All paediatric patients require good vision for motor and language skills, and cognitive development; in fact, it is estimated around 80% of learning occurs through vision.1 The provision of well-fitting paediatric spectacles is clearly vital in ensuring that the child achieves the best possible visual outcome, particularly during the critical period.
Children’s head and facial features have very different proportions compared to adults’ and, as a result, scaled down versions of adult frames are not suitable for paediatric patients. Three-dimensional stereophotogrammetry has recently been used to describe facial measurements for a range of paediatric patients, and percentiles were calculated according to age, gender and ethnicity.2
Children may also present with head and facial measurements that differ significantly from the age-related norms, due to a broad range of eye disorders, systemic conditions or dysmorphic features that occur in isolation. Furthermore, some conditions are associated with an increased risk of an eye or vision problems, and so not only are these patients more likely to need glasses, but the fit may be more challenging.
The aim of this article is to discuss two paediatric conditions that require more specialist dispensing and give a wider understanding of the pathology, ophthalmic management and the many interacting factors that affect frame, lens choice and spectacle fitting.
Craniosynostosis
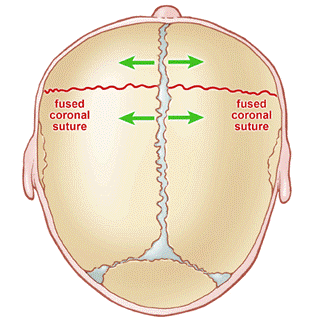
A newborn’s skull is made up of several bony ‘plates’, which are separated by sutures (see figure 1). These sutures allow the head to change shape during delivery, and permit rapid brain growth during the first few years of life.
Figure 1: Anatomy of a newborn skull
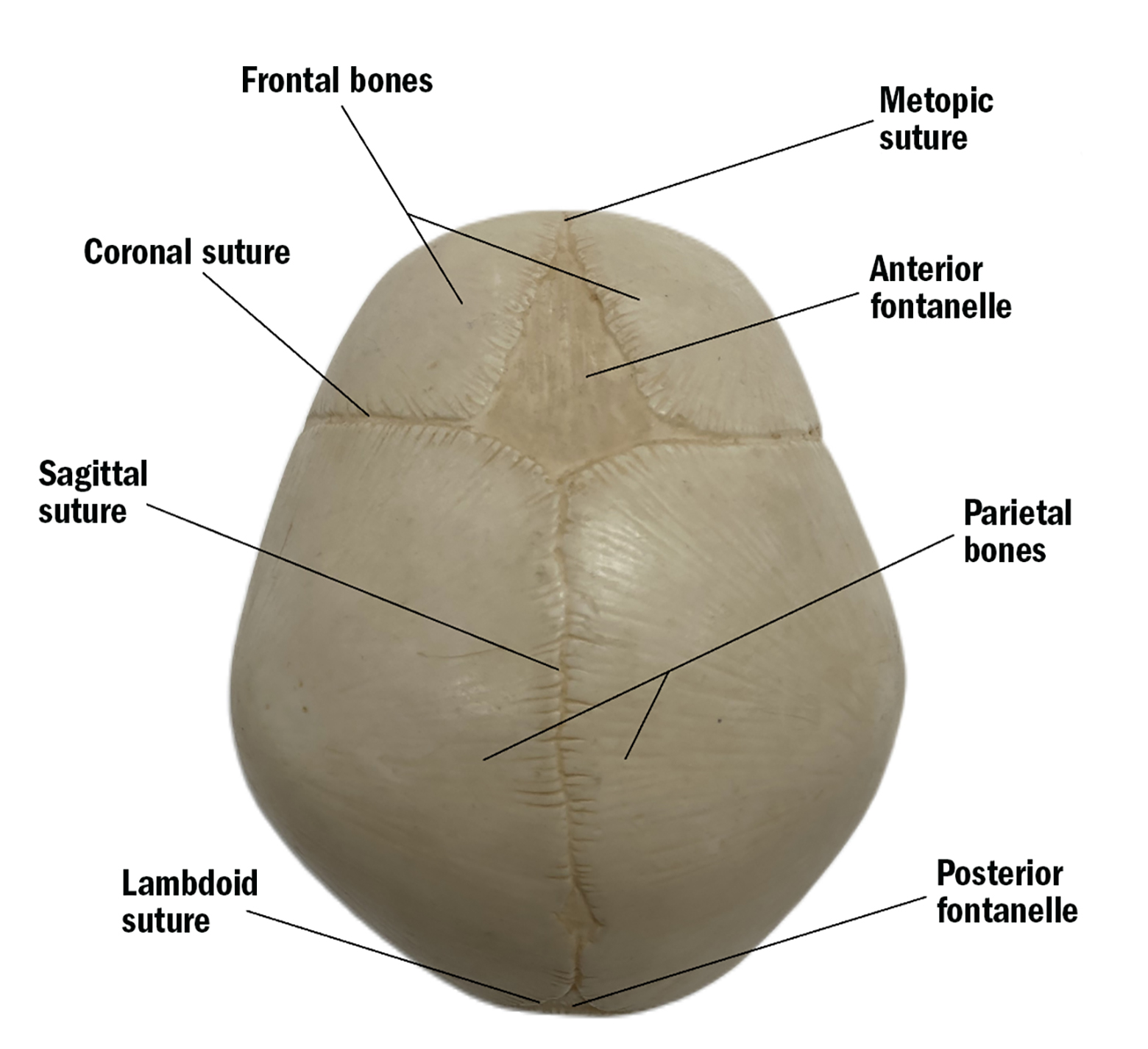
The sutures close when new bone is deposited along the leading edge. The sutures begin fusing during early childhood and fuse completely by adulthood once the brain has finished growing. This forms the protective ‘helmet’ for the brain, known as the skull.
In craniosynostosis however, either one or a number of these sutures fuse prematurely (weeks or months before birth). When a suture fuses prematurely, it inhibits growth perpendicular to the suture and causes compensatory overgrowth parallel to the suture.3 As a result, there will be abnormalities in the skull morphology, which can then affect the development of the brain and neurological structures.
The skull and face shape will depend on which suture has fused prematurely. Some examples of the different types of craniosynostosis are shown in figures 2, 3, and 4. In addition to the atypical head shape, a patient may also have shallow orbits, midface hypoplasia (underdevelopment of upper jaw, cheekbones and eye sockets), lower forehead retrusion and exophthalmos.4
Figure 2: Sagittal craniosynostosis Premature closure of the sagittal suture causes a narrow, elongated skull (scaphocephaly)
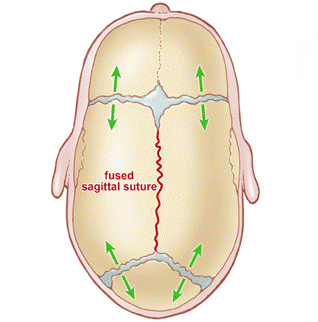
Figure 3: Unicoronal craniosynostosis Premature closure of the left coronal suture has caused compensatory overgrowth of the right forehead
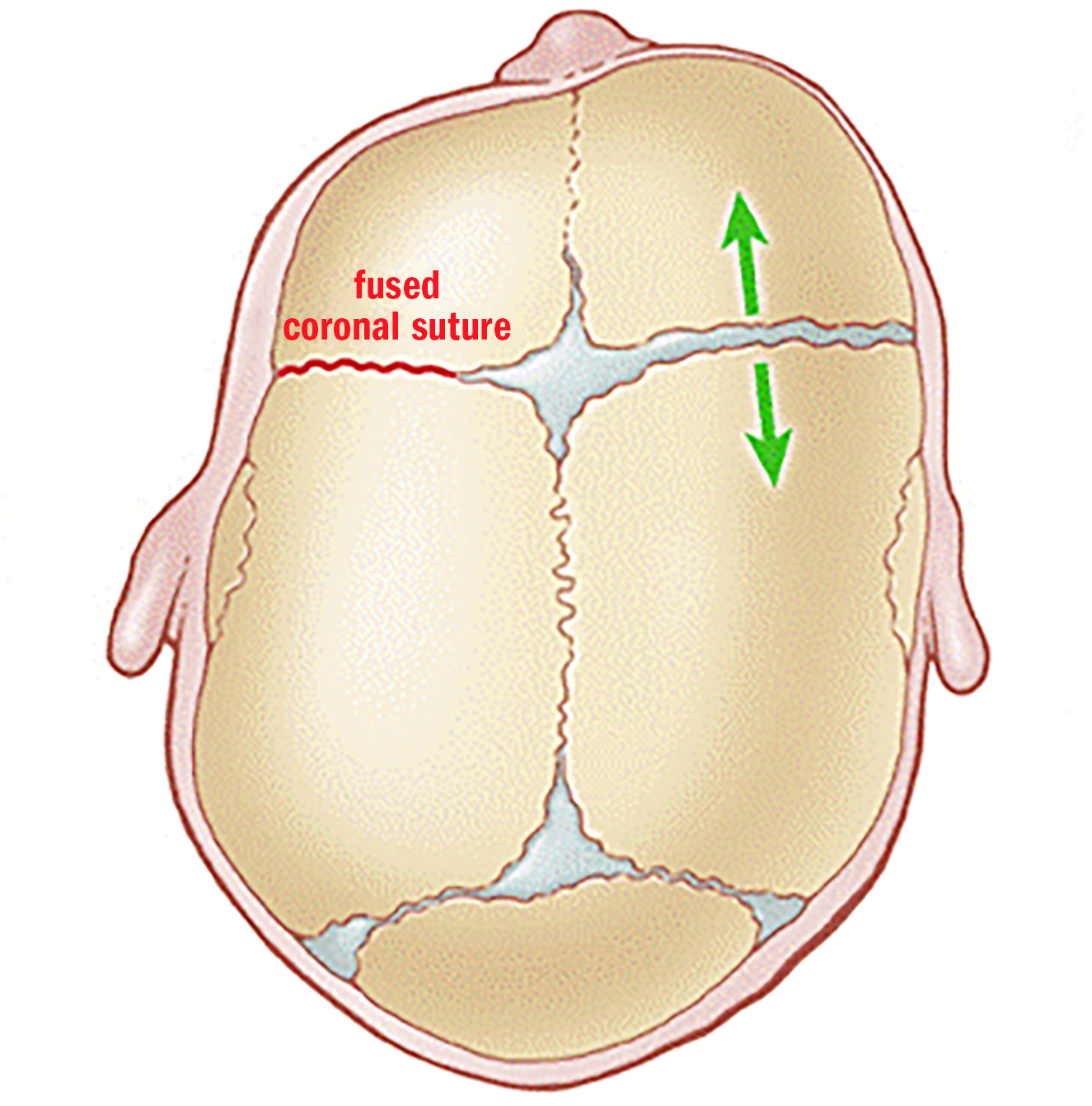
Figure 4: Bicoronal craniosynostosis Premature fusion of the two coronal sutures causes the head to become short and wide (brachycephaly). The forehead and eye sockets have a flatter appearance
Prevalence
Craniosynostosis is a relatively common congenital defect thought to affect around one in 2,000-2,500 births worldwide.3 Craniosynostosis can occur in isolation or as a part of a genetic syndrome (around 15% of cases).
Isolated cases are most likely to affect a single suture, while multiple sutures are more likely to be affected when there is an associated genetic syndrome.3 Aperts, Crouzon and Pfeiffer syndrome are examples of common craniofacial syndromes.
Referral
Craniosynostosis is an important condition for all health care professionals to be aware of. Timely referral is essential for optimal treatment and beneficial patient outcomes. However, often patients with craniosynostosis are not referred at all or are referred too late.5
Common ophthalmic manifestations of craniosynostosis
- Patients with craniosynostosis are more likely to have ametropia and in particular astigmatism. A study by Hinds et al6 found that 67.2% of patients with syndromic craniosynostosis had astigmatism greater than 1.00D, and 18.9% had anisometropia of greater than 1.00D. Patients that have shallow orbits are more likely to have hypermetropia due to a shorter axial length.7
- Shallow orbits can cause lagophthalmos and exophthalmos, which may result in corneal exposure. This, combined with a lack of adequate tear film protection, can lead to exposure keratopathy and a patient may suffer from corneal ulcers and scarring. Meticulous lubrication of the eye is needed for these patients, day and night. For patients with Crouzon syndrome, it can even be possible for subluxation of the eyeball to occur.3
- Optic neuropathy may result from raised intracranial pressure (ICP). Raised ICP can cause visual impairment, cognitive impairment, disability and even death.8 It has been suggested that raised ICP occurs when skull growth isn’t keeping up with brain growth, resulting in papilledema and optic atrophy.4 Vision loss caused by optic atrophy is a relatively common occurrence in this group of patients. The risk of raised intracranial pressure occurring is thought to be 30-40% in syndromic craniosynostosis.9 Other clinical signs of raised ICP include headache, vomiting, behavioural changes and lethargy – although children may not show any of these symptoms.4
- Patients are more likely to have a strabismus (the most common being an exotropia).3 Hind et al found that 43.6% of patients with craniosynostosis had a strabismus compared with 2.7% incidence of manifest strabismus in the general population, and 48 of 99 patients demonstrated ‘V’ pattern.6
- As a result of all the ophthalmic problems that can occur in patients with craniosynostosis, patients are more likely to develop amblyopia. Hind et al6 found that 76.7% of patients with syndromic craniosynostosis achieved a best corrected visual acuity of ≥ 6/12 in the better eye. Longitudinal population data shows the prevalence of amblyopia in children aged between four and 11 years to be 2.9%.10
- Around 45% of patients with craniosynostosis will have hypertelorism (wide separation of the orbits).11
Other clinical features
Other common features in patients with craniosynostosis include syndactyly, hearing loss, dental abnormalities, cleft palates, and increased respiratory effort as a result of the midface hypoplasia – this can cause more perspiration, respiratory obstruction and sleep apnoea.
Ophthalmic management
The main priority for the ophthalmology team will be checking the optic discs for signs of papilledema (which may indicate raised ICP), and ensuring the front surface of the eyes are kept moist and not damaged through exposure.
It is important that visual development proceeds normally to avoid the development of amblyopia – this may involve surgery to correct strabismus, patching, and the prescribing/dispensing of spectacles.
Patients with craniosynostosis will need to see the ophthalmology team around twice a year until they are around eight years, and yearly after the age of eight.3 After the age of eight, brain growth slows, as does the risk of amblyopia.
Monitoring for raised ICP
Direct intraparenchymal monitoring is the gold standard for measuring ICP however, this is a surgical procedure that carries risks of bleeding, infection and device failure.12
The ophthalmology team have a number of non-invasive methods to aid in the diagnosis of raised intracranial pressure including optical coherence tomography (particularly looking at the retinal nerve fibre layer), electrodiagnostics (using Visual Evoked Potential testing) and optic nerve sheath diameter measurements.
Treatment for raised ICP
If a patient is found to have raised ICP, they may require decompressive surgery or a cerebral spinal fluid (CSF) shunt to be fitted. A CSF shunt has a catheter that drains excess fluid from the brain to another part of the body where it is absorbed by the circulatory system. A shunt may make a visible lump under the skin.
A patient with craniosynostosis may undergo several surgeries during their childhood in order to allow normal cerebral growth and development and correct the atypical head shape.
Dispensing considerations
The choice of frame will be dictated by which sutures have fused prematurely and the resultant head shape, however the following is a summary of some of the challenges that may be faced when dispensing a patient with craniosynostosis:
- Head shape – abnormal, often asymmetric, the length and width can be smaller or larger than average.
- Proptosis and flat bridges create negative bridge projections that can cause the eyelashes or even the globe to rub on the back of the spectacle lenses.
- Increased perspiration due to narrow airways and increased respiratory effort can cause glasses to slip (and can also impede patching).
- Hypertelorism means patients are likely have a large pupil distance.
- Ear anomalies – position is often low and small. May wear hearing aids.
- Syndactyly of the hands may make it harder for patients to place, remove or adjust their glasses.
- Surgery – this can often drastically change a patient’s head and facial features.
- Shunts – pressure on a shunt should be avoided.
- Anisometropic and amblyopic prescriptions –vertical optical centre heights are important but centring the lenses can be more difficult.
Tomato glasses (or similar) can work very well for this patient group. Figure 5 provides a summary of the fitting features of Tomato glasses and how they suit the features commonly found in patients with craniosynostosis.
Figure 5: The benefits of using Tomato glasses for patients with craniosynostosis

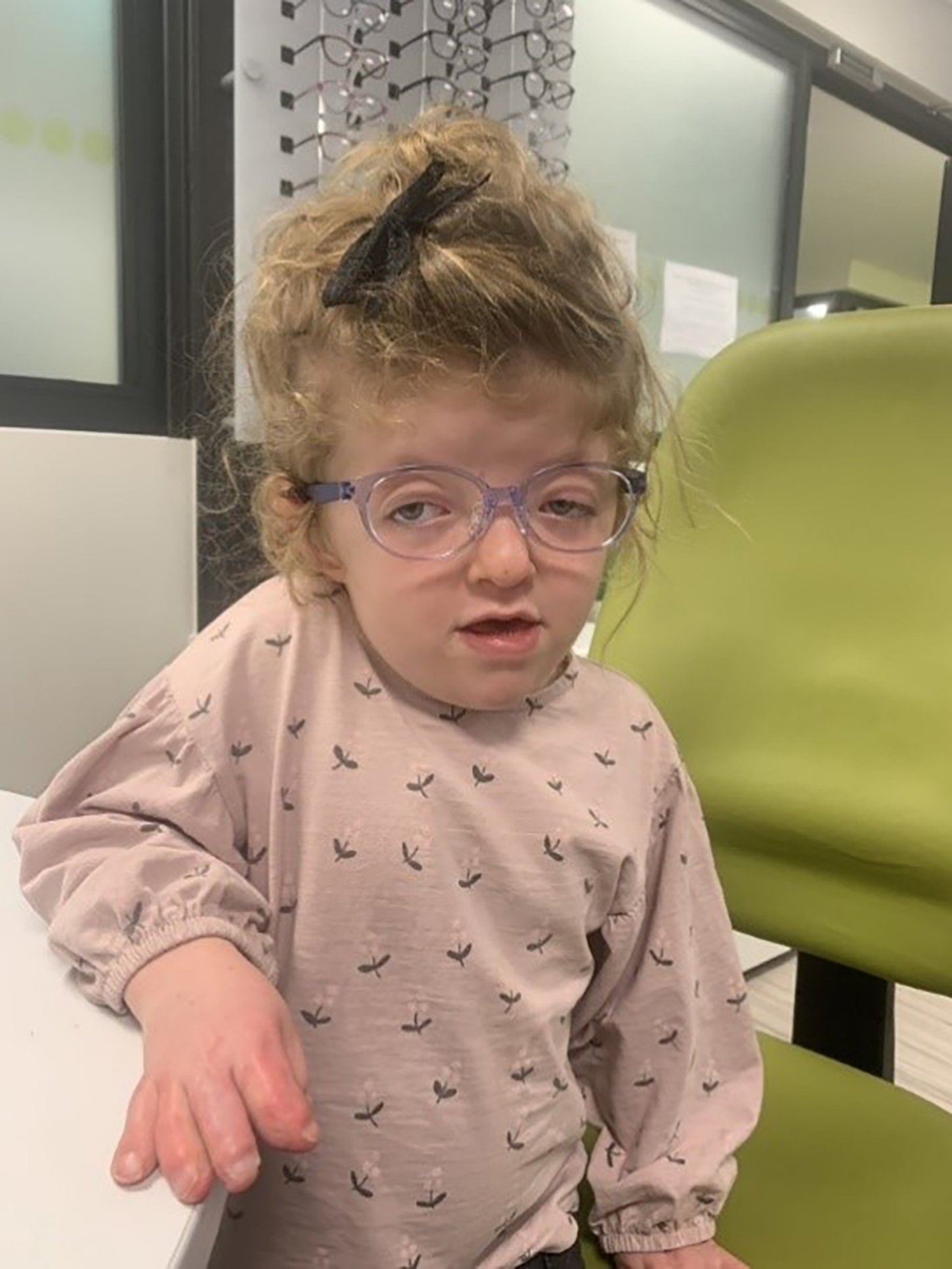
An example of a Tomato frame on a patient with Aperts syndrome can be seen in figure 6. The frames are robust and so can withstand the limited dexterity that may arise as a result of the syndactyly.
Figure 6: A young girl with Aperts syndrome wearing a Tomato C frame
Congenital cataract
Prevalence
Congenital cataract occurs in about one to six cases per 10,000 births in industrialised countries.13 It is one of the largest treatable causes of low vision worldwide.
Causes
Most cases of bilateral congenital cataract are the result of a genetic mutation with the most common mode of inheritance being autosomal dominance.14
Inherited congenital cataract may occur in isolation (70%), as part of another ocular abnormality (15%) or as part of a syndrome (15%).14 Other causes include trauma, environmental factors or iatrogenic factors.
Referral
Depending on the density and position of the cataract, they can interfere with visual development and lead to deprivation amblyopia. Prompt referral and treatment is necessary to ensure good visual outcomes.
As part of the NHS screening programme, a newborn is checked for a red reflex within 72 hours of birth and at five to eight weeks of age.15
Treatment
While intraocular lens implantation is routine for adult cataract patients, the situation is very different for paediatric patients and has changed greatly over the years.
For infants born with congenital cataract, the timing of the cataract surgery is crucial – too late and the visual outcome may be reduced. Too early and there is an increased risk of the patient developing glaucoma, as well as risks from anaesthetics.16 Surgery is normally between six and 12 weeks, depending on whether the cataract is bilateral or unilateral.17
The use of intraocular lenses to treat infantile cataract only began in the 2000s. Although there was a lack of research and evidence, it was thought the use of IOLs would improve the visual outcome and reduce the risk of secondary glaucoma (the main risk associated with cataract surgery).
It then became the standard treatment for paediatric patients with cataract around the world. Since then however, there have been two key studies18,19 that have looked at the visual outcomes and risks of using IOLs compared to leaving a patient aphakic and treating them with contact lenses/glasses.
The research has shown IOL implantation does not improve visual outcomes or reduce the risk of the patient developing glaucoma; but IOL implantation is associated with an increased likelihood of requiring further surgery (due to intraocular proliferation of remnant lens epithelial cells or inflammatory cells).
It is becoming increasingly recognised that exposure to general anaesthetics can adversely affect a child’s neurodevelopment20 and so should be limited wherever possible.
Consequently, the Royal College of Ophthalmologists now recommends that infants with cataracts who are under the age of two should be left aphakic and treated with contact lenses and glasses; and implantation of IOLs should be reserved for infants that are aged two or over.20
Although contact lenses may give superior optical correction and allow the prescription to be changed as a child becomes less hyperopic, spectacles will still be required for times when contact lens wear is not possible or for families who are unable to use contact lenses.
What does this mean for dispensing opticians? Dispensing opticians will be increasingly required to dispense glasses for infants who are aphakic, some of whom may be as young as six weeks old.
Young infants who have been left aphakic, will generally have a prescription of around +20.00DS. For young infants the prescription is normally adjusted for near focus (overcorrected by around plus two). As the child ages a bifocal may be required to allow both near and far focus.
Dispensing considerations
There are many challenges that arise from dispensing such a high prescription to a young infant, and the patient’s family should be made aware of these before the surgery.
Effective communication is essential, and the family should be given an idea of the type of frame their child will wear following the cataract surgery, the design and thickness of the aphakic lenses and the time taken to manufacture the lenses.
This will help to manage their expectations and help everything to run smoothly following the surgery. The following factors should be considered when selecting frame and lenses:
- Sourcing a frame small enough with the right dimensions may be challenging, particularly for premature babies or those with microcephaly. The smallest frame available in the UK is the Jellybeanz 54 in a 32 eye (see figure 7). The sides supplied on the Jellybeanz frame are likely to be too long and require shortening. Once cropped to give the right length to tangents, ideally the sides will retain the silicone covering as these tend to be more comfortable and give more support. Other suitable infant frames include the Swissflex Loop Baby and Tomato Baby (see figures 8 and 9).
- Before the age of three months, an infant’s neck muscles are too weak to be able to support their own head.21 The spectacles dispensed must be stable enough to remain in the correct position, while the infants head is supported by the parent or caregiver. Frames that are too large or that have long sides will be pushed forward while the infant’s head is being supported.
- Newborn infants grow extremely quickly – their head size can grow half an inch every month for the first six months22 and so it is imperative that the fit is checked regularly, and larger glasses dispensed as required.
- Full aperture lenses may not be available in the high powers required for the aphakic prescriptions. Lenticular lenses have the advantage of availability in higher powers and help to keep edge thickness and weight down. There are however, down sides to lenticular lenses including the following:
- Magnification will make objects look closer and bigger, the patient’s eyes will also look bigger, although for patients with microphthalmia this may be beneficial.
- Roving ring scotoma reduces field of view.
- Jack-in-the-box phenomenon (the presence of the annular scotoma leads to objects suddenly appearing in and out of a patient’s field of view).
Figure 7: A Jellybeanz 52 frame in a 32 eye from Atlantic Optical
Figure 8: A Swissflex Loop Baby frame. The sides can be cropped to the required length to tangents, and a strap can be fitted onto the curl sides

Figure 9: A young infant with aphakia wearing a Tomato Baby frame and 1.6 index lenticular lenses from Optimum Coatings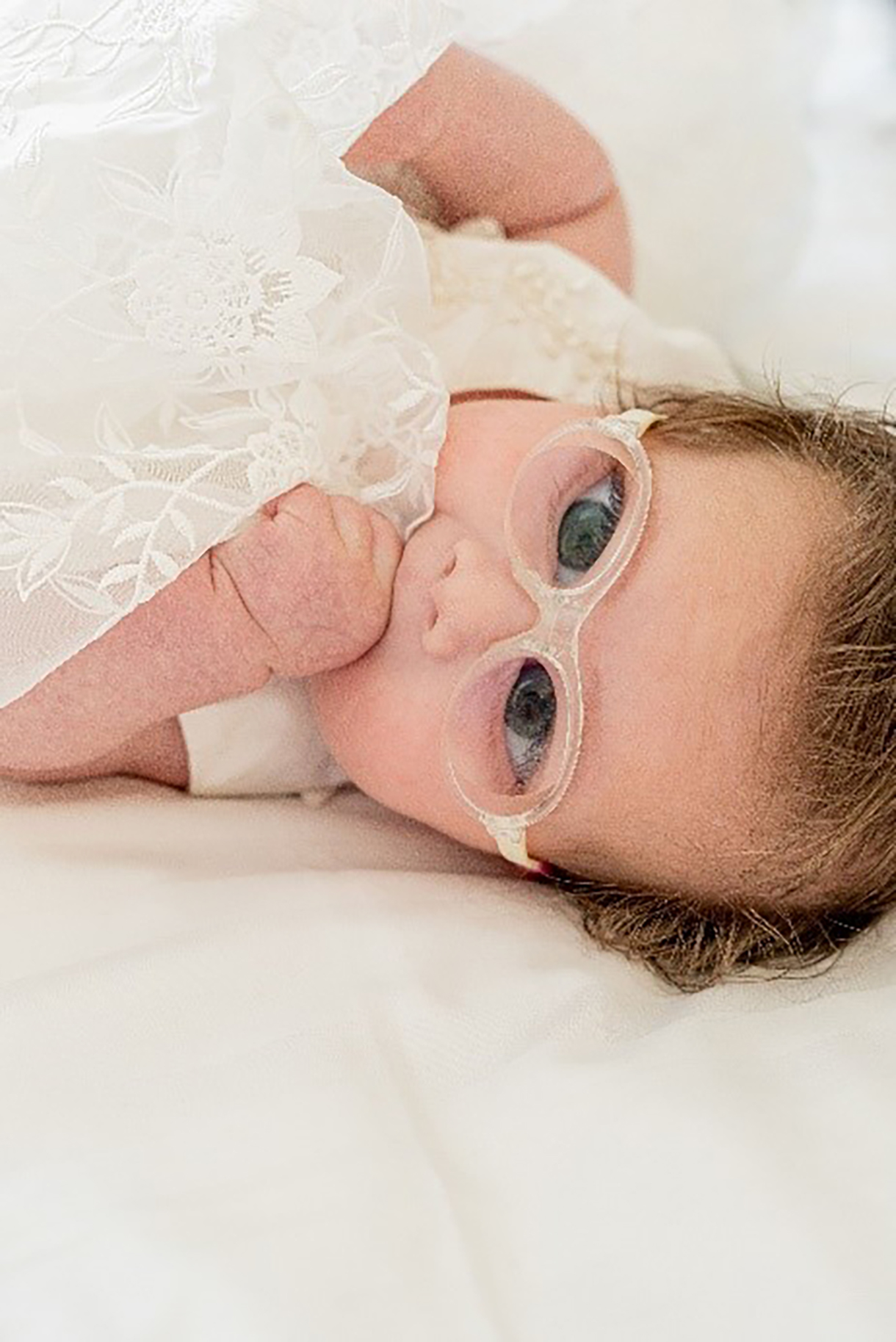
Despite the disadvantages of lenticular lenses, a good cosmetic outcome can still be achieved. The following may be helpful when dispensing lenticular lenses:
- Dispense a frame with approximately the same depth as the bowl.
- The prescription will influence the bowl size. Generally, the higher the prescription the smaller the bowl will be.
- Order surfaced lenses for the optimum cosmetic effect.
- Avoid decentring the lenses (match the frame box centre distance with patient pupillary distance).
- The pupils should be positioned on or just above the horizontal centre line.
- When dispensing lenticular bifocals, a high segment top will increase the thickness at the top rim. Dispensing a frame with an appropriate crest height will counteract this.
- Plastic frames will help to hide and support the lenses.
- Fit the glasses with as small a vertex distance as possible. This will maximise field of view, reduce spectacle magnification and retinal image size, and reduce distortion and chromatic aberrations.
- Always include a UV filter.
- Button size is usually 34mm or 38mm.
- Anti-reflection coatings will reduce surface lens reflection and improve light transmission.
- Single vision lenticulars are available in higher index materials, it is always worthwhile to contact your lens manufacturer regarding availability.
- Lenticular lenses can also act as low vision aids, increasing the magnification factor and improving the visual acuity.
Dispensing to children falls within a restricted category under the Opticians Act 1989,23 and it is clear that any child presenting with either of the conditions discussed in this article present several dispensing challenges.
Craniosynostosis requires a knowledge and understanding of anthropometric facial development as both head and facial measurements differ greatly from the age-related norms. There may also be binocular vision concerns, and surgeries to facilitate a more normal cerebral growth and development.
Congenital cataract now with the current evidenced based approach to leave very young children aphakic treating with contact lenses/glasses as IOLs do not improve visual outcomes or reduce the risk of glaucoma, means we will see more aphakic paediatric patients presenting to high street practices.
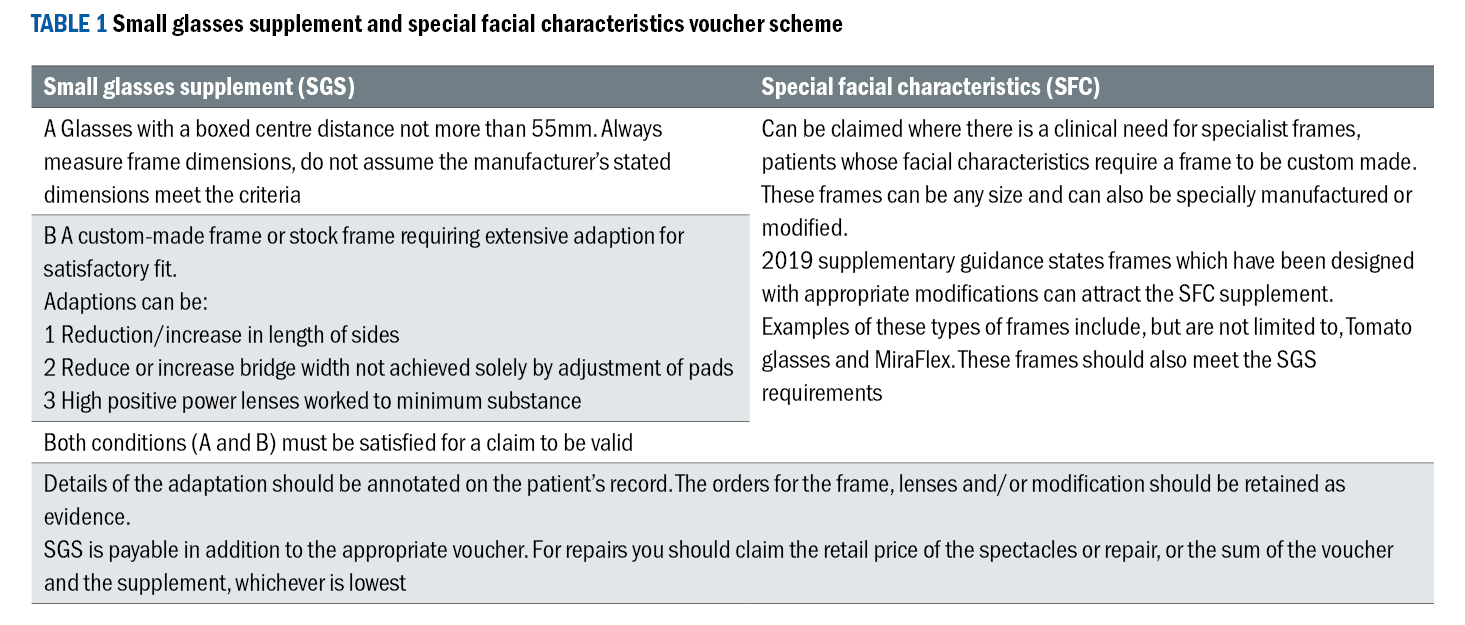
Knowledge and availability of paediatric frames, specific bridge sizes, eye sizes, side type and side lengths is essential. Understanding the relationship between facial measurements and frame measurements and what modifications can be made to a frame to improve fit as well as how the NHS small glasses supplement (SGS) and special facial characteristics (SFC) voucher scheme can help parents with support towards the cost of modifications (see table 1).24,25
 The training and skillset of the dispensing optician are in greater demand now more than ever, especially with the increased pressure on the Hospital Eye Service.
The training and skillset of the dispensing optician are in greater demand now more than ever, especially with the increased pressure on the Hospital Eye Service.
Conclusion
Offering local, easy to access, high quality dispensing care for patients with specialist dispensing needs can make a huge difference to a patient and their family. If carried out successfully, with positive, considered language, where the patient is central to any discussion and decisions, we can help to improve a child’s confidence and sense of autonomy.
This can lay a good foundation for future attitudes towards eye care, spectacle compliance and future medical appointments. Not only this, but with the appropriate frame and lenses, we can add symmetry to a patients face, minimise or accentuate certain features, and show a patient’s personality and sense of style.
These patients are likely to need long term eye care and practitioners are in a wonderful position to build a rapport with these children and help them to develop and reach their full potential.
- Jessica Gowing BSc (Hons) FBDO is a qualified dispensing optician who has worked in both private practice and a hospital setting. She is a senior dispensing optician at Great Ormond Street Hospital in London where she has been working since 2014. Gowing works as part of a multi-disciplinary team to provide a specialist service for children with a wide range of conditions and complex prescriptions and fitting requirements. Gowing also presents lectures and workshops on paediatric dispensing.
References
- Vision to Learn. UCLA Study: Impact Analysis of Vision to Learn. Available at: https://visiontolearn.org/impact/ucla-study-impact-analysis-of-vision-to-learn/ [Accessed 23rd Jan 2024].
- Thompson, A. Paediatric facial anthropometry applied to spectacle frame design. Aston University. 2021. Dissertation. https://web.archive.org/web/20220506051335id_/https://publications.aston.ac.uk/id/eprint/43749/1/THOMPSON_ALICIA_JANE_2021.pdf
- Taylor D, Hoyt G. Paediatric Opthalmology and Strabismus. London: Elsevier, 2005. 354-367.
- Duan M, Skoch J, Pan BS, Shah V. Neuro-Ophthalmological Manifestations of Craniosynostosis: Current Perspectives. Eye and Brain. 2021;13:29-40. https://doi.org/10.2147/EB.S234075
- Mathijssen IM. Guideline for Care of Patients With the Diagnoses of Craniosynostosis: Working Group on Craniosynostosis. J Craniofac Surg. 2015, 26(6): 1735-807. Available at: https://journals.lww.com/jcraniofacialsurgery/fulltext/2015/09000/guideline_for_care_of_patients_with_the_diagnoses.3.aspx
- Hinds AM, Thompson DA, Rufai SR, Weston K, et al. Visual outcomes in children with syndromic craniosynostosis: a review of 165 cases. Eye (Lond). 2021; 36 (5):1005-1011. https://doi.org/10.1038%2Fs41433-021-01458-5
- Tay T, Martin F, Rowe N, et al. Revalence and causes of visual impairment in craniosynostotic syndromes. Clin Experiment Ophthalmol. 2006; 34 (5): 434–440. https://doi.org/10.1111/j.1442-9071.2006.01242.x
- Rufai SR, Jeelani N, Bowman R, Bunce C et al. Recognition of intracranial hypertension using handheld optical coherence tomography in children (RIO Study): a diagnostic accuracy study protocol. BMJ Open. 2022; 12(1). https://bmjopen.bmj.com/content/12/1/e048745
- Brendan FJ, Jordan W, Swanson JB, Yang W, et al. Intraoperative intracranial pressure monitoring in the pediatric craniosynostosis population. J NeurosurgPediatr. 2018; 22(5): 475-480. https://doi.org/10.3171/2018.5.PEDS1876
- 10Kvarnstrom G, Jakobsson P, Lennerstrand G. Visual screening of Swedish children: An ophthalmological evaluation. 2002; 79(3): 240-244. https://doi.org/10.1034/j.1600-0420.2001.790306.x
- 11Dufier J, Vinurel M, Renier D, et al. Ophthalmologic complications of craniofacial stenoses. Apropos of 244 cases. J Fr Optalmol. 1986; 9: 273-280
- 12Tamburrini G, Calderelli M, Massimi L, Santini P, et al. Intracranial pressure monitoring in children with single suture and complex craniosynostosis: a review. Childs Nerv Syst. 2005; 21: 913-921. https://doi.org/10.1007/s00381-004-1117-x
- 13Gilbert C, Foster A. Childhood blindness in the context of VISION 2020 – The Right to Sight. Bull World Health Organ. 2001; 79(3): 227-232. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2566382/pdf/11285667.pdf
- 14Bell SJ, Oluone N, Harding P, Moosajee M, Congenital cataract: a guide and clinical management. Therapeutic Advances in Rare Disease. 2020; 1: 1-22. https://doi.org/10.1177/2633004020938061
- 15NHS England. Newborn and infant physical examination (NIPE) screening programme handbook. Available at: https://www.gov.uk/government/publications/newborn-and-infant-physical-examination-programme-handbook [Accessed 13th March 2024]
- 16Scott R, Lambert M. The timing of surgery for congenital cataracts minimising the risk of glaucoma following cataract surgery while optimising the visual outcome. Jaapos. 2016; 20(3): 191-192. https://doi.org/10.1016/j.jaapos.2016.04.003
- 17Lambert SR, Buckley EG, Drews-Botsch C, DuBois L, The infant aphakia treatment study: design and clinical measures at enrolment. Arch Ophthalmol. 2010; 128(1): 21-7. https:// doi.org/10.1001/archophthalmol.2009.350
- 18Chak M, Rahi JS, et al. Incidence of and Factors Associated with Glaucoma after Surgery for Congenital Cataract: Findings from the British Congenital Cataract Study. Ophthalmology. 2008; 115(6): 1013-1018. https://doi.org/10.1016/j.ophtha.2007.09.002
- 19DiMaggio C, Sun L, Li G. Early Childhood Exposure to Anesthesia and Riak of Developmental and Behavioural Disorders in a Sibling Birth Cohort. Anesth Analg. 2011; 113(5): 1143-1151. https://doi.org/10.1213%2FANE.0b013e3182147f42
- 20Royal College of Ophthalmologists. Paediatric Cataracts. Available at: https://www.rcophth.ac.uk/wp-content/uploads/2023/01/College-News-July-2022-FOCUS.pdf [Accessed 21st January 2024]
- 21Beaver M, Brewster J, Jones P, Keene A, et al. Babies and Young Children Book 1 Development 0-7. England: Stanley Thornes (Publishers); 1994. P.32
- 22World Health Organisation. Head Circumference for Age. Available at: https://www.who.int/tools/child-growth-standards/standards/head-circumference-for-age [Accessed 3oth January 2024]
- 23Great Britain. Opticians Act 1989: Elizabeth II. Chapter 44. London: The Stationery Office; 1989.
- 24Association of British Dispensing Opticians. Making Accurate Claims in England 2022. Available at: https://www.abdo.org.uk/wp-content/uploads/2022/05/MACE-guide-2022.pdf [Accessed 20th January 2024]
- 25NHS England. Small glasses supplement – Additional guidance. Available at: https://www.england.nhs.uk/publication/small-glasses-supplement-additional-guidance/ [Accessed 20th January 2024]
