedema.
In addition, there is evidence that lesions in the visual pathway proximal to the lateral geniculate nucleus can lead to retrograde degeneration of retinal ganglion cells.4 Consequently, discriminating the various causes of optic neuropathy may be clinically challenging.
A study by Zangerl et al5 discusses the distinctive patterns in optical coherence tomography (OCT) images for pre and post-chiasmal lesions. The distinct differences they have found indicates that a suitably trained AI algorithm may be able to differentiate the various optic neuropathies and assist in clinical diagnosis.
Ageing population, under and overdiagnosis of glaucoma
Age is a recognised risk factor for glaucoma, and the increasing prevalence of both glaucoma and neurodegenerative conditions in the elderly implies that primary and secondary retrograde degeneration due to lesions in the visual pathways, may be difficult to differentiate.
A study by Tong et al6 showed macular ganglion cell deterioration, due to ageing, starts in the late 30s. Therefore, discriminating disease-related and ageing-related retinal ganglion cell loss remains challenging. Despite advances in diagnostic testing, glaucoma remains difficult to detect, resulting in approximately 50% of patients worldwide remaining undiagnosed.7
This underdiagnosis is attributed to factors such as patients’ lack of awareness, limited access to specialised eye care, and financial constraints. Overdiagnosis is also prevalent, with a study reporting 60% of the Caucasian population being misdiagnosed and treated for glaucoma.8
Various factors contribute to this misdiagnosis, including different levels of clinical training, lack of quality imaging instruments and the confounding effects of other diseases affecting the optic nerve in addition to glaucoma.8 There are several conditions that can mimic glaucoma, known as ‘glaucoma mimickers’.
Examples include congenital conditions, such as optic disc coloboma, optic disc pit, optic disc drusen, morning glory disc anomaly, superior segmental optic nerve hypoplasia, megalopapilla.
Additionally, high myopia, retinal conditions, such as retinitis pigmentosa, branch retinal artery occlusion, branch retinal vein occlusion and central retinal vein occlusion; neuro-ophthalmological disorders, including intracranial tumours or intracranial space-occupying lesions; inflammatory conditions, such as optic neuritis; hereditary, such as hereditary optic neuropathy, autosomal dominant optic atrophy; ischemic conditions like hypoxic ischemic encephalopathy, ischemic optic neuropathy, and toxic/nutritional optic neuropathy can mimic glaucoma.9
Apart from the neuro-ophthalmological diseases, primary neurological diseases may also mimic glaucoma.
Neurodegenerative diseases mimicking glaucoma
Neurodegenerative diseases can manifest as structural alterations reminiscent of glaucoma. For instance, similar features like other optic nerve head pathologies, RNFL loss, and ganglion cell loss may be observed in these conditions.10,11
OCT is crucial for diagnosis in such cases, but distinguishing conditions like ischaemic RNFL loss from glaucoma is challenging (figure 1).12

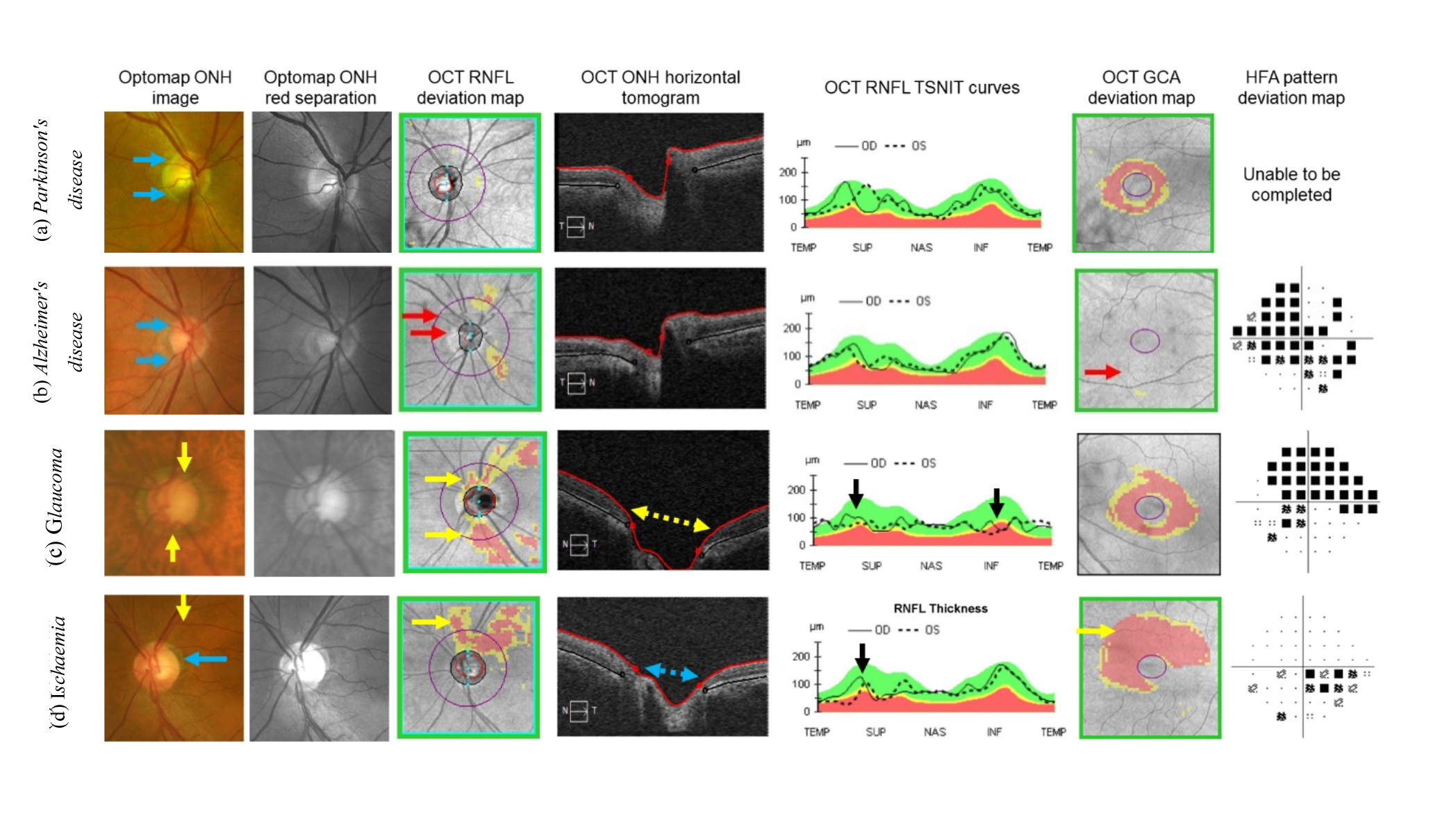
Gaps in knowledge exist, regarding the trajectory of retinal manifestations in cognitive neurodegenerative diseases, which complicates the management plans.13 Therefore, it is crucial to understand these patterns well and identify the disease-specific retinal lesions in order to accurately diagnose and differentiate between them.
- a) Parkinson’s disease
- b) Alzheimer’s disease
- c) Glaucoma
- d) Ischaemia
Prevalence, meta-analyses and the demand for AI
Recent studies utilising OCT images to diagnose glaucoma focused on assessing RNFL and ganglion cell (GC) thickness changes.12 However, there is an overlap in these changes with neurodegenerative diseases like dementia and Parkinson’s. Some key points to note:
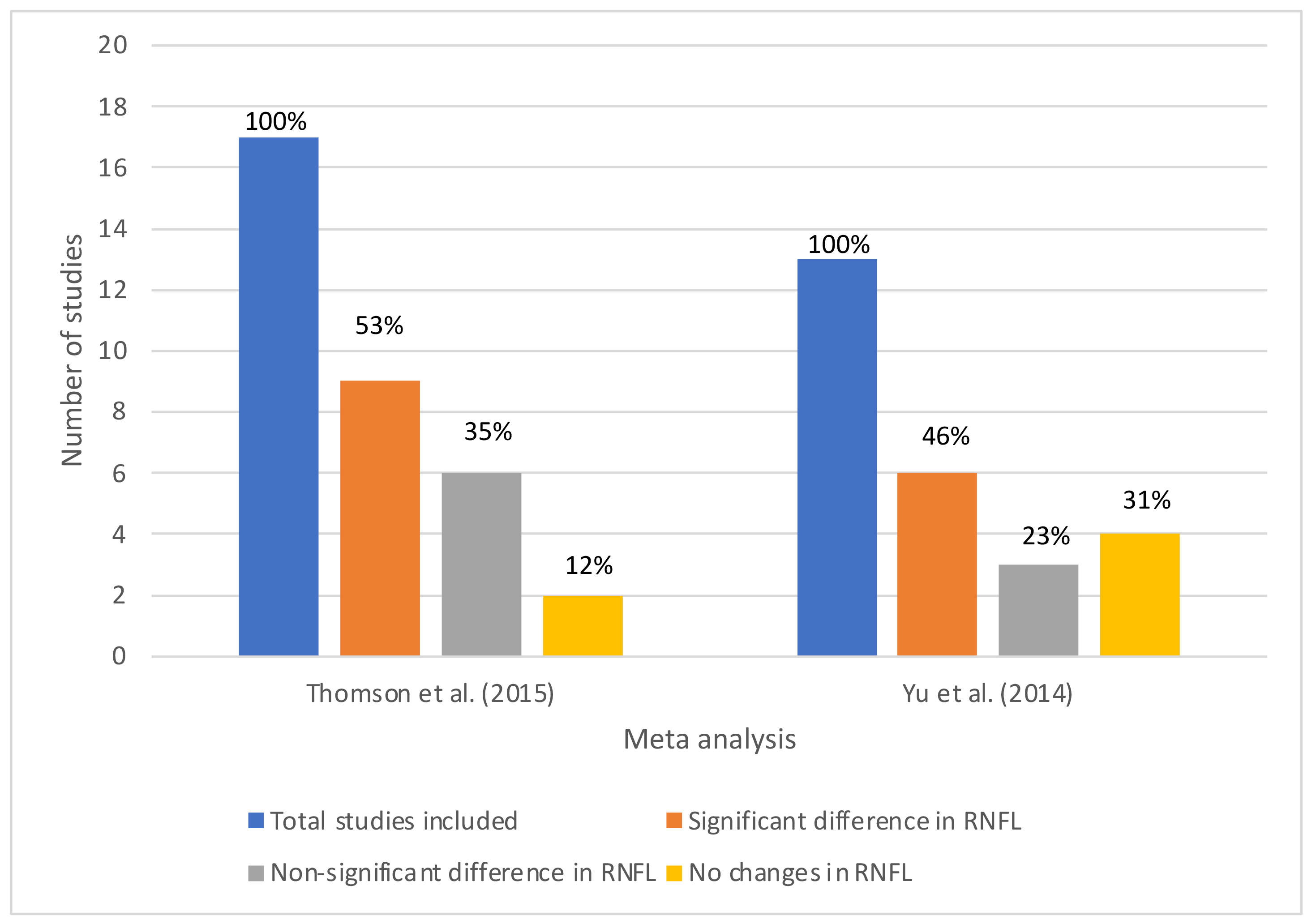
- Meta-analyses conducted on dementia14 and Parkinson’s disease15 diagnoses have revealed significant reduction in RNFL thickness due to these conditions.
- Some studies demonstrate considerable RNFL changes due to neurodegenerative diseases, while others indicate non-significant or no changes (figure 2), emphasising the need for further research.14,15
- The varied nature of findings in meta-analyses emphasises the need for more advanced analysis techniques for diagnosing neurodegeneration using retinal imaging.
- The pattern of loss in RNFL and ganglion cell complex appears to be different in glaucoma and neurodegenerative diseases,5 and adding an AI component would allow the development of algorithms that could assist clinicians to better differentiate between the conditions.
- Many studies currently rely on traditional statistical methods. There is a crucial need to integrate advanced AI techniques, including machine learning and deep learning, which have demonstrated superior performance compared to traditional statistical analysis.12
- AI applications can potentially bridge the knowledge gap for clinicians and enhance decision-making through automated computer algorithms while diagnosing neurodegenerative diseases and glaucoma.
Figure 2: Outcomes of meta-analyses detecting RNFL changes in dementia and Parkinson’s patients, (left) Thomson et al,14 (right) Yu et al15
AI in Glaucoma and Neurodegenerative
Disease Diagnosis
Retinal imaging modalities
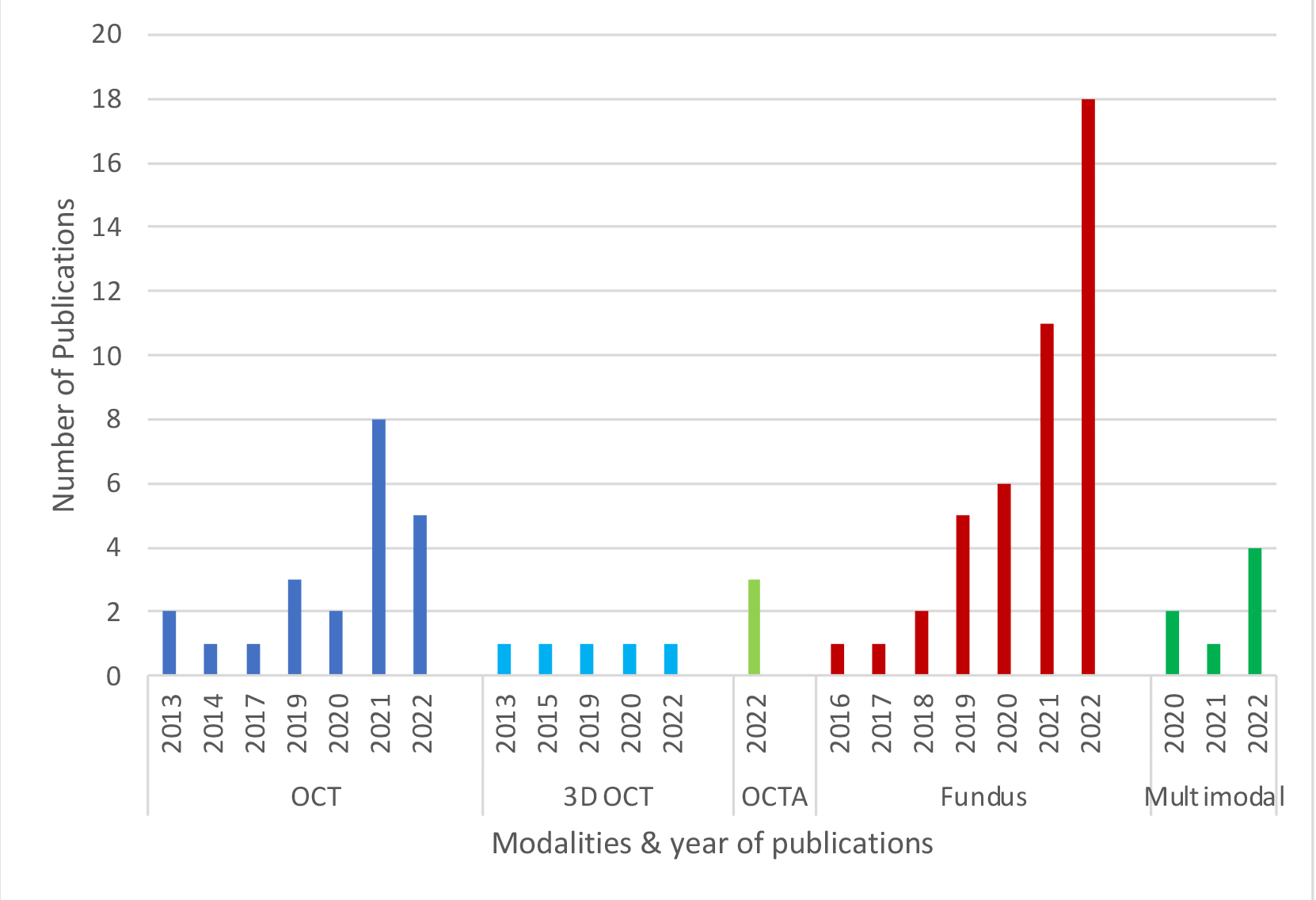
Researchers have utilised various retinal imaging modalities for diagnosing glaucoma and neurodegenerative diseases using AI. Colour fundus imaging is the most commonly employed modality, followed by OCT, and OCT angiography (OCTA).12 Some key points regarding AI applied to the variety of retinal image modalities follow:
- Recent trends from our analysis (figure 3) suggest a consistently increasing number of studies using colour fundus images, while the number of OCT-based studies appears to be variable.
- Multi-modal imaging studies have been on the rise, even though still relatively small in number.12
Figure 3: Number of studies incorporated, categorised by modality, within the past decade (with the absence of publications in the intervening years for each respective modality)
Extracted retinal features
One of the pivotal stages in machine learning is feature extraction, where specific parameters are derived from input data for the machine learning model. In the case of diagnosing glaucoma and neurodegenerative diseases, various features have been identified for different imaging modalities. Key features used for AI-based diagnosis follow:
- OCT-based studies predominantly focus on mean RNFL thickness features, computing the thickness values in four quadrants and 12 clock hours. Macular thickness and ganglion cell-inner plexiform layer (GC-IPL) thickness are less commonly studied in this context.
- Fundus-based studies, on the other hand, emphasise segmentation-based features like cup-to-disc ratio and rim area, or deep learning extracted features from image pixel values. Additionally, image-specific texture features are also extracted, which measure patterns, smoothness, brightness, and darkness in the retinal images to aid in understanding eye conditions.
Interpretation of diagnostic results
Interpretation of diagnostic results requires understanding of key metrics such as sensitivity, specificity, accuracy, and the area under the Receiver Operating Characteristic (ROC) curve (AUC). For example, sensitivity measures the ability of a test or diagnostic system to correctly identify individuals with the condition.
A high sensitivity means there are few missed cases with the disease. On the other hand, specificity measures its correctness in identifying those without the condition (ie healthy). Accuracy is the overall measure of correctness in the diagnosis of glaucoma and healthy conditions in this application.
A sensitivity of 80% for glaucoma diagnosis means the diagnostic test accurately identifies 80 out of 100 individuals with the condition. Conversely, a specificity of 90% indicates the correct identification of 90 out of 100 individuals without the condition (ie healthy).
Overall, if the accuracy is 85%, it means the diagnostic test correctly identifies individuals with or without the condition 85% of the time. AUC condenses sensitivity and specificity into a single score and an AUC value close to 1.00 signifies excellent diagnostic performance. Therefore, the higher the AUC score, the better the diagnostic system is in the diagnosis of the condition.
AI-based diagnostic performance
A review of the studies from the past 10 years reveals various factors influence the effectiveness of AI-based classifiers in diagnostics (figure 4). When comparing the diagnostic capabilities of AI techniques, it is noteworthy that deep learning-based approaches consistently exhibit superior performance compared to traditional machine learning-based diagnostic models.
Figure 4: Overall diagnostic performance of the recent AI-based studies diagnosing glaucoma and neurodegenerative diseases. Four relative comparisons are shown in sub-figures for different factors. (a) Based on type of AI approaches (Machine Learning (ML), Deep Learning (DL) and ML+DL), (b) based on type of validation sets to test the AI model’s performance (internal data, external data) [accuracy has been plotted, as AUC was not reported in the corresponding studies], (c) based on different modalities (OCT, 3D OCT, OCTA, colour fundus, multi-modal), (d) based on the type of dataset (private, public, or a combination of both)
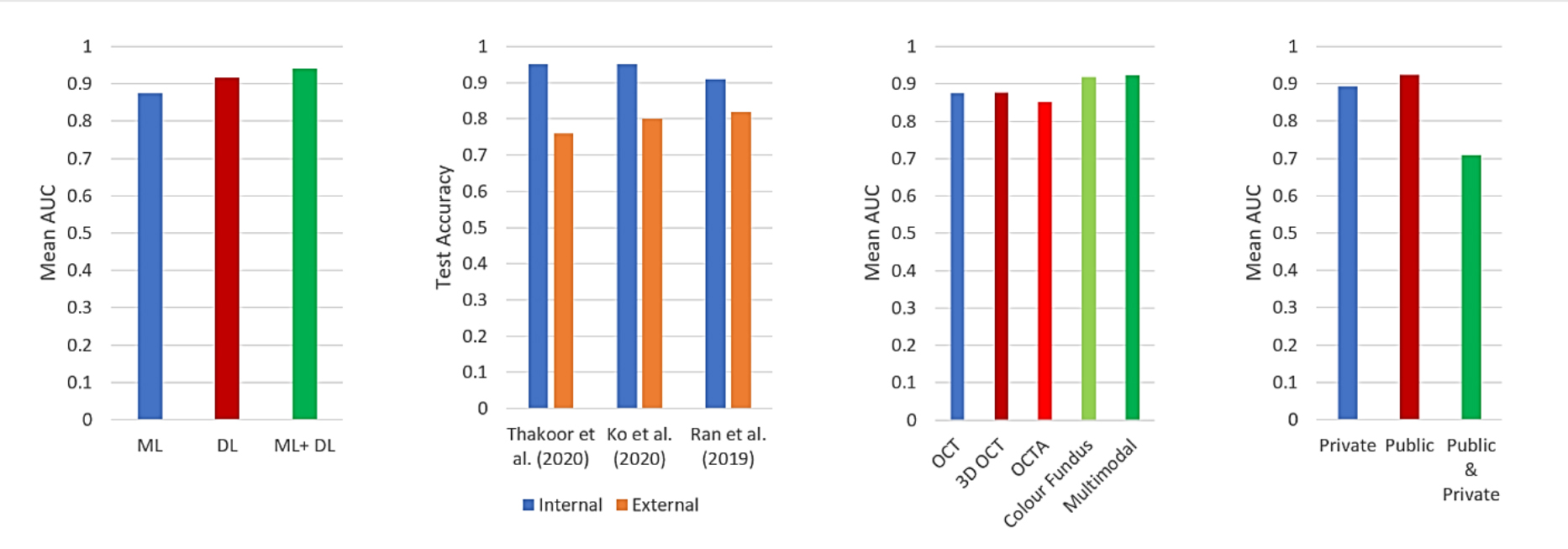 In certain scenarios, the integration of conventional learning methods, when combined with multiple deep learning models, has proven beneficial (figure 4a).16,17 Regardless of the AI approach employed, the relative performance across different modalities highlights the advantages of leveraging multimodalities (figure 4c).
In certain scenarios, the integration of conventional learning methods, when combined with multiple deep learning models, has proven beneficial (figure 4a).16,17 Regardless of the AI approach employed, the relative performance across different modalities highlights the advantages of leveraging multimodalities (figure 4c).
Feature fusion, involving extracted features from various imaging modalities like OCT and fundus photography, as well as health-related data (eg age, sex, etc) plays a pivotal role in this context.18 This emphasises the added value of integrating clinical features such as health data16 and visual field (VF) tests19-22 with imaging modalities like OCT and fundus images.
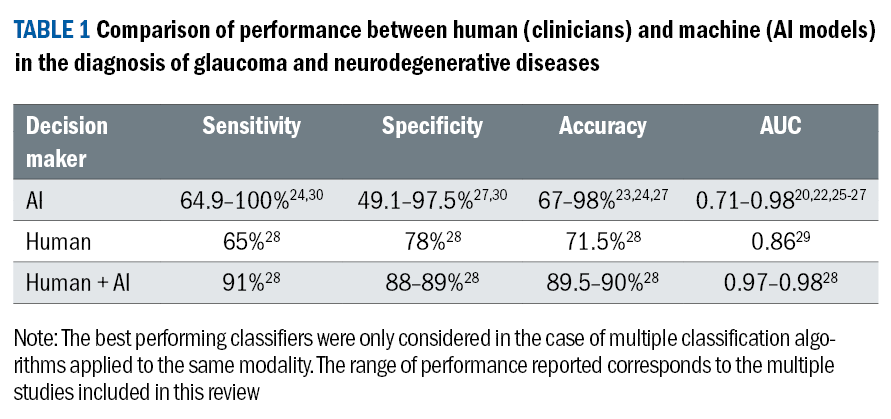
However, it is crucial to acknowledge that the potential overlap in characteristic changes between glaucoma and neurodegenerative diseases can impact overall diagnostic performance. Overall, the AI-supported diagnostic accuracy spans from 67-98%,23,24 and the AUC ranges from 0.71-0.98.20,22,25-27 This performance surpasses the average human diagnostic performance of 71.5% accuracy28 and 0.86 AUC (table 1).29
Limitations and discrepancies across studies
The use of AI in medical diagnosis, particularly in glaucoma and neurodegenerative diseases, has shown promise, but various limitations and inconsistencies exist. These include age disparities between normal and diseased groups and small sample sizes in diseased groups3,10 and challenges specific to deep learning models, such as imbalanced training sets31 and exclusion of poor-quality images.31,32
Traditional machine learning studies often lack feature selection33-35 in diagnosing glaucoma and neurodegenerative diseases. Additionally, there is variability in the use of ground-truth references, with different studies employing diverse methods for diagnosis, such as the use of glaucoma specialists36 and VF defects.26,28,37
Some studies included glaucoma suspects in the glaucoma group,37,38 and these diversities makes it challenging to generalise the performance of AI models across different studies, emphasising the need for caution in interpretation. See table 2.

Future considerations
This review of the AI-based studies on glaucoma and neurodegenerative disease diagnosis shows the need for improved model generalisability across diverse populations and settings, emphasising the importance of multi-ethnic training datasets.12 The examination indicates that only a limited number of studies have employed multiple modalities, emphasising the need for further research in this domain.
Biomedical engineers have recently devised a novel imaging technique known as fluorescent hyperspectral imaging, demonstrating potential for early glaucoma detection.39 Alongside OCT, fundus and ultra-wide field images, the integration of artificial intelligence-based techniques can enable the fusion of multimodal features, providing valuable support for clinicians in practical decision-making.
Utilising interpretable artificial intelligence is essential for deciphering ML-based results, particularly in cases of overlapping diseases. Paucity of research solutions exists in the creation of user-friendly artificial intelligence applications designed for the real-time diagnosis of glaucoma, glaucoma staging and neurodegenerative diseases.
Developing user-friendly AI apps to identify severity levels in glaucoma using AI could help address these limitations. Considering the increasing prevalence of smartphone-based artificial intelligence technology in telehealth systems and the potential inclusion of personal and sensitive information in private medical data, it is crucial to address legal and ethical concerns.
Ensuring privacy preservation41 in AI-based diagnostic models would help addressing this issue. Key future considerations for AI applied to glaucoma and neurodegenerative disease diagnosis are summarised below:
- Address biases to improve generalisability, considering ethnicity and machine-specific datasets.
- Explore new modalities and promote multi-modal analysis, including fluorescent hyperspectral imaging.
- Utilise explainable AI for interpreting overlapping diseases and understanding disease progression.
- Develop user-friendly AI apps for real-time diagnosis, emphasising mobile accessibility.
- Shift focus from binary classification to consider severity levels and glaucoma types for improved diagnosis.
- Highlight the importance of ethical considerations, especially privacy preservation, in smartphone-based AI technology and telemedicine.
Conclusion
This article reviews recently published articles on AI-based diagnosis of glaucoma and neurodegenerative diseases, highlighting the significant contribution of AI to diagnosis, which surpasses human performance. AI methods, especially machine learning and deep learning presents opportunities for more precise real-world clinical decision-making.
The article emphasises the need for future research to enhance AI models in generalisability and ‘explainability’, aiming for practical deployment in clinical applications.
- Md Mahmudul Hasan is a PhD student in the School of Computer Science and Engineering (CSE), University of New South Wales (UNSW), Sydney, Australia. He is supervised by Professor Erik Meijering, Professor Arcot Sowmya and Professor Michael Kalloniatis.
- Jack Phu is lecturer, School of Optometry and Vision Science, University of New South Wales (UNSW) and Research Fellow, School of Medicine (Optometry), Deakin University, Waurn Ponds, Victoria, Australia.
- Arcot Sowmya is Professor and Head of School of Computer Science and Engineering, University of New South Wales (UNSW).
- Erik Meijering is Professor of Biomedical Image Computing, School of Computer Science and Engineering, University of New South Wales (UNSW).
- Michael Kalloniatis is Professor, School of Medicine (Optometry), Deakin University, Waurn Ponds, Victoria, Australia, and adjunct Professor, School of Optometry and Vision Science, University of New South Wales (UNSW), Australia.
For further information, readers may be interested in reading an associated peer reviewed publication: Hasan, MM, Phu, J, Sowmya, A, Meijering, E, & Kalloniatis, M (2024).
Artificial intelligence in the diagnosis of glaucoma and neurodegenerative diseases. Clinical and Experimental Optometry, 1-17.
References
- Honavar SG. Artificial intelligence in ophthalmology-Machines think! Indian Journal of Ophthalmology 2022; 70: 1075.
- Fingert JH, Alward WL, Kwon YH et al. No association between variations in the WDR36 gene and primary open-angle glaucoma. Archives of Ophthalmology 2007; 125: 434-436.
- Tham Y-C, Li X, Wong TY et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology 2014; 121: 2081-2090.
- Marchesi N, Fahmideh F, Boschi F et al. Ocular neurodegenerative diseases: Interconnection between retina and cortical areas. Cells 2021; 10: 2394.
- Zangerl B, Whatham A, Kim J et al. Reconciling visual field defects and retinal nerve fibre layer asymmetric patterns in retrograde degeneration: an extended case series. Clinical and Experimental Optometry 2017; 100: 214-226.
- Tong J, Phu J, Khuu SK et al. Development of a spatial model of age-related change in the macular ganglion cell layer to predict function from structural changes. American Journal of Ophthalmology 2019; 208: 166-177.
- Mitchell P, Smith W, Attebo K et al. Prevalence of open-angle glaucoma in Australia: the Blue Mountains Eye Study. Ophthalmology 1996; 103: 1661-1669.
- Founti P, Coleman AL, Wilson MR et al. Overdiagnosis of open-angle glaucoma in the general population: the Thessaloniki Eye Study. Acta Ophthalmologica 2018; 96: e859-e864.
- Senthil S, Nakka M, Sachdeva V et al. Glaucoma Mimickers: a major review of causes, diagnostic evaluation, and recommendations. Seminars in Ophthalmology 2021; 36: 692-712.
- La Morgia C, Di Vito L, Carelli V et al. Patterns of retinal ganglion cell damage in neurodegenerative disorders: parvocellular vs magnocellular degeneration in optical coherence tomography studies. Frontiers in neurology 2017; 8: 710.
- Gupta N, Yücel YH. Glaucoma as a neurodegenerative disease. Current opinion in ophthalmology 2007; 18: 110-114.
- Hasan MM, Phu J, Sowmya A et al. Artificial Intelligence in the Diagnosis of Glaucoma and Neurodegenerative Diseases. Clinical and Experimental Optometry 2024: 1-15. DOI: https://doi.org/10.1080/08164622.2023.2235346
- Heijl A, Bengtsson B, Hyman L et al. Natural history of open-angle glaucoma. Ophthalmology 2009; 116: 2271-2276.
- Thomson KL, Yeo JM, Waddell B et al. A systematic review and meta-analysis of retinal nerve fiber layer change in dementia, using optical coherence tomography. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring 2015; 1: 136-143.
- Yu J-g, Feng Y-f, Xiang Y et al. Retinal nerve fiber layer thickness changes in Parkinson disease: a meta-analysis. PloS one 2014; 9: e85718.
- Mehta P, Petersen CA, Wen JC et al. Automated detection of glaucoma with interpretable machine learning using clinical data and multimodal retinal images. American Journal of Ophthalmology 2021; 231: 154-169.
- Sánchez-Morales A, Morales-Sánchez J, Kovalyk O et al. Improving glaucoma diagnosis assembling deep networks and voting schemes. Diagnostics 2022; 12: 1382.
- An G, Omodaka K, Hashimoto K et al. Glaucoma diagnosis with machine learning based on optical coherence tomography and color fundus images. Journal of healthcare engineering 2019; 2019: 1-9.
- Christopher M, Belghith A, Weinreb RN et al. Retinal nerve fiber layer features identified by unsupervised machine learning on optical coherence tomography scans predict glaucoma progression. Investigative Ophthalmology and Visual Science 2018; 59: 2748-2756.
- Yi S, Zhang G, Qian C et al. A multimodal classification architecture for the severity diagnosis of glaucoma based on deep learning. Frontiers in neuroscience 2022; 16: 982.
- Yousefi S, Goldbaum MH, Balasubramanian M et al. Glaucoma progression detection using structural retinal nerve fiber layer measurements and functional visual field points. IEEE Transactions on Biomedical Engineering 2014; 61: 1143-1154.
- Huang X, Sun J, Gupta K et al. Detecting glaucoma from multi-modal data using probabilistic deep learning. Frontiers in Medicine 2022; 9: 923096.
- Wang X, Jiao B, Liu H et al. Machine learning based on optical coherence tomography images as a diagnostic tool for Alzheimer’s disease. CNS Neuroscience and Therapeutics 2022; 28: 2206-2217.
- Salam AA, Khalil T, Akram MU et al. Automated detection of glaucoma using structural and non structural features. SpringerPlus 2016; 5: 1-21.
- Xu J, Ishikawa H, Wollstein G et al. Three-dimensional spectral-domain optical coherence tomography data analysis for glaucoma detection. PloS one 2013; 8: e55476.
- Maetschke S, Antony B, Ishikawa H et al. A feature agnostic approach for glaucoma detection in OCT volumes. PloS one 2019; 14: e0219126.
- Kim SJ, Cho KJ, Oh S. Development of machine learning models for diagnosis of glaucoma. PloS one 2017; 12: e0177726.
- Gong D, Hu M, Yin Y et al. Practical application of artificial intelligence technology in glaucoma diagnosis. Journal of Ophthalmology 2022; 2022: 5212128.
- Xiong J, Li F, Song D et al. Multimodal machine learning using visual fields and peripapillary circular oct scans in detection of glaucomatous optic neuropathy. Ophthalmology 2022; 129: 171-180.
- Barella KA, Costa VP, Gonçalves Vidotti V et al. Glaucoma diagnostic accuracy of machine learning classifiers using retinal nerve fiber layer and optic nerve data from SD-OCT. Journal of Ophthalmology 2013; 2013: 1-7.
- Lin M, Hou B, Liu L et al. Automated diagnosing primary open-angle glaucoma from fundus image by simulating human’s grading with deep learning. Scientific Reports 2022; 12: 14080.
- Sun S, Ha A, Kim YK et al. Dual-input convolutional neural network for glaucoma diagnosis using spectral-domain optical coherence tomography. British Journal of Ophthalmology 2021; 105: 1555-1560.
- Wu CW, Chen HY, Chen JY et al. Glaucoma detection using support vector machine method based on Spectralis OCT. Diagnostics 2022; 12: 391.
- Sharma P, Ninomiya T, Omodaka K et al. A lightweight deep learning model for automatic segmentation and analysis of ophthalmic images. Scientific Reports 2022; 12: 1-18.
- Kooner KS, Angirekula A, Treacher AH et al. Glaucoma diagnosis through the integration of optical coherence tomography/angiography and machine learning diagnostic models. Clinical Ophthalmology 2022; 16: 2685-2697.
- Manassakorn A, Auethavekiat S, Sa-Ing V et al. GlauNet: Glaucoma Diagnosis for OCTA Imaging using a New CNN Architecture. IEEE Access 2022; 10: 95613-95622.
- Medeiros FA, Jammal AA, Mariottoni EB. Detection of progressive glaucomatous optic nerve damage on fundus photographs with deep learning. Ophthalmology 2021; 128: 383-392.
- Thakoor KA, Li X, Tsamis E et al. Strategies to improve convolutional neural network generalizability and reference standards for glaucoma detection from oct scans. Translational Vision Science and Technology 2021; 10: 16-16.
- Glaucoma Australia Media Release. Glaucoma Australia to fund promising new imaging technology. In, 2021. Date accessed 16 December 2023.
- Dias DT, Ushida M, Battistella R et al. Neurophthalmological conditions mimicking glaucomatous optic neuropathy: analysis of the most common causes of misdiagnosis. BMC Ophthalmology 2017; 17: 1-5.
- Lim JS, Hong M, Lam WST et al. Novel technical and privacy-preserving technology for artificial intelligence in ophthalmology. Current Opinion in Ophthalmology 2022; 33: 174-187.
