The previous article in this series (Optometric Practice – The natural home of dry eye part 1) discussed a number of ocular surface conditions that can mimic typical dry eye disease (DED) symptoms, and the importance of identifying true DED. This masquerading of dry eye can often lead to delayed and inaccurate diagnosis with inappropriate management options prescribed.
On deeper examination of the wide condition of ‘dry eye’ it is evident this is an umbrella term covering more than one form, the most prevalent being evaporative dry eye (EDE) and a smaller number of cases aqueous deficient dry eye (ADDE),1 although both forms can exist simultaneously as mixed dry eye.
The prevalence of dry eye disease has historically been underestimated, probably because it remained a hidden condition, not reported by patients, who have self-treated via the pharmacy, supermarket, or internet, with varying degrees of success. Under-reporting of this condition could have been due to eye care professionals (ECPs) not asking the right questions and a lack of patient awareness that this condition for the majority of patients is best diagnosed and managed within optometric practice.2
Perhaps the most convincing evidence of the increasing prevalence of DED in the UK is from a study reported in the summer of 2023 – The epidemiology of dry eye disease in the UK: The Aston dry eye study,3 which found that around one third of UK adults have DED. Interestingly, a recent review by Stapleton et al (2024)4 on DED in the young population also noted DED may be underdiagnosed in this age group, prevalence found to be slightly lower than in adults but symptomatic DED was remarked as common.
Risk factors
While the large population of people with a chronic dry eye condition (and/or other forms of ocular surface disease) are self-treating, without a definitive diagnosis, they are probably not making their condition directly worse, however, the fact that the precise cause of their symptoms is yet to be identified gives the specific condition time to progress, sometimes to the point where it is not reversible.
You may recall from part 1 in this series how it was found that meibomian gland disease (MGD) was whole or partially responsible for 86% of dry eye disease,1 just some of the many ophthalmic risk factors in the progression of MGD are; chronic blepharitis (anterior or posterior), contact lens wear and Demodex folliculorum.5
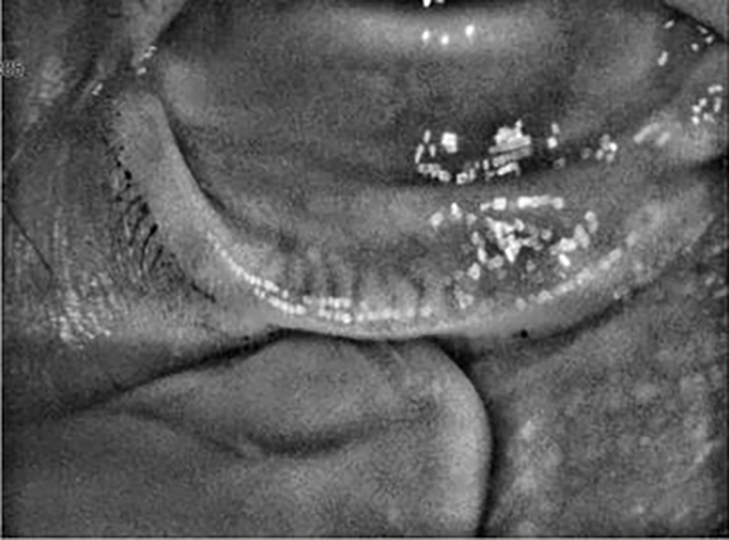
Figure 1: Meibomian gland loss
Systemic risks include an imbalance in omega-3 to omega-6 fatty acids, a Niedernolt, placebo-controlled, double-masked trial showed when omega-3 intake was supplemented that tear break-up time (TBUT), ocular surface disease index (OSDI) score, and meibum score improved.6
Other risk factors for progressing MGD and, hence, evaporative dry eye (EDE) are not so easily modifiable, such as hormone balance and age. However, if detected early enough, a blepharitis condition, duration of contact lens wear/lens design, demodex infestation and even the patient’s dietary intake of omega-3 etc could be considered to be modifiable. As in so many aspects of health, the key is to diagnose early and accurately.
Patients may take an overly simplistic view of their ‘dry eye’ symptoms, taking the phrase (the author wishes this term ‘dry eye’ had never been invented) as the be all and end all of whatever is irritating their eye and looking for a quick fix. However, as ECPs, with a greater knowledge base, we know a detailed focused exam is in the best interests of the patient, equally through experience and past conversations with patients it is human nature for them to look for a cheap, easy and quick answer to their symptoms.
In these situations, if the ECP at the dispensing desk, or via a phone conversation, deduces it is not an ocular emergency and the patient is reluctant to arrange a specific appointment, good advice may be, assuming it is an EDE condition (specific cause as yet unknown), and an eye drop formulation may help to address symptoms, and improve tear film homeostasis, detail on artificial tear formulations will be covered later in this series.
Sometimes, to illustrate to patients’ similar symptoms can be due to a number of conditions, an analogy of dental pain could be used, they will appreciate that until they are sitting in the dentist’s chair, the specific cause of this extremely distracting long-term pain, only temporarily helped by oral pain medication, is unknown.
The causes listed by the website NHS inform lists; tooth decay/cavities, a cracked tooth, lose or broken fillings, receding gums (the author likes this analogy as meibomian glands also atrophy with age), periapical abscess, periodontal abscess, ulcers on gums, sore or swollen gums around a tooth that is breaking through, etc.7 The same applies to ocular surface symptoms, where the specific causes can be many and extremely varied.
A practical approach to ‘Duty of Care’
It is good practice and good practice building, when recommending an initial approach with eye drops alone, without a specific focused exam, to instruct the patient on how, and how frequently, to instil the recommended drops, then to agree to call the patient for a follow-up conversation in two to three weeks’ time.
If the patient is not at that stage noticing a significant improvement in symptoms, an appointment would be strongly indicated. A logbook of the advice given is recommended, which will enable follow-up calls to be scheduled, this concept can be used by all practice staff, trained support, dispensing opticians and optometrists.
All information should also be fully annotated in the patient’s record, when there is no existing record one should be created ensuring clear, up to date and accessible information for all of the practice team involved in the patient’s care.8 Instructions on eye drop instillation frequency and techniques, along with follow-up attention and interest differentiates the care provided to patients by optical practices compared to an internet seller, or the checkout at the supermarket.
The structured assessment approaches
At the follow-up call, if as in many cases, the drops provided do not make a significant difference to symptoms, or no difference at all, and the only symptomatic relief is through frequent instillation of the drops due to the residence time of topical eye drops being relatively short (document this in the logbook) try to avoid suggesting other drops without a clear rationale.
The patient should be strongly advised to attend a dry eye assessment/tear clinic appointment/ocular surface exam etc, focused on the
condition. It should be carefully explained to the patient the exam is not a ‘sight-test’ with the aim of issuing a spectacle prescription, which is probably still for most patients their only experience of stepping into an eye care exam room.
An excellent starting point for investigation into the type of ocular surface symptoms, their specific origin and degree, is the use of one of the several ocular surface/dry eye questionnaires available, some of them lend themselves to general screening use, others are more detailed.
Whichever one used will convey to the patient the appointment is something apart from their normal eye exam, their condition is taken seriously, and is being assessed in a structured manner. All give a score which can be used to measure the degree of patient’s symptoms and, if wished, compared to a follow-up score as treatments progress.
Whichever one used will not, on its own be sufficient to make a diagnosis, no single test can. Questionnaires can be completed by the patient, often with a little guidance as to what the question is asking, outside the exam room prior to the focused clinical examination.
Recalling the 32.1% prevalence of DED among UK adults3 the shorter type of questionnaire used as a screening tool for all patients can provide extremely useful information identifying those patients with previously unreported symptoms, affording an opportunity to provide a proactive approach to management options.
Which questionnaire to use?
There are a number of validated ocular surface/dry eye questionnaires available. The most commonly used in research and clinical trials over a number of years is the OSDI,9 which the author has used in practice for some time. The OSDI is a 12-item frequency-based questionnaire, covering the areas of physical symptoms, vision and environments.
It does not however ask questions related to the intensity of the symptoms or watery eyes. It is for this reason that the author, in the process of researching these articles, is considering a change to the Dry Eye Questionnaire (DEQ-5),9 which is shorter, perhaps quicker and does contain questions on intensity of symptoms and watery eyes.
A cross-sectional population-based study of 329 participants concluded the performance of the DEQ-5 questionnaire in discriminating symptoms of dry eye is comparable to the OSDI questionnaire and that the DEQ-5 questionnaire is a valid measure of dry eye symptoms and can be used as a dry eye symptoms assessment tool in clinical and epidemiological studies.9
Both questionnaires are reported on in the Tear Film and Ocular Surface Society (TFOS) Dry Eye Workshop (DEWS) II Diagnostic Methodology Subcommittee report where they recommend a positive result being a DEQ-5 score of six or above or an OSDI score of 13 or higher.10 Other popular questionnaires are the McMonnies Questionnaire, Standardised Patient Evaluation of Eye Dryness (SPEED), Symptoms Assessment in Dry Eye (SANDE).11
How about DED signs?
There is no single objective test to confirm DED, it is far more a case of building a picture from the constituent parts of the jigsaw. When it comes to tests, the guiding principle is to work from least to most invasive, for instance there is little accuracy in measuring non-invasive tear break up time after conducting a Schirmer test.
However, sometimes it is simply not possible to conduct all the tests in a perfect order in one appointment, one or two tests may have to be deferred to the follow-up visit. To arrive at a diagnosis, it is certainly not necessary, or desirable from the patient’s perspective, to conduct every test. In an attempt to give some form of protocol to the test routine, after the questionnaire, an order could be:
Measure tear prism/marginal tear film height/tear meniscus height (TMH)
The latter seems to be the more accepted terminology in 2023. The height of the tear meniscus is a generally accepted method to evaluate tear film volume, especially in dry eye diagnoses and management.12
A study of 97 elderly subjects produced an average TMH value of close to 0.2mm, as compared to a small number of individuals with rather greater TMH values of up to 0.7mm, this average value, also seen in other studies, might therefore be considered as indicative of a normal tear volume, with a few individuals having reflex tearing.13
Based on the data and literature values, a lower cut-off limit for normal would be ≤0.1mm, while ≥0.25mm would indicate reflex tearing and/or sub-optimal tear drainage.
How to measure TMH? Reliability was studied using 20 subjects (26.8 ± 5.6 years) by two observers using optical coherence tomography (OCT), slit lamp microscope image analysis, and with a reticule at low (8x) and high (32x) magnification.14
TMH was also evaluated by both observers by comparing TMH to thickness of the lid margin (lid-ratio grade) and to the number of eyelashes fitting in the tear meniscus. The most reliable method to measure TMH was OCT followed by slit lamp using a reticule. TMH cannot be reliably evaluated by comparing it against lid margin thickness or number of eyelashes.14
Non-invasive tear break up time (NITBUT)
If the measuring of TMH can be done with very little or no stimulus to reflex tear production, then it may be considered that the validity of results from a NITBUT is worthwhile, if not the decision needs to be made as to which is conducted and perhaps which deferred.
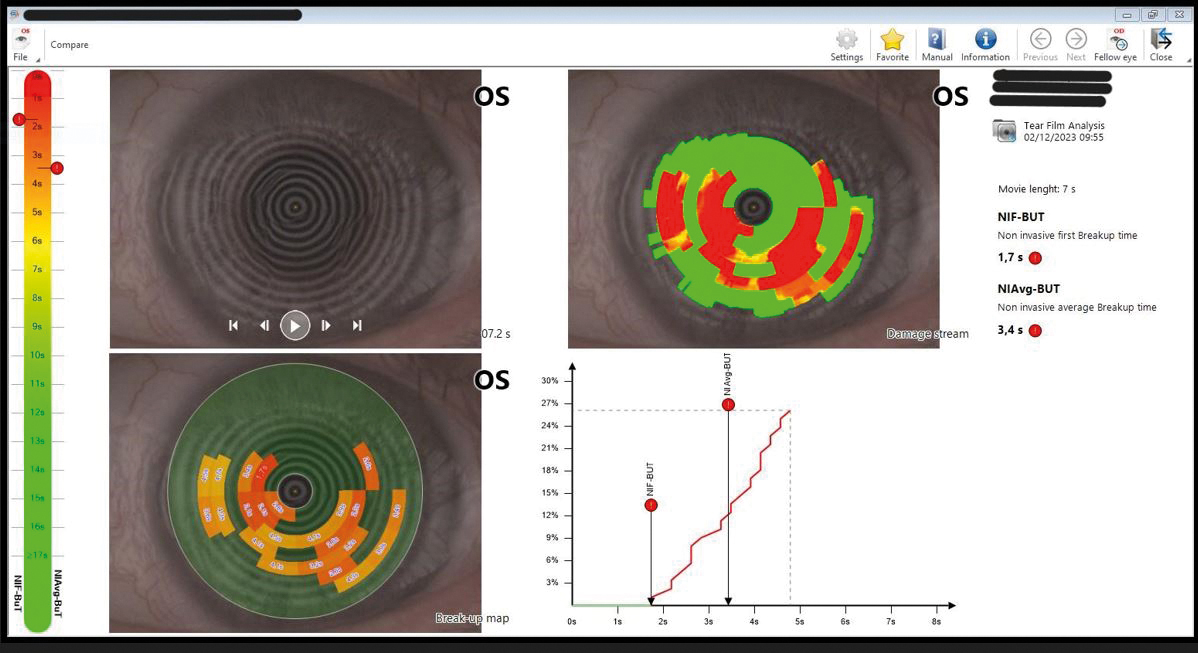
Figure 2: Abnormally short NITBUT capture
As a caution, to place the NITBUT before TMH measure, it has been shown that delayed blinking in a NITBUT test can significantly increase the TMH, in the same study the average NITBUT was 16.7 ± 7.6 seconds.15
To further complicate the decision-making process on which test is more appropriate at this point, this type of test could confound the results of a Schirmer test (see below: Tear sampling – what can it tell us?), although probably not in a gross aqueous hypo-secretor.
The test is by non-invasively projecting a grid or other pattern onto the tear film, the time interval following a complete blink to the first occurrence of breaks or a change in the reflected grid image is defined as the breakup time, this reflected image change is best done automatically.
A study investigating the agreement and repeatability of four different instruments (Tearscope plus, Polaris, EasyTear Viewplus, Keratograph 5M) in the measurement of NITBUT, showed their measurement of tear stability were reasonably repeatable and give similar average results.16
For those with a Oculus Keratograph 5M this enables measurements of both NITBUT and TMH and enables examination of the meibomian glands, a simple Placido disc or one position keratometer can also be used to give a gross assessment of NITBUT. The latter two options however are not as accurate or repeatable as an automatic instrument.
Tear sampling – what can it tell us?
Tear sampling is by its very nature invasive, and conducting one form of these tests may be thought of as interfering with the reliability of another form, or even other types of tear/ocular surface tests carried out subsequently, so it is a rational choice to defer some for follow-up visits if they are indicated.
Let us take as an example the Schirmer test (originated in 1903), in my opinion, unfairly criticised by some, who state the very act of hanging paper threads from the inferior lid stimulates tear production. However, if the TMH value is low, the Schirmer test may be indicated at that visit or at the very least at the first follow-up visit.
The early identification of an aqueous production deficiency is vital, as it has implications for the treatment of ocular surface conditions and possibly, if within the triad of dry eyes, dry mouth, and evidence of an autoimmune process mediating these and other clinical manifestations – Sjögren’s syndrome (SS)17 or other autoimmune disease.
It might be thought that if the patient’s tear production is still low under the abnormal stimulation during the test it would indeed indicate they are an aqueous hypo-secreter, it is these patients that need identifying, not the normal who will tear profusely.
The Schirmer test measures the five-minute wetting of a standardised strip of filter paper placed in the lower lateral fornix of the eyelid and serves as an indicator of basal (anesthetised) or stimulated (unanaesthetised) tear flow. The unanaesthetised Schirmer test is supportive of SS when wetting measures 5mm or less in either eye.17
As the level of aqueous production can be part of a larger systemic diagnosis, as discussed above, the author likes to repeat the test on a separate occasion before arriving at a conclusion.
There are other forms of tear sampling or Point of Care (POC) testing that have been developed more recently, one is InflammaDry (Quidel Corporation), which measures the presence of MMP-9 on the ocular surface. MMP-9 is an enzyme important for tissue remodelling in normal physiological processes like wound healing and bone development. 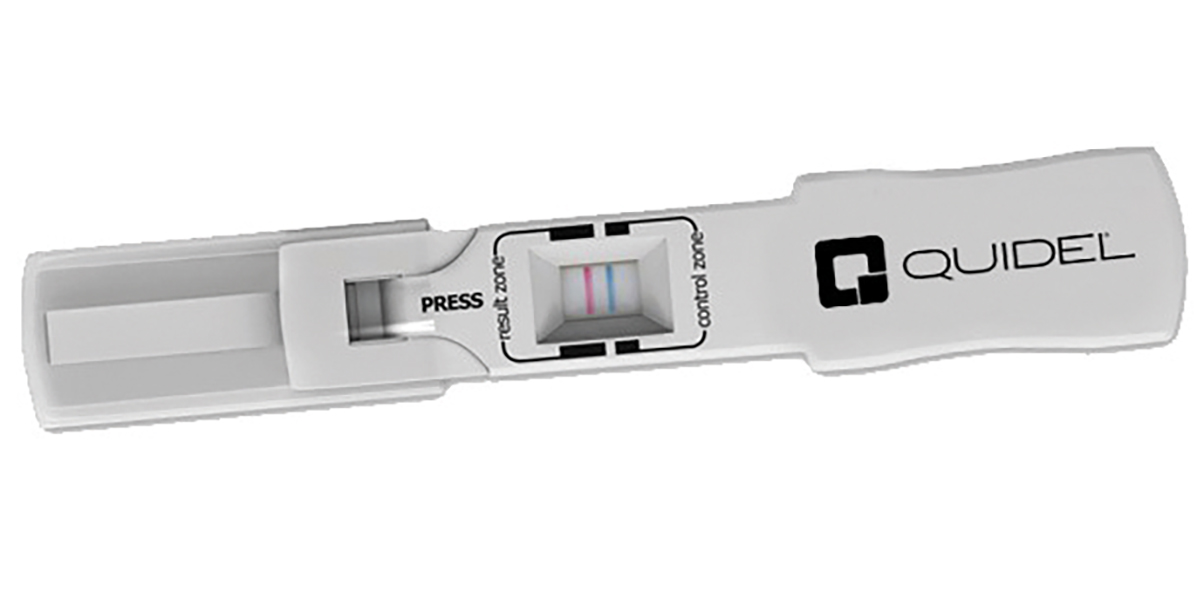
Figure 3: ImflammaDry with postive test
However, elevated levels of MMP-9 are also understood to play a pathogenic role in inflammatory disease, and away from the eye; arthritis, cardiovascular diseases, pulmonary diseases and cancer.18
The test should be performed prior to administering ocular anaesthetic, topical dyes, or Schirmer test, (so again no perfect order to conduct these tests in, some may need to be deferred until a follow-up visit). In the test, the sample collector is dabbed in multiple locations along the palpebral conjunctiva until the sampling fleece is saturated with tears.
Next, the sample collector is assembled to the test cassette, then the test pad is dipped into a buffer solution for 20 seconds for activation. After 10 minutes the test window is examined for a) blue line to indicate the tear sample will give a reliable result and b) the presence or indeed strength of a red line indicating the presence of MMP-9.
If this sounds vaguely familiar it will be because the world has become used to the concept of lateral flow tests since the arrival of
Covid-19. Osmosis is defined as the movement of a solvent across a semipermeable membrane toward a higher concentration of solute. When a cell is submerged in water, the water molecules pass through the cell membrane from an area of low solute concentration to high solute concentration.
For example, if the cell is submerged in saltwater, water molecules move out of the cell. If a cell is submerged in freshwater, water molecules move into the cell.19 Imagine a corneal epithelial cell with a given concentration of solutes within it, sitting under a tear film that may have a higher concentration of solutes due to evaporation, remember EDE is the dominant form of DED,2 the epithelial cell will lose water.
Osmolarity is a term given to the total concentration of penetrating solutes (capable of moving across the cell wall) and non-penetrating solutes (not capable of moving across the cell wall) and its measurement is expressed as Osm/L (the number of osmoles (Osm) of solute per litre (L)).
In the field of tear film osmolarity we are using milliosmole (mOsm), which is 1/1,000 of an osmole, therefore it is thought normal mean tear film osmolarity values would be in a range from 270 to 315 mOsm/L with an overall average of 300mOsm/L.15 with variation between normal right and left eyes is of 6.9 ± 5.9 mOsm/L.19
However, the range and which values are thought to be relevant is still under discussion, for instance in a 2010 prospective, multisite clinical study (10 sites in the European Union and the United States), normal, mild/moderate, and severe dry eyes were said to have average tear osmolarity values of 302 ± 8mOsm/L, 315 ± 11mOsm/L and 336 ± 22mOsm/L, respectively.20
Osmometers are instruments that can collect a tear sample and analyse its electrical impedance producing an osmolarity value, the first generation TearLab Osmolarity System and the more recent ScoutPro Osmolarity System (both Trukera Medical) are both examples .
So far we have considered some of the initial tests that can be employed to determine if the patient’s ocular surface condition is dry eye and if so which form and its degree. Remember not every test is required and there is no perfect order, least invasive early – if possible, and make a note of other tests to be carried out at the follow-up visit(s).
In the following article in this series we will discuss the widely used, almost ubiquitous, fluorescein breakup time test and ocular surface stains. We will also look at lid margin examination, including the meibomian glands appearance, meibography and more.
- Andrew D Price FBDO(Hons)CL MBCLA, CEO of ADP Consultancy and ADP-EyeCare is a clinician providing contact lens and dry eye clinics, a principal investigator in clinical trials, professional services consultant, educator and author. He received certification in ophthalmic assisting during work in ophthalmology practice in the USA and has worked in laser vision and ophthalmology clinics in the UK and USA. He has published and presented over 300 lectures and workshops, in the UK and abroad and was involved in developing Eye Drops Database. Andrew is also a past ABDO National Clinical Committee and Optometry Wales Board member and has been a guest lecturer for ABDO and Anglia Ruskin University.
References
- Lemp, MA, Crews, LA, Bron, AJ, Foulks, GN, & Sullivan, BD (2012). Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 31(5), 472-478.
- Bradley JL, Stillman IO, Pivneva I, Guerin A, et al. Dry eye disease ranking among common reasons for seeking eye care in a large US claims database. Clinical Ophthalmology. 2019;13: 225-232. https://doi.org/10.2147%2FOPTH.S188314
- Vidal-Rohr M, Craig JP, Davies LN, Wolffsohn. The epidemiology of dry eye disease in the UK: The Aston dry eye study. Contact Lens and Anterior Eye. 2023; 46(3): 101837 https://doi.org/10.1016/j.jtos.2017.05.003
- Stapleton F, Velez FG, Lau C, Wolffsohn JS. Dry eye disease in the young: A narrative review. The Ocular Surface. 2024;31: 11-20. https://doi.org/10.1016/j.jtos.2023.12.001
- Debra A Schaumberg, Jason J Nichols, Eric B Papas, Louis Tong, Miki Uchino, Kelly K Nichols. Investigative Ophthalmology & Visual Science. March 2011;.52:1994-2005. https://doi.org/10.1167/iovs.10-6997e
- Macsai MS. The role of omega-3 dietary supplementation in blepharitis and meibomian gland dysfunction (an AOS thesis). Trans Am Ophthalmol Soc. 2008;106:336–356. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2646454/
- NHS Inform. Toothache. Available from: https://www.nhsinform.scot/illnesses-and-conditions/mouth/toothache/ Accessed [17th January 2024]
- General Optical Council. s.8 Maintain adequate patient records, Standards of Practice for Optometrists and Dispensing Opticians. London: General Optical Council; 2016 p. 13
- Akowuah PK, Adjei-Anang J, Nkansah EK, Fummey J, et al. Comparison of the performance of the dry eye questionnaire (DEQ-5) to the ocular surface disease index in a non-clinical population. Contact Lens and Anterior Eye.2022;45(3):. https://doi.org/10.1016/j.clae.2021.101441.
- Amparo F, Schaumberg DA, Dana R. Comparison of two questionnaires for dry eye symptom assessment: The Ocular Surface Disease Index and the Symptom Assessment in dry eye. Ophthalmology. 2015;122:7:1498-1503.
- Dana R, Meunier J, Markowitz JY, Joseph C, et al Patient-reported burden of dry eye disease in the United States: Results of an online cross-sectional survey. American Journal of Ophthalmology. 2020;216: 7-17. https://doi.org/10.1016/j.ajo.2020.03.044
- Wolffsohn JS, Arita R, Chalmers R, Djalilan A, et al TFOS DEWS II Diagnostic Methodology report. The Ocular Surface. 2017; 15(3): 539-574. https://doi.org/10.1016/j.jtos.2017.05.001
- Michael J Doughty, Mohammad Laiquzzaman, Emil Oblak, Norman Button. The tear (lacrimal) meniscus height in human eyes: a useful clinical measure or an unusable variable sign? Contact Lens and Anterior Eye.2002; 25(2): 57-https://doi.org/10.1016/S1367-0484(01)00005-4.
- Niedernolte B, Trunk L, Wolffsohn JS, Pult H, Bandlitz S. Evaluation of tear meniscus height using different clinical methods. Clin Exp Optom. 2021 Jul;104(5):583-588. doi: 10.1080/08164622.2021.1878854.
- Wang J, Palakuru JR, Aquavella JV. Correlations Among Upper and Lower Tear Menisci, Non-invasive Tear Break-up Time, and the Schirmer Test, American Journal of Ophthalmology. 2008; 145(5): 795-800, https://doi.org/10.1016/j.ajo.2007.12.035
- Bandlitz S, Peter P, Pflugi T, Jaeger K, et al. Agreement and repeatability of four different devices to measure non-invasive tear breakup time (NIBUT). Contact Lens and Anterior Eye. 43(5). 2020, Pages 507-511. ISSN 1367-0484, https://doi.org/10.1016/j.clae.2020.02.018
- Baer AN, Hall JC. 138 — Sjögren Syndrome. In: Marc C Hochberg, Alan J Silman, Josef S Smolen, Michael E Weinblatt, Michael H Weisman. (eds) Rheumatology (Sixth Edition). USA: Mosby Ltd; 2015, Pages 1131-1143
- Nicole L Lanza, Felipe Valenzuela, Victor L Perez, Anat Galor, The Matrix Metalloproteinase 9 Point-of-Care Test in Dry Eye. The Ocular Surface. 2016;14(2):, 189-195. https://doi.org/10.1016/j.jtos.2015.10.004
- Marieb E. Essentials of human anatomy and physiology 9th Edn. America: Pearson Education; 2009. P.77
- Wilcox DP, Argueso P, Georgiev GA, Holopainen JM et al. TFOS DEWS II Tear Film Report. The Ocular Surface.2017;15(3): 366-403 https://doi.org/10.1016/j.jtos.2017.03.006
- Sullivan BD, Whitmer D, Nichols KK, Tomlinson A, et al. An Objective Approach to Dry Eye Disease Severity. Invest. Ophthalmol. Vis. Sci. 2010;51(12):6125-6130. https://doi.org/10.1167/iovs.10-5390
