This report explores the multifaceted applications of artificial intelligence (AI) in retinal image analysis for clinical purposes, with a primary focus on cardiovascular risk profiling and ocular disease screening. It aims to provide a brief overview of the potential of AI for clinical purposes in optometry, and primary care, highlighting some potential benefits, challenges and implications for optometrists.
The integration of AI in healthcare has partly been driven by two things: firstly, the enormous amount of data available including image data and, secondly the improved analytical ability to handle not only numerical but also image-based data. The capability to handle large data sets with multiple timepoint information may well revolutionise diagnostic capabilities and patient management.
In optometry, AI’s impact is particularly noteworthy in retinal image analysis. While fundus cameras may have not been the norm 20 years ago, most primary care optometrists would not be without them now. So why does it seem such a daunting step to consider the integration of AI in utilising the images obtained more efficiently? The answer to this question is not particularly complex.
The reason can partly be found in the fact that while we moved swiftly from ophthalmoscopy to retinal image capture, we have failed to effectively use the images beyond traditional visual inspection. While fundus photography has allowed us to store and review images, we failed to utilise their full potential including measures such as calibre assessment, lesion location/count and dimensional parameterisation.
Technological advances and AI enable us to not only use retinal images more effectively but to also integrate all other clinical information (eg concomitant disease, age, gender, ethnicity, geographic location, systemic disease, diet and more) to further enhance their clinical capabilities.
Retinal image analysis and AI: Technological foundations
When referring to retinal imaging technologies, this does not exclusively mean fundus photography but also includes optical coherence tomography (OCT), autofluorescence imaging, widefield imaging and any other form of 2D and 3D capture of retinal features. These images serve as a rich source of information for assessing ocular health and can provide information about systemic conditions, as well as their progression, over time.
AI algorithms, particularly deep learning models, have demonstrated remarkable capabilities in analysing retinal images. These algorithms can detect subtle changes (eg features and dimensions), quantify structures, and identify biomarkers associated with cardiovascular risk and ocular diseases, such as diabetic retinopathy. But how do they work? It is not simply a matter of replicating a ‘clinician’s’ workflow.
When referring to AI algorithms we often use terms such as machine learning, deep learning, convolutional neural networks, supervised and unsupervised approaches – but what are they? In brief, the term machine learning was coined in 1959 by Arthur Samuel describing a method capable of learning and extracting ‘patterns’ from data. For example, extracting retinal features based on a set of rules such as differences in colour, location and size.
Another example would be providing advice to patients based on a set of rules, which may include a list of symptoms and clinical outcome measures to choose a suitable lifestyle intervention, drug or treatment method. In fact, such a system was developed as early as 19841 and aimed at choosing the appropriate antibiotic based on preset rules, however, it was never clinically implemented.
Such systems are not new to medicine and are largely based on ‘expert knowledge’, ie the decision-making of experienced clinicians who use a set of facts and rules (eg clinical classifiers, guidelines, diagnostic cut-off values) which have been implemented and ‘learned’ by the algorithm. How does this process work?
In the case of retinal image interpretation, the machine learning approach requires a set of ‘input parameters’ such as, for example, optic nerve head (ONH) size, lesion colour, or other ‘features’ alongside a set of ‘training’ images. Identification of certain features through the algorithm will be used to classify each image.
The sensitivity and specificity of the algorithm can then be assessed against a clinician or other standard. However, this method is not without limitations, the major one being the benchmark it is measured against: a human. It will not come as a surprise that as humans we also make mistakes. If humans are set as the benchmark, it is unlikely that the algorithm will be capable of significantly outperforming us.
While this may well ensure a ‘safe’ operation, it does not facilitate identification of ‘pre-clinical’ biomarkers, which may be present (but not obvious to a human observer) prior to manifest disease/ lesion development. Deep learning and convolutional neural networks offer further insight into the data being assessed as they are able to analyse and extract information beyond serving as a mere ‘classifier’.
They train and learn beyond a set of input parameters and therefore enable us to identify ‘pre-clinical’ markers, which can be useful for treatment/lifestyle and other interventions prior to manifest disease. Primary optometric care has an important ‘gatekeeper’ role in that patients may see us more regularly than their GP.
This is particularly true for those without manifest disease or without yet recognised disease. The data to derive cardiovascular risk parameters is largely captured via history and symptoms and targeted questions about family history and current lifestyle. Retinal images and scans obtained can help refine the risk profiling and act as a powerful tool for patient communication and motivation to make healthy lifestyle choices.
Linking retinal findings to cardiovascular health
Are we already assessing the retinal microcirculation? The short answer is yes. But the reality often involves a brief rubric completion, detailing visual appearance and the arterio-venous ratio as estimated visually, not using a standardised protocol. NICE guidelines and NHS patient information on hypertension and diabetes all state the eye is an organ which can be damaged if systemic conditions are left untreated and due to disease duration.
Hence, it seems neglectful not to assess these vessels with the level of accuracy needed to provide more impactful patient advice and ability to effectively monitor disease and treatment impacts. Retinal microvascular changes have long been identified as signs of cardiovascular risk.
Prior to retinal photography, the visual grading of retinal blood vessel appearance had been suggested as a way to assess the impact of hypertension on the ocular circulation2 by assessing vessel appearance, size, reflection and shape (and consideration of hypertension status).3 Using a visual grading system is always prone to errors owing to observer experience and visualisation tools used.
With the introduction of retinal image capture, digitisation and automated analyses, large scale population studies around the world have demonstrated the potential of retinal calibre analyses when assessing hypertension, diabetes and stroke.4 The central retinal arteriolar diameter was consistently shown to be narrower than in patients with hypertension4 compared to those with normal blood pressure.
Besides showing the diagnostic and monitoring potential of retinal images, image capture also highlighted the differential impact of systemic diseases on the arterial and venous circulation and the need to look at both these factors rather than just computing the arterio-venous ratio.
This is so, because the vessel ratio may be unaltered despite changes on both the arterial and venous side. While the results for arterial narrowing and their link with hypertension and its correlation with blood pressure appears clearer, venous dilation has been shown to be linked with diabetes, diabetes duration, diabetic retinopathy, and stroke.4
Other vessel parameters such as tortuosity, density, branching angles and length to diameter ratios have also been found useful in assessing the impact of systemic disease on the eye, but a lack of standardised assessment of these parameters has made it more difficult to compare study outcomes.
The calibre assessment of central retinal artery equivalent and vein equivalent has seen more standardisation5,6 whereby a concentric ring around the optic disc is used (see figure 1). But could such calibre measurements in primary care replicate the findings from large scale research studies? Indeed, they can.
Figure 1: Showing standardised measurement zone for including the six largest arterioles and venules coursing through the measurement zone in a concentric circle around the optic disc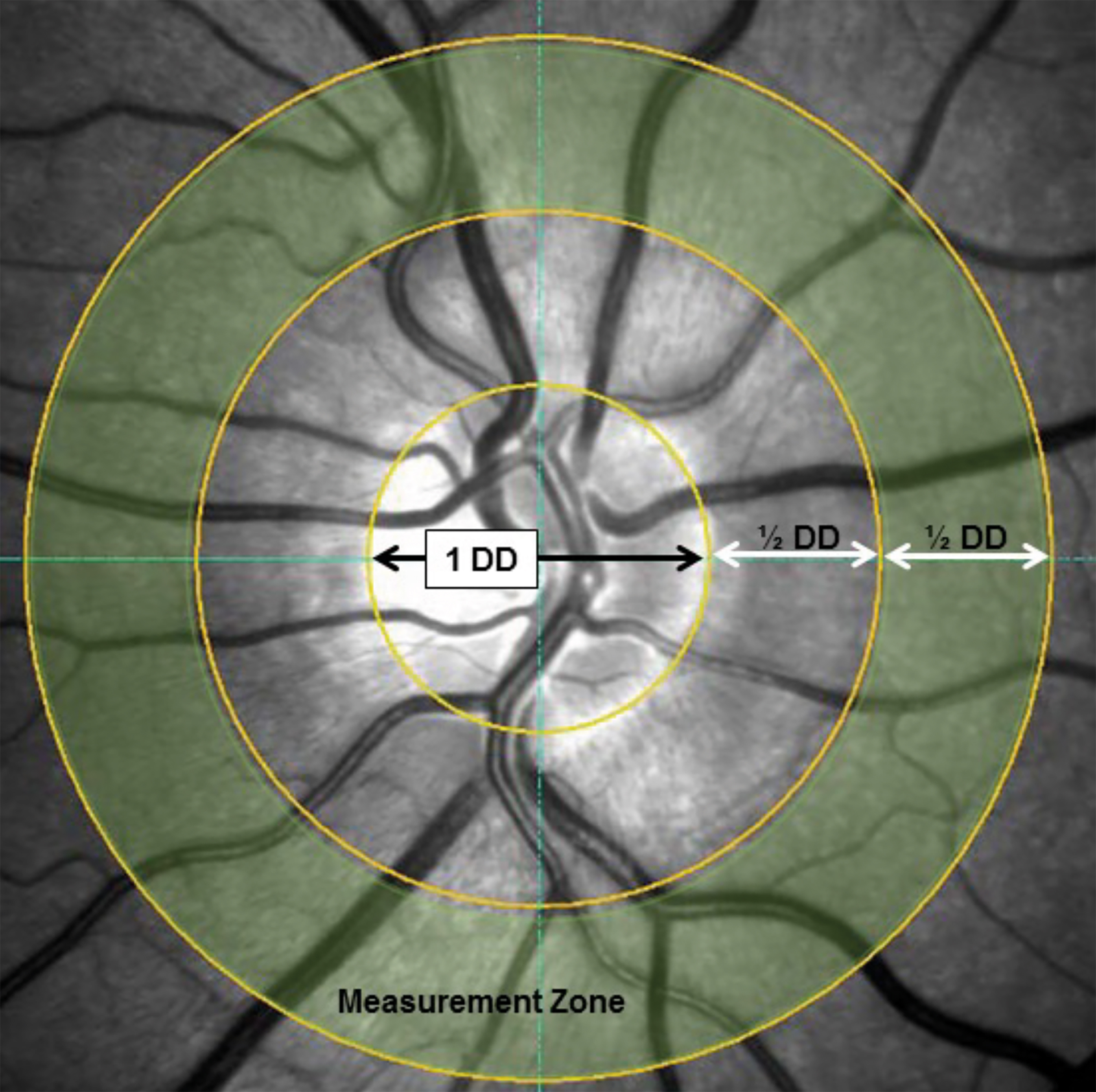
French and colleagues conducted a small-scale study using retinal images captured in primary care alongside the information to compute cardiovascular risk score, they showed that individuals with the narrowest arterial calibres did indeed have a significantly worse cardiovascular risk score in the short (10 years) and long term (30 years).7 They also showed that the those with narrower retinal arterioles were more likely to have hypertension than those without.
Larger studies using retinal images captured and deposited in the UK biobank have also shown the predictive capability of the retinal vasculature regarding cardiovascular parameters8 and cardiovascular outcomes such as myocardial infarction.8,9 Both papers suggested that implementing AI algorithms to unlock their clinical potential would be well suited for primary optometric care.
AI-driven retinal image analysis may allow optometrists to detect early signs of hypertensive retinopathy, atherosclerosis, and other vascular alterations, without the error of a visual subjective method, and contribute to a more comprehensive cardiovascular risk profile. This may allow for a more targeted referral and may help with the implementation of preventative measures to avoid further complications.
Screening for ocular diseases
While the UK has a dedicated diabetic eye screening programme (DESP), there is no defined screening programme for other ocular diseases. This is partly due to cost vs clinical benefit but also owing to the parameters assessed. For glaucoma, for instance, there are several parameters which need to be assessed and not one single test has full diagnostic power. Considering that AI may help us through identification of pre-clinical markers this may well change.
Diabetic retinopathy, a common complication of diabetes, is a leading cause of vision impairment worldwide. With increasing numbers of people suffering from diabetes in the UK and worldwide, AI may help in driving down the screening costs and making screening more accessible. Early detection and intervention are the key for preventing irreversible damage and diabetes related visual impairment.
By now there are several FDA cleared AI algorithms with high accuracy in detecting and classifying diabetic retinopathy from retinal photographs. While some rely only retinal images others already implement visual function, OCT, disease status, type, duration and medication to provide a more refined output.
When it comes to AI and other eye disease such as age-related macula degeneration (AMD), Stargardt’s disease, retinitis pigmentosa, retinopathy of prematurity, and glaucoma, there is still work to do as the disease presentation can vary and currently used diagnostic parameters are not standardised for all conditions requiring several specialist tests.
Limitations – why is AI not infallible?
There are a number of challenges when it comes to implementing AI driven systems. While they can process a vast amount of data, much faster than any human, AI systems are not without fault. One of the first hurdles when considering retinal image analysis is the image quality, which can be affected by differences in camera settings, population characteristics (eg degree of cataracts) and the training of the individual obtaining the image.
To provide some context, studies using retinal images from the UK biobank have reported that ~20% of the images were not suitable for analysis due to ‘insufficient’ image quality.8 On a positive note, the level of ungradable images from a primary care optometry sample collected in Denmark did not perform any worse than the biobank study.10
Apart from the level of cataracts there are other population-based factors to consider, such as ethnicity. For example, algorithms developed using a large Indian population may not be suitable for a Chinese or European population, the algorithm’s performance may be significantly poorer than when used on a sample similar to that used for training the algorithm.
This is unsurprising, especially considering other clinical data where we have already gained sufficient understanding of differences with respect to ethnicity, for example, BMI cut-off values used for a predominantly white population are not suitable for a South Asian population. The same is true when we consider the risk of developing diabetes mellitus and other conditions.
When assessing retinal images and extracting retinal vascular parameters there are other challenges beyond the image quality. Good contrast is essential to identity the vessel boundaries, and this can vary depending on fundus pigmentation, retinal thickness (eg choroid showing through in high myopes or areas of retinal thinning) and reflections on vessel boundaries due to inner limiting membrane/epiretinal membrane reflections, location of aneurysms and haemorrhages.
For accurate calculation of retinal vessel diameters, the correct identification and differentiation of arteries and veins is also necessary as veins are normally larger in diameter than arteries and vice versa; incorrect classification could lead to erroneous outputs. While the identification and segmentation can be supported by knowledge about size and oxygenation (darker vessels=lower saturation=veins), individual anatomical features can further impact this process in particular when vessels are in close proximity to each other (see figure 2).
Figure 2: Left image showing segmentation errors; white box: due to close proximity the artery and vein have been segmented as one vessel and labelled as vein; white arrow: the retinal artery has been labelled as retinal artery. Right image for reference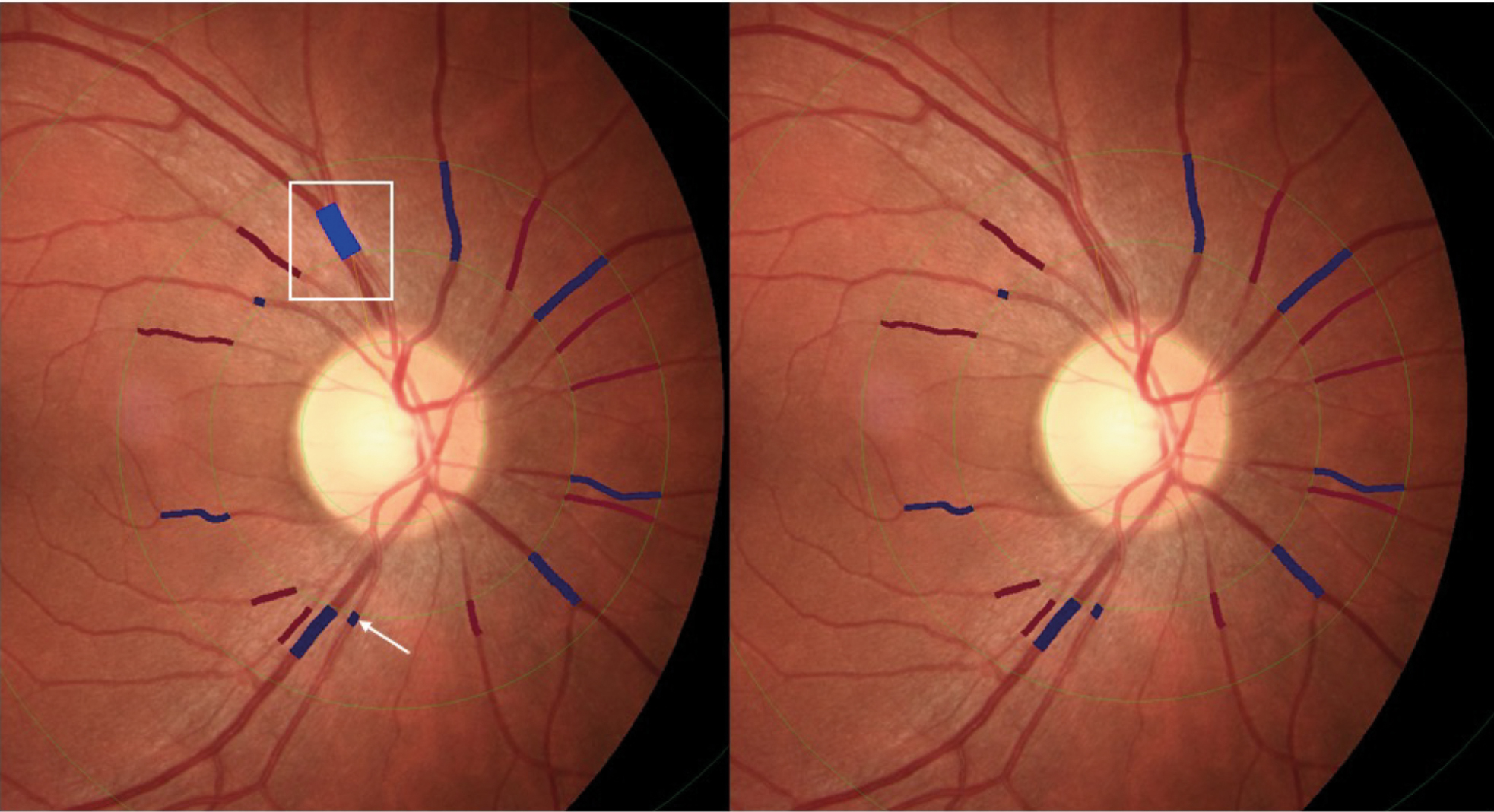
But let us assume we could tackle all these challenges, how do we, as practitioners, feel about implementing AI? How do health care providers/ insurers feel about supporting and reimburseming AI assessments? And lastly how would patients accept recommendations based on AI systems?
Implementation of AI for cardiovascular risk profiling and ocular disease screening in primary optometric care
The accuracy of AI algorithms relies on the quality and standardisation of the input data and type of AI used. Efforts to establish standardised protocols for retinal imaging and data collection are key to enhance the reliability and generalisability of AI applications in optometry. As with many other test modalities FDA approval, NICE recommendation and reimbursement options would support a smooth transition.
But beyond approvals, most clinicians would prefer accessibility to the decision-making process of the algorithm so to appreciate what, how and with what rationale the output has been produced. Acceptance from clinicians is more likely when they can follow the decision-making process. As it stands however, we are already using AI in many situations: risk profiling, likelihood ratios, data consolidation across multiple tests such as risk progression outputs and others.
Yet, the integration of AI into optometric primary care and healthcare provision raises ethical considerations surrounding patient privacy, consent and data security. Optometrists must adhere to regulatory frameworks and establish transparent communication with patients regarding the use of AI in retinal image analysis. But on the other hand, we as practitioners are still liable for our clinical decision-making, whether it is based on AI or not. This is an area where more professional guidance is necessary to clarify liability, transparency and privacy.
Integrating AI tools seamlessly into the clinical workflow is crucial for their effective utilisation as it helps overcome some of their limitations (ie limitations in database diversity). This requires widespread implementation of interoperability with electronic health records to ensure that optometrists can easily access and interpret AI-generated insights during patient consultations.
Such outputs benefit from the consideration of the entire patient health record including retinal image derived parameters and have the potential to provide primary care optometrists with comprehensive information about cardiovascular risk and ocular disease status assisting with more targeted decision-making when referring and managing patients.
A patient-centric approach utilising AI in primary care optometry can facilitate improved patient engagement in screening and risk profiling processes. Furthermore, it has the potential to foster awareness and motivation among patients to be active participants in their healthcare journey. Optometrists can use AI-generated visualisations to support patient education when explaining retinal findings and what they mean in relation to their general health.
As AI algorithms and applications improve and expand, ongoing education and training programs are essential to equip optometrists with the knowledge and skills needed to effectively integrate AI into their practice. The integration of AI in primary care optometry using retinal imaging output would be one step closer to a personalised medicine approach.
It has the potential to provide valuable insights into cardiovascular risk profiling, ocular disease progression, treatment efficacy and monitoring, ultimately facilitating an improved patient management. If optometry embraces these technological and analytical advancements this may pave the way for a more integrated and effective approach to both preventive eye care and overall health management.
- Dr Rebekka Heitmar is a reader in optometry and vision sciences, within the Department of Optometry and Vision Sciences, School of Applied Sciences at the University of Huddersfield. Dr Heitmar has a strong research background and is a past recipient of the College of Optometrists’ Neil Charman Medal for her cross-disciplinary approach to ocular haemodynamics research.
References
- Buchanan BG and Shortliffe EH. (1984) Rule-based expert systems: the Mycin experiments of the Stanford heuristic programming project. USC? Information Sciences Institute, Marina del Rey, CA 90292, USA.
- Walsh JB. Hypertensive retinopathy. Description, classification, and prognosis. Ophthalmology. 1982;89:1127–1131.
- Scheie HG. Evaluation of ophthalmoscopic changes of hyper-tension and arteriolar sclerosis. AMA Arch Ophthalmol 1953;49(2):117–138.
- French CJD and Heitmar R. Associations of retinal vessel calibre with cardiovascular disease: a systematic literature review. Optometry in Practice 2017;18(3):155-170.
- Hubbard LD, Brothers RJ, King WN et al. (1999) Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities study. Ophthalmology 106, 2269–80.
- Knudtson MD, Lee KE, Hubbard LD et al. (2003) Revised formulas for summarizing retinal vessel diameters. Curr Eye Res 27,143-9.
- French C, Cubbidge RP, Heitmar R. The application of arterio-venous ratio (AVR) cut-off values in clinic to stratify cardiovascular risk in patients. Ophthalmic Physiol Opt. 2022 Jul;42(4):666-674.
- Rudnicka AR, Welikala R, Barman S, Foster PJ, Luben R, Hayat S, Khaw KT, Whincup P, Strachan D, Owen CG. Artificial intelligence-enabled retinal vasculometry for prediction of circulatory mortality, myocardial infarction and stroke. Br J Ophthalmol. 2022 Dec;106(12):1722-1729.
- Andres Diaz-Pinto, Nishant Ravikumar, Rahman Attar, Avan Suinesiaputra, Yitian Zhao, Eylem Levelt, Erica Dall’Armellina, Marco Lorenzi, Qingyu Chen, Tiarnan D. L. Keenan, Elvira Agrón, Emily Y. Chew, Zhiyong Lu, Chris P. Gale, Richard P. Gale, Sven Plein & Alejandro F. Frangi. Predicting myocardial infarction through retinal scans and minimal personal information. Nature Machine Intelligence volume 4, pages55–61 (2022)
- Freiberg J, Welikala RA, Rovelt J, Owen CG, Rudnicka AR, Kolko M, Barman SA; FOREVER consortium. Automated analysis of vessel morphometry in retinal images from a Danish high street optician setting. PLoS One. 2023 Aug 24;18(8):e0290278.
