Cataract surgery is among the most common operations performed worldwide and while in general it is felt to be a very successful procedure, there is a known range of potential post-operative complications. In the UK, an increasing proportion of cataract post-operative evaluation is undertaken by optometrists. It is therefore vital that optometrists are familiar with the major complications of cataract procedures.
This article discusses the most common complications, with reference to the National Ophthalmology Database (NOD) Audit, undertaken and published annually by the Royal College of Ophthalmologists, based on figures for cataract surgery funded by the NHS in England and Wales.1 At present the main focus of the NOD relates to untoward surgical events, with less focus on post-operative outcomes.
What is the size and nature of the problem?
The latest NOD report details surgery of over 291,920 cataract operations conducted in 158 surgical facilities in the year 2021-22.2 Unfortunately, it does not have follow-up data on more than 62% of these, which means that the true incidence of complications remains unknown.
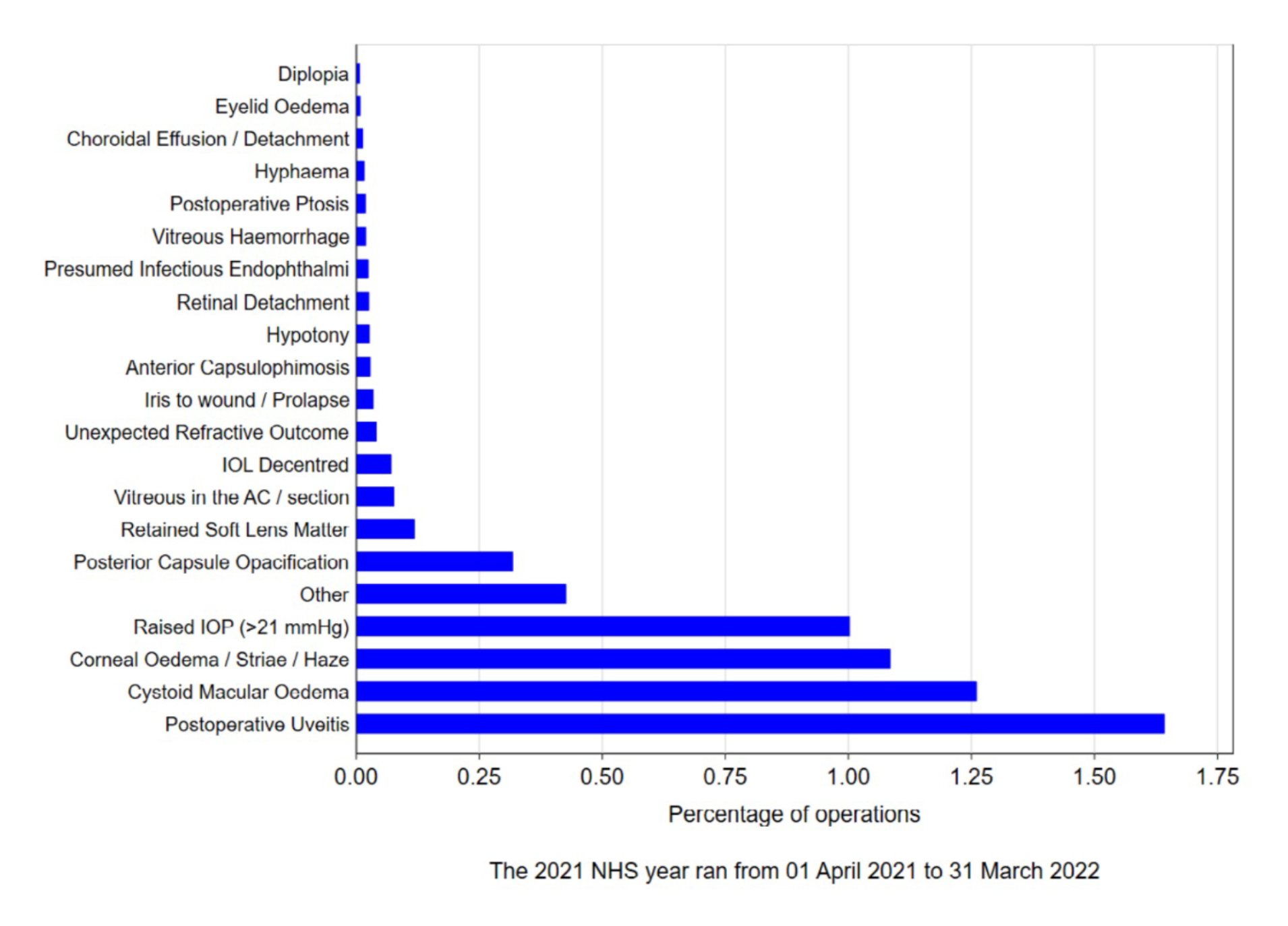
The incidence and type of post-operative complication is shown in figure 1, taken from the NOD Audit report of 2023.2 The four commonest post-operative complications were persistent uveitis, cystoid macular oedema, corneal oedema and raised intraocular pressure.
This review will examine complications in order of decreasing frequency, with a particular emphasis on these four, describing their mechanism, cause and presenting features.
Figure 1: Post-operative complications reported to the sixth National Ophthalmology Database Cataract surgery audit (reproduced with permission from the RCOphth)
Post-operative uveitis
Mechanism: The blood-aqueous barrier is maintained by tight junctions in the blood vessels of the iris, ciliary body and Schlemm’s canal; loss of this barrier allows leakage of inflammatory cells and protein (flare) into the anterior chamber.
Cataract surgery leads to release of inflammatory mediators and therefore some degree of post-operative inflammation in the anterior chamber occurs in all patients. A tapering dose of topical steroids are prescribed prophylactically, and typically inflammation resolves within one month of surgery.
White blood cells are best observed with a small bright conical section (illumination at approximately 45 degrees to observation) in a darkened room under high magnification; they resemble a beam of sunlight streaming through a room, illuminating airborne dust particles.
Anterior chamber activity can be graded according to the SUN classification;3 one or two cells in the anterior chamber of a quiet eye are not unusual at the four-week post-op check.
According to the NOD, 1.2% of patients still have uveitis at their post-op check (at four weeks). This may be asymptomatic, or present with pain, photophobia, deceased vision and patient anxiety.4 Risk factors include surgical technique, degree of iris pigmentation, diabetes,5-12 rheumatoid arthritis; or previous uveitis.13
This may cause cystoid macular oedema, posterior synechiae (with a subsequent increased risk of glaucoma14) and elevated intraocular pressure caused by inflammatory cells clogging the trabecular meshwork.
Management: After excluding local causes (retained cortical lens material or IOL malposition causing iris chafing),15 most cases of post-operative uveitis are treated in a similar way to idiopathic acute anterior uveitis. The aims are to reduce inflammation in the anterior chamber and provide symptomatic relief.
Topical corticosteroids16,17 are used to reduce inflammation, typically prescribed on a tapering dose over a six-week period starting at six times a day, the dose being tapered to prevent a rebound inflammatory response. Studies have shown that ketorolac eye drops may be as effective as prednisolone at controlling inflammation and more effective at reducing flare.16
Cyclopentolate may be a useful adjunct because it relieves pain by immobilising the iris and ciliary muscle and prevents posterior synechiae through mydriasis.18 Oral anti-inflammatories such as ibuprofen may also be used to reduce pain and inflammation if there are no contraindications.
The presence of a hypopyon implies fulminating inflammation, including possible endophthalmitis (see below) and needs immediate referral to eye casualty.
Cystoid macular oedema
Mechanism: Post-operative cystoid macula oedema (CMO), was first described in the early 1950s.19 Initially, there is macula thickening caused by leakage of fluid from dilated peri-foveal capillaries into the intra-retinal spaces, which progress onto cystoid-shaped spaces within the outer plexiform and inner nuclear layers of the retina.20
The pathogenesis of CMO is uncertain with current consensus being post-surgical inflammation as the most likely cause; arachidonic acid released from the uvea starts the inflammatory cascade and inflammatory mediators then disperse into the vitreous and disrupt the blood-retinal barrier.21
Any disruption of the ocular blood-aqueous22 and/or blood-retinal barrier23 can increase the likelihood of post-operative CMO, whether pre-operative (older age, male sex, uveitis, prostaglandin analogue use24), peri-operative (capsule rupture,25 dense cataracts,26 use of a pupil expansion device27 and iris trauma28) or post-operative (including diabetes, corneal haze26 and severe postoperative inflammation28).
The incidence of post-operative CMO after uncomplicated cataract surgery ranges from 1.17%25 to 4.2%.26 It peaks between four to six weeks post-operatively and is usually self-limiting,29 although persistent CMO lasting up to one year can occur30 and cause permanent vision loss.31
The typical clinical picture is of a patient experiencing improved vision immediately after surgery, only to notice blurriness after about four to six weeks, sometimes accompanied by mild photophobia, central scotomas and metamorphopsia.
Visual acuity is reduced with a loss of foveal reflex, macular thickening and peri-foveal yellow cystic spaces, especially with red-free illumination. If the condition persists, it can show as a lamellar macula hole, while severe CMO can also cause optic disc swelling.32
Post-operative CMO is described as ‘clinically significant’ where there is a symptomatic reduction in best corrected visual acuity (BCVA) of Snellen 6/12 (logMAR 0.3) or worse.29 Although fluorescein angiography is the gold standard for diagnosis, optical coherence tomography (OCT) is non-invasive and more commonly used.33,34
If the post-operative BCVA is reduced, or there is unplanned hyperopia, an OCT is essential as CMO may well not be seen clinically and is almost certainly under-reported. Higher risk patients may benefit from post-operative prophylactic treatment to reduce the risk, using non-steroidal anti-inflammatories (NSAIDs) in combination with topical steroids.35-37
Intravitreal anti-VEGF has also been shown to have prophylactic benefit38 and intravitreal corticosteroids have been used in refractory cases.32 Management: Typical treatment for post-operative CMO is topical ketorolac trometamol 0.5% and prednisolone acetate 1% eyedrops four times daily.
Duration of treatment is normally eight to 12 weeks, with the drops tapered off over three weeks once CMO has resolved.39
Corneal oedema/striae/haze
Mechanism: The corneal stroma and endothelium are key for corneal transparency. The stroma makes up 90% of corneal thickness40 and the lattice arrangement of its collagen fibres minimise light scatter,41 while the endothelium pumps water and ions in and out of the stroma to maintain the cornea’s state of dehydration.42
Corneal oedema is a common complication of cataract surgery,41 typically reaching a maximum three days after surgery43 and resolving within one month,43 although in some individuals it can take up to one year to clear.44
It occurs due to a breakdown in the tight junctions of the corneal endothelial cells with a resulting relative influx of water from the aqueous humour into the cornea.41 It is more common with a pre-existing reduction in endothelial cell count (as in Fuchs’, iridocorneal endothelial (ICE) syndrome, pseudoexfoliation and trauma.)41
Phacoemulsification surgery also causes a reduction in endothelial cell count, especially if excessive energy is used.41 2,500 endothelial cells/mm2 is a normal level for human adults and if the levels drop below 400-500 cells/mm2 40 corneal oedema is inevitable.45 Other risk factors include the surgeon’s experience, duration of surgery and the viscosurgical device used during surgery.41
Patients with corneal oedema typically present with pain, photophobia, watery eyes, and blurred vision.41 Slit lamp reveals central corneal haze with or without striae in Descemet’s membrane.13,41 Sometimes oedema in the subepithelial and epithelial layers causes bullae (pseudophakic bullous keratopathy).46 Pachymetry may help in confirming the diagnosis.41
Management: Prolonged use of post-operative steroid drops13 plus topical lubricants. Hypertonic sodium chloride eye drops or ointment41 can reduce oedema in early cases of bullous keratopathy47 although more advanced cases may require endothelial transplantation or even penetrating keratoplasty.48
Raised IOP (>21mmHg)
Mechanism: The reported incidence of one-day post-cataract surgery spikes in intraocular pressure (IOP) ranges from 0.31%49 to 22.4%;50 this usually reverts to normal after 24 hours.51 Pre-operative risk factors include high myopia,52 baseline high IOP53 and history of glaucoma treatment.49
Intra-operative factors include inadequate removal of viscoelastic material,54 intra-operative floppy iris syndrome55 and other peri-operative complications.50 Oral acetazolamide or topical hypotensive agents maybe prescribed in the immediate post-operative period to mitigate the risk of IOP spikes.56,57
Raised IOP post-cataract surgery can be caused by pigment dispersion following sulcus placement of a single-piece acrylic intraocular lens (IOL),58,59 pseudo-exfoliation syndrome,60 previous acute angle-closure glaucoma61 and by response to post-surgical steroid eyedrops.62-64
Although the exact cause remains unclear65 ‘high steroid-responders’ may have an IOP rise of greater than 15mmHg.66 The incidence has been reported as 18% to 36% ¬ more in those with primary open angle glaucoma and with risk factors including a family history of glaucoma, high myopia, and diabetes.65
Management: Increased IOP usually dissipates in one to three weeks once the steroid drops are stopped,67 but other causes for post-cataract ocular hypertension may require ocular hypotensive treatment to the operated eye, for several weeks or months post-surgery, or even IOL exchange surgery if complications arise from sulcus placement of single-piece acrylic IOL.68
Posterior capsular opacification (PCO)
Posterior capsular opacification (PCO) is a relatively common post-operative complication of cataract surgery in which the lens epithelial cells (LEC) that remain after cataract surgery proliferate, undergo abnormal differentiation and migrate along the posterior capsule.69,70 The NOD shows increasing rates for PCO at one, three, and five years (4.4%, 19.7% and 34.0% respectively).71
Mechanism: PCO is readily visible by retro illumination on the slit lamp in pearl or fibrous form.69,70,72 The pearls result from pre-equatorial LEC’s and the fibrous form from epithelial mesenchymal transformation of the residual anterior LEC’s.69,70 PCO can be classified from grade 1 (mild PCO) to 4 (dense PCO).73,74
Risk factors include hydrophilic IOLs, axial length >26mm, high myopia (large capsular bag), lower IOL powers, prior
vitrectomy, younger age, and female gender. Square-edged intraocular lenses (IOL), which prevent the migration of the LEC’s to the central part of the capsule, may be effective in reducing the risk.69,71
Management: PCO is treated with YAG laser capsulotomy.69,75 It is advisable to wait at least three months after cataract surgery before referral for laser.76 The laser beam is fired in short bursts to create a cruciate, circular or u-shaped central opening in the capsule.69 Risks include transiently raised IOP, anterior uveitis, CMO, lens pitting and retinal detachment.69,70
Retained soft lens matter
Mechanism: Retained lens matter results from incomplete removal of the crystalline lens during cataract surgery. Nuclear or epinuclear fragments may nestle in the anterior chamber angle, or in the vitreous cavity after posterior capsular rupture.77 They are often small, curved and translucent and may be difficult to detect even with gonioscopy.
The incidence of retained soft lens fragments ranges from 0.18% to 0.19%.78,79 Risk factors include complex cataract surgery, surgical inexperience, male sex, smoking and mature cataract.78-80 Retained lens fragments may cause raised intraocular pressure, corneal oedema, uveitis, retinal detachment, CMO and endophthalmitis,77,81-83 sometimes presenting up to one year later.84
Management: Urgent referral is indicated if the optometrist suspects retained lens matter85 and posturing may be indicated so the fragment remains visible.86 Early surgical removal of the lens fragment is usually indicated,82,83,85 typically performed by a vitreoretinal surgeon if there is posterior capsular rupture as vitrectomy may be required. Smaller fragments may be managed medically with observation or topical corticosteroids.79,82
Vitreous in the AC/Section
Mechanism: Posterior capsular rupture or zonular dehiscence during cataract surgery can allow vitreous strands to move into the anterior chamber.87 The incidence of such intraoperative vitreous ‘loss’ varies from 1% to 5%:87,88 risk factors include pseudoexfoliation,89 dense cataract, posterior polar cataract, previous ocular trauma and Marfan’s syndrome.87
Vitreous in the anterior chamber increases the risk of corneal decompensation, glaucoma, cystoid macular oedema, retinal detachment and endophthalmitis.81,87,90
Patients may complain of blurred vision, and IOP is raised in more than half of cases.89 Ten percent of cases of vitreous prolapse occur more than three months after surgery and are classified as ‘late onset’.91
Management: In the absence of concerning signs or symptoms, a routine referral should be made to ophthalmology. Treatment options include observation, Nd:YAG laser vitreolysis, IOL repositioning, anterior vitrectomy or posterior pars plana vitrectomy.91
IOL Decentred
Mechanism: An implanted intraocular lens (IOL) should be centred on the eye’s visual axis but IOLs can decentre from their initial position, with magnitude ranging from mild malposition to severe subluxation.
Dilated slit-lamp assessment may show one or both IOL haptics positioned outside the capsular bag in the case of mild IOL malposition, whereas mild subluxation usually shows lens zonules and part of the IOL-bag complex within the pupil, with possible anterior segment inflammation.89,92
Severe subluxation may result from an unnoticed posterior capsular rupture or tear allowing the IOL to move into the vitreous cavity,93 thus appearing aphakic.
Early dislocation (within three months of surgery) is usually associated with intraoperative complications93,94 whereas late dislocation is associated with progressive zonular weakness, commonly due to pseudoexfoliation, or other ophthalmic and systemic risk factors.95-97
Anterior89 and posterior95 capsulotomy reduces the risk of late dislocation, possibly due to a reduction in capsular contraction. The most common symptom of IOL decentration is polyopia (seeing multiple images) caused by the off-centre IOL optic within the pupil.
Other symptoms include reduced visual acuity, haloes, light sensitivity, ghosting and glare.93 The incidence of IOL decentration ranges from 0.05% to 3%.93,94 In a large South Korean population-based study group, the highest incidence was in males aged between 50-59 years old, possibly associated with activity-related trauma in this group.94
Management: When the optometrist sees or suspects IOL dislocation, referral to an ophthalmologist is required, usually routinely, unless there is sudden visual loss and/or inflammation. Ophthalmology treatment options include observation, surgical repositioning and removal of the IOL with vitrectomy,98 with a subsequent anterior chamber or an iris fixated/scleral-fixated posterior chamber IOL.93
Unexpected refractive outcome
Despite continued advances in surgery, residual refractive error after cataract surgery remains a primary source of dissatisfaction and litigation.99 In a 10-year study of NHS patients, postoperative refraction was within 1D of target refraction for 88% of eyes and within 0.50D for 62% of eyes.100
According to the College of Optometrists: ‘The patient’s refractive status and final visual acuity should form the basis of the data submission back to the operating centre for local and national audit. Return of post-operative data outcome continues to be crucial…'
However, refractive outcome in the NODS is only presented as 0.02% of patients having had an unexpected refractive outcome. This conflicts with other published results, where between 14%101 and 38%100 are within 0.5D of target.
The exact mechanisms for unwanted refractive outcome are complex and beyond the scope of this review.
They include errors in calculation due to inaccurate pre-operative measurements, limitations of calculation formulae, corneal ectasia102 and previous corneal refractive surgery.103 In addition, careful pre-operative discussion is necessary to understand each patient’s preferences.
Creating emmetropia in a non-dominant eye or a former myope may result in patient dissatisfaction.104 Pre-existing corneal astigmatism masked by lenticular astigmatism will become manifest following surgery;105 this effect may be reduced if the surgeon aligns their corneal incision with the axis of the astigmatism.106 Human error can result in the wrong IOL being inserted.107
Management: In the absence of asthenopia, anisometropia or patient dissatisfaction, no specific action is indicated other than to inform the operating centre so that their post-operative return to the NOD can be more accurate.
Symptomatic ‘refractive surprise’ may be managed with spectacles, contact lenses, refractive surgery, or further intraocular surgery (explantation, IOL replacement or piggy-back lens).108
Iris to wound/prolapse
Iris prolapse may be caused by raised intraocular pressure (secondary to trauma, coughing or retained viscoelastic109) coupled with inadequate wound construction or closure109,110 causing the iris to move anteriorly to touch or protrude through the corneal incision (wound).
Slit lamp examination shows a distorted iris, with iris trapped on, in, or protruding through the wound. A Seidel test is required to establish whether aqueous is still actively leaking. Iris prolapse increases the risk of endophthalmitis and epithelial ingrowth syndrome109,110 and iris to wound adhesions can lead to distortion of the pupil, anterior synechiae, corneal vascularisation, persistent anterior uveitis, and even secondary angle-closure glaucoma.111
Iris prolapse is an uncommon complication following phacoemulsification cataract surgery, with an incidence between 0.04%78- 0.1%.2 The incidence is higher in cases of manual extracapsular cataract extraction surgery,109 probably due to an increased susceptibility of the limbal incisions to raised IOP,112 whereas the more traditional scleral incisions are closer to the iris root, which makes them less susceptible to iris prolapse.110
Management: Optometrists should refer distorted pupils to ophthalmology, urgently if the iris is in contact with the wound or as a same-day emergency if the iris is protruding externally through the wound.
Ophthalmology treatment options include observation, for small iris adhesions to the wound, topical miotics in very mild cases111 or surgical repositioning of the iris and sutured wound closure.110
Anterior Capsulophimosis
Mechanism: Anterior capsular phimosis (ACP) is the fibrosis and constriction of the anterior capsule due to the proliferation of anterior capsule LECs. It occurs rapidly within six weeks of cataract surgery and slows down thereafter.113
Slit lamp assessment reveals fibrosis and wrinkling of the anterior capsule with reduced capsulorhexis diameter. Patients may be asymptomatic or describe progressive visual loss.114 ACP has an incidence of 1.4% to 5%.75
Surgical risk factors include a smaller curvilinear capsulorrhexis, hydrophobic IOLs and retained LECs.113 There is also an increased risk with weakened lens zonules ¬ pseudoexfoliation, Marfan’s syndrome and high myopia.115
Management: Urgent referral for ophthalmology management, including YAG laser anterior capsulotomy69,115,116 to disrupt the centripetal contraction forces,117 restore vision and reduce the risk of future IOL displacement: severe cases may require surgical excision.117
Hypotony
Mechanism: Ocular hypotony, (hypotension), is very low IOP averaging 5mmHg or less.118 It is a rare complication of cataract surgery, typically due to wound leak, large choroidal effusion or rhegmatogenous retinal detachment.119,120
A Seidel test should be performed to check for leaking aqueous,121 and slit lamp examination may show a shallow anterior chamber with a trembling of the IOL (pseudophacodonesis) or iris (iridodonesis) during ocular movements. Corneal oedema may be noted as a result of corneal endothelial dysfunction.122,123
Due to the reduction in axial length, fundus assessment may show radial choroidal-retinal folds, particularly around the fovea,124 and possible optic disc swelling.125 The low IOP may allow the choroidal vessels to leak or rupture thus creating a choroidal effusion or haemorrhagic detachment.126
Refractive error may show a hyperopic shift due to reduced axial length or a myopic shift if there is anterior displacement of the intra ocular lens.127
Management: A leaking wound may be surgically repaired, and any secondary complications are managed separately. Some patients can tolerate long periods of hypotony without loss of vision, yet others may develop keratopathy, maculopathy and/or choroidal effusion with a resulting loss of vision. Chronic hypotony may result in phthisis bulbi.128,129
Retinal detachment (RD)
Mechanism: The incidence of post-surgical (pseudophakic) RD has reduced with modern surgical techniques130 to approximately 0.2% within the first year,131 although the risk may remain increased for 10 years after surgery.130
It is increased by patient factors (including younger age,131,132 male sex,131,132 long axial length132,133 and high myopia131) and surgical complications (including posterior capsular rupture with vitreous loss133 or zonular dehiscence).
The pathophysiology involves vitreous changes during and after phacoemulsification, which causes a reduction in the stabilising effect of the vitreous body by the crystalline lens and posterior capsule; it also destabilises the vitreous through biochemical changes, which result in increased syneresis.134
These result in an increased incidence of PVD, retinal tears and RD. PVD occurring prior to surgery might reduce the risk of a postoperative RD.134
Management: Post-operative RDs are referred as an ophthalmic emergency and managed by a vitreoretinal specialist.
Endophthalmitis
Mechanism: Endophthalmitis is an intraocular inflammation of the vitreous and anterior chamber.15 Post-operative
endophthalmitis (POE) is categorised as ‘acute onset’ if it occurs within six weeks of surgery or ‘delayed onset’ if it occurs after six weeks.15 Acute POE represents 70%135 of cases of POE and is one of the most serious complications of cataract surgery, often resulting in severe visual impairment.136
Endophthalmitis must be considered in the differential diagnosis of post-operative anterior uveitis or ptosis with pain.
Symptoms include visual loss, pain, redness, and photophobia. Signs of POE include eyelid oedema, conjunctival hyperaemia, corneal haze, cells and flare in AC (hypopyon if severe) and possibly a reduced fundus view due to vitritis.137,138
Upper lid swelling is one of the most suggestive signs of endophthalmitis, especially when accompanied by rapidly progressing severe inflammation and reduced vision. Delayed onset POE has similar but more mild signs and symptoms: it is often less painful and by definition has a delayed onset, with a low-grade but progressive uveitis with only mild vitreous activity.15
POE is a rare complication of cataract surgery in the UK, with an incidence of 0.03% to 0.14%.139,140 POE is usually infective and is often caused by Staphylococci (acute onset) or Propionibacterium acnes (delayed-onset),141,142 which originate from surgical instruments, irrigating fluids or accidental contact with the external ocular surfaces.15
Surgical risk factors include longer operating times, silicone intraocular lenses, posterior capsular rupture, wound leakage and a self-sealing clear corneal incision.136 Ophthalmic conditions such as blepharitis also increase the risk of POE so these should be managed before surgery.140,143 Systemic conditions with reduced resistance to infections also increase the risk of POE.
Perioperative intracameral antibiotics such as cefuroxime lower the risk of POE144-147 and postoperative antibiotic drops such as levofloxacin are typically given to further reduce risk.144
Management: Acute POE is an ophthalmic emergency requiring immediate referral to eye casualty and usually hospital admission.138 Vitreous and/or aqueous culture (and possibly PCR testing) are used to identify the causative organism.148
Treatment includes antibiotics and steroids (topical, intravitreal and systemic) and surgery may be required to remove the IOL, capsule and vitreous.138 Chronic POE is often managed with topical steroid and antibiotics.137
Vitreous haemorrhage
Mechanism: Extravasated blood in the vitreous cavity149 can be associated with a myriad of conditions, such as diabetic retinopathy, vasculitis, choroidal neovascular membrane, hypertension, trauma or valsalva.150,151
It is a rare complication of cataract surgery but can result from vitreous traction, a PVD and/or retinal break which may disrupt retinal vessels directly.150 In complicated surgery, haemorrhage may extend into the vitreous from any adjacent intra-ocular structure,152 after trauma to the iris or ciliary body.
Clinical examination typically reveals reduced VA152 and red blood cells in the vitreous cavity.150 Symptoms may include floaters, haziness, shadows, seeing a red hue and photopsia.150 The clearance of blood from the vitreous chamber happens slowly, at a rate of 1% per day,153 although resolution is faster if the vitreous is liquified or absent.
Management: Refer to secondary care as an emergency, primarily to manage a possible retinal tear or detachment. Depending on the cause ophthalmic management includes152 laser photocoagulation, cryotherapy, intra-vitreal anti-VEGF injections and pars-plana vitrectomy.154
Ptosis
Mechanism: Ptosis following cataract surgery occurs in up to 20% of patients and is due to disinsertion of the levator aponeurosis (LPA) from its attachment at the superior and anterior part of the tarsal plate in the upper eyelid.155,156
This may be explained by the anatomical ‘stress’ caused by the constriction of the levator muscle (due to the eyelid speculum) while the superior rectus, which it is closely associated with, is affected by inferior rotation of the globe during surgery.
Patients often report reduced vision in the superior field and ‘tired’ eyes.157 The causes of LPA disinsertion are multifactorial and the evidence is inconclusive but factors such as the use of an eyelid speculum, bridle suture, post-operative eyelid oedema, retrobulbar anaesthesia and longer surgery time may contribute.155-167
Management: Post operative ptosis is likely to self-resolve within six months, but may take up to a year.168-170 Ptosis that persists for six months or more after cataract surgery and interferes with central vision may require surgical correction depending upon severity.157,169,171
Choroidal effusion/detachment
Mechanism: A choroidal effusion is an abnormal accumulation of fluid between the choroid and sclera (the suprachoroidal space), often as a result of hypotony.172 Other risk factors include myopia, anti-coagulation therapy, valsalva and old age.173 Choroidal effusions may result from transudation of serum into the suprachoroidal space (serous effusion), potentially causing anterior displacement of the lens-iris diaphragm and a resultant myopic shift.174
‘Haemorrhagic’ choroidal effusion is a rare but devastating complication of ophthalmic surgery, with rapid bleeding from ruptured choroidal vessels into the suprachoroidal space, either acutely during surgery or in the days following surgery,175,176 typically with severe pain and a marked reduction in acuity.174,177
Modern surgical techniques with small incisions185 have significantly reduced the incidence of acute choroidal effusions177,178 to 0.1%.179 On clinical examination choroidal effusions appear elevated in a four-lobed presentation because of the firm attachment of the choroid to the vortex veins. B-scan echography is used to differentiate choroidal effusion from retinal detachment.180
Management: Intraoperatively, acute choroidal effusions can precede an expulsive haemorrhage, which may be prevented or limited by immediate closure of the incision.181,182 Small effusions may be observed as they often resolve post-operatively183,184 as the IOP increases to normal levels.174 Larger effusions may require surgical drainage or treatment with topical and oral steroids.174
Eyelid oedema
Mechanism: Acute post-operative eyelid oedema is rare following cataract surgery.
It can be caused by sutures rubbing on the tarsal conjunctiva (typically used for a large corneal incision), an underlying post-operative uveitis, eyelid compression by the lid speculum against the superior orbital rim, allergic reaction to post-operative eye drops or local trauma caused by removing the sticky surgical drapes from an area of blepharochalasis or sensitive eyelid skin.
Potential causes of eyelid oedema should be identified and managed prior to cataract surgery.186
Management: The oedema is typically transient, and resolution is aided by suture removal or by using post-operative corticosteroid and non-steroid inflammatory eye drops.159,166,169,187 Eyelid oedema accompanied by pain should alert the clinician to possible endophthalmitis.
Diplopia
Mechanism: Diplopia following cataract surgery is rare, with incidence ranging from 0.093%-0.99%.188 Depending on the cause it may resolve spontaneously within a number of days or weeks.189
Monocular diplopia is typically caused by a subluxed/decentred IOL,190,191 macula disease (eg AMD, post-op CMO, ERM),188-190,192 induced astigmatism,190,192 corneal oedema/scarring190,192 or pupillary deformation.189,191,192
Binocular diplopia is more common than monocular diplopia; its causes are considered in table 1.190
Table 1: Potential causes of post operative binocular diplopia (in order of decreasing frequency)

Management: Patients should be referred back for ophthalmology management (acutely if nerve palsy or lens subluxation is suspected). Subsequent management depends on the cause, including further surgery such as IOL repositioning, IOL exchange or piggyback IOL implantation.188
A cohort of patients are observed for a few months to see if they regain fusional ability.188 This may be supplemented with an updated spectacle lens correction190,191 and orthoptic exercises to improve convergence190 and fusion.188
Prismatic correction is commonly used188,190 in Fresnel form until the deviation has been stable for three months; prisms may also be used as a temporary measure before strabismus surgery.188
Botulinum toxin188-190 has been used successfully as an alternative to prisms and surgery. For incomitant deviations, strabismus surgery with adjustable sutures is the mainstay of treatment, once the deviation is stable.188-190
Summary
This review describes the common major complications of cataract surgery that are likely to be encountered by optometrists undertaking post-op evaluations, as published in the NOD.
While the most important single measurement at the post-surgery examination remains the visual acuity, it is important to check for all of these conditions in every patient.
Not only does this promote better patient care in a timely fashion, but it will increase the accuracy of future audits of post-cataract surgery complications.
- Christian Dutton, MScClinOpt FCOptom DipTp(IP) Higher Cert Glauc, is a Specsavers clinical performance consultant supporting community practices and clinicians across the South of England. He has a held a variety of posts as a clinical, organisational and volunteer optometrist and was awarded fellowship of the College of Optometrists for ‘promoting good practice locally and internationally’.
- Simon Hardman-Lea, MBBS MA FRCS FRCOphth, is a consultant ophthalmologist and clinical medical director for Evolutio Care Innovations. He has specialist interests in ocular surface disease and neuro-ophthalmology and in the development of community ophthalmology and
telemedicine. - Jasraj Singh Bhangra, BSc MCOptom DipTp(IP), is a clinical optometrist who works for Evolutio Ophthalmology and Western Eye Hospital and occasionally works as a locum optometrist.
- Jo-Sheetal Thethy, BSc(Hons.) MCOptom DipTp(IP), is a specialist optometrist based at Optegra Eye Hospital in North London. She has a special interest in managing post operative cataract complications and performing YAG capsulotomies. She has supported eye casualty clinics with Evolutio Care Innovation and delivered AMD injections at the Prince Charles Eye Unit for the Royal Berkshire Hospital. She enjoys training colleagues and has volunteered with various UK Eye Charities carrying out overseas projects in Sri Lanka, Zambia and Ethiopia.
- Kin Mun Tam, MSc BSc MCOptom DipTp(IP) DipGlauc Prof Cert Med Ret Prof Cert LV, is a specialist clinical optometrist for Evolutio community ophthalmology, and also practices in secondary care. He has a special interest in glaucoma.
References
- The Royal College of Ophthalmologists (2016) ‘National Ophthalmology Database Audit. Year 1 Annual Report – Piloting of the National Ophthalmology Database Audit Methodology.’ Available at: https://www.nodaudit.org.uk/u/docs/20/muedbbgqwv/NOD%20Audit%20Annual%20Report%202016.pdf (Accessed 21 November 2023).
- The Royal College of Ophthalmologists (2023) ‘National Ophthalmology Database Audit. Year 7 Annual Report – The Sixth Prospective Report of the National Ophthalmology Database Audit National Cataract Audit’ Available at: https://nodaudit.org.uk/sites/default/files/2023-08/NOD%20Cataract%20Audit%20Full%20Annual%20Report%202023.pdf (Accessed 27 January 2024).
- Jabs, D. Nussenblatt, R. Rosenbaum, J. & & Standardization of Uveitis Nomenclature (SUN) Working Group (2005) ‘Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop,’ American Journal of Ophthalmology, 140(3), pp. 509-516. Available at: https://pubmed.ncbi.nlm.nih.gov/16196117/ (Accessed 20 October 2023).
- Mohammadpour, M. Jafarinasab, M. Javadi, M. (2007) ‘Outcomes of acute postoperative inflammation after cataract surgery,’ European Journal of Ophthalmology, 17(1), pp. 20-28. Available at: https://pubmed.ncbi.nlm.nih.gov/17294379/ (Accessed 21 November 2023).
- Wellen, K. Hotamisligil, G. (2005) ‘Inflammation, stress, and diabetes,’ Journal of Clinical Investigation, 115(5), pp. 1111–1119. Available at: https://pubmed.ncbi.nlm.nih.gov/15864338/ (Accessed 21 November 2023).
- Doxey, D. Nares, S. Park, B. Trieu, C. Cutler, C. Iacopino, A. (1998) ‘Diabetes-induced impairment of macrophage cytokine release in a rat model:potential role of serum lipids,’ Life Sciences, 63(13): pp. 1127-1136. Available at: https://pubmed.ncbi.nlm.nih.gov/9763208/ (Accessed 21 November 2023).
- Fortes, Z. Farsky, S. Oliveira, M. Garcia-Leme, J. (1991) ‘Direct vital microscopic study of defective leukocyte-endothelial interaction in diabetes mellitus,’ Diabetes, 40(10), pp. 1267-1273. Available at: https://pubmed.ncbi.nlm.nih.gov/1936589/ (Accessed 21 November 2023).
- Pereira, M. Sannomiya, P. Leme, J. (1987) ‘Inhibition of leukocyte chemotaxis by factor in alloxan-induced diabetic rat plasma,’ Diabetes, 36(11), pp. 1307-1314. Available at: https://pubmed.ncbi.nlm.nih.gov/3666321/ (Accessed 21 November 2023).
- Sannomiya, P. Pereira, M. Garcia-Leme, J. (1990) ‘Inhibition of leukocyte chemotaxis by serum factor in diabetes mellitus: selective depression of cell responses mediated by complement-derived chemoattractants,’ Agents and Actions, 30(3), pp. 369-376. Available at: https://pubmed.ncbi.nlm.nih.gov/2167002/ (Accessed 21 November 2023).
- Takamura, Y. Tomomatsu, T. Arimura, S. Tomomatsu, Y. Matsumura, T. Takihara, Y. Inatani, M. (2013) ‘Anterior capsule contraction and flare intensity in the early stages after cataract surgery in eyes with diabetic retinopathy,’ Journal of Cataract & Refractive Surgery, 39(5), pp. 716–721. Available at: https://pubmed.ncbi.nlm.nih.gov/23541898/ (Accessed 21 November 2023).
- Ino-ue, M. Azumi, A. Shirabe, H. Tsukahara, Y. Yamamoto, M. (1994) ‘Laser flare intensity in diabetics: correlation with retinopathy and aqueous protein concentration,’ British Journal of Ophthalmology, 78(9), pp. 694–697. Available at: https://bjo.bmj.com/content/78/9/694 (Accessed 21 November 2023).
- Liu, Y. Luo, L. He, M. Liu, X. (2004) ‘Disorders of the blood-aqueous barrier after phacoemulsification in diabetic patients,’ Eye, 18(9), pp. 900–904. Available at: https://www.nature.com/articles/6701349 (Accessed 21 November 2023).
- Tsaousis, K. Panagiotou, D. Kostopoulou, E. Vlatsios, V. Stampouli, D. (2016) ‘Corneal oedema after phacoemulsification in the early postoperative period: A qualitative comparative case-control study between diabetics and non-diabetics,’ Annals of Medicine and Surgery, 5, pp. 67-71. Available at: https://pubmed.ncbi.nlm.nih.gov/26865977/ (Accessed 21 November 2023).
- Edelsten, C. Lee, V. Bentley, C. Kanski, J. Graham, E. (2002) ‘An evaluation of baseline risk factors predicting severity in juvenile idiopathic associated uveitis and other chronic anterior uveitis in early childhood,’ British Journal of Ophthalmology, 86(1), pp. 51-56. Available at: https://pubmed.ncbi.nlm.nih.gov/11801504/ (Accessed 21 November 2023).
- Maalouf, F. Abdulaal, M. Hamam, R. (2012) ‘Chronic postoperative endophthalmitis: a review of clinical characteristics, microbiology, treatment strategies, and outcomes,’ International Journal of Inflammation, 2012: 313248. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3328945/ (Accessed 21 November 2023).
- Hirneiss, C. Neubauer, A. Kampik, A. Schönfeld, C. (2005) ‘Comparison of Prenisolone 1%, Rimexolone 1% and Ketorolac Tromethamine 0.5% after cataract extraction –A prospective, ramdomised, double-masked study,’ Graefe’s Archive for Clinical and Experimental Ophthalmology, 243(8), pp. 768-773. Available at: https://pubmed.ncbi.nlm.nih.gov/15756571/ (Accessed 21 November 2023).
- The College of Optometrists (2021) ‘Clinical Management Guidelines - Uveitis (anterior)’. Available at: https://www.college-optometrists.org/clinical-guidance/clinical-management-guidelines/uveitis_anterior (Accessed 21 November 2023).
- Agrawal, R. Murthy, S. Sangwan, V. Biswas, J. (2010) ‘Current approach in diagnosis and management of anterior uveitis,’ Indian Journal of Ophthalmology, 58(1), pp.11-19. Available at: https://pubmed.ncbi.nlm.nih.gov/20029142/ (Accessed 21 November 2023).
- Irvine, S. (1953) ‘A newly defined vitreous syndrome following cataract surgery,’ American Journal of Ophthalmology, 36(5), pp. 599–619. Available at: https://pubmed.ncbi.nlm.nih.gov/13040458/ (Accessed 21 November 2023).
- Flach, A. (1998) ‘The incidence, pathogenesis and treatment of cystoid macular edema following cataract surgery,’ Transactions of the American Ophthalmological Society, 96, pp.557. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1298410/ (Accessed 21 November 2023).
- Grzybowski, A. Sikorski, B. Ascaso, F. Huerva, V. (2016) ‘Pseudophakic cystoid macular edema: update 2016,’ Clinical Interventions in Aging, 11, pp. 1221. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5025006/ (Accessed 21 November 2023).
- Ursell, P., Spalton, D. Whitcup, S. Nussenblatt, R. (1999) ‘Cystoid macular edema after phacoemulsification: relationship to blood-aqueous barrier damage and visual acuity,’ Journal of Cataract & Refractive Surgery, 25(11), pp. 1492–1497. Available at: https://pubmed.ncbi.nlm.nih.gov/10569164/ (Accessed 21 November 2023).
- Fleissig, E. Cohen, S. Iglicki, M. Goldstein, M. Zur, D. (2018) ‘Changes in choroidal thickness in clinically significant pseudophakic cystoid macular edema,’ Retina, 38(8), pp.1629–1635. Available at: https://www.researchgate.net/publication/317961052 (Accessed 21 November 2023).
- McCafferty, S. Harris, A. Kew, C. Kassm, T. Lane, L. Levine, J. Raven, M. (2017) ‘Pseudophakic cystoid macular edema prevention and risk factors,’ BMC Ophthalmology, 17(1), pp. 16. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5319126/ (Accessed 21 November 2023).
- Chu, C. Johnston, R. Buscombe, C. Sallam, A. Mohamed, Q. Yang, Y. (2016) ‘Risk Factors and Incidence of Macular Edema after Cataract Surgery - A Database Study of 81984 Eyes,’ Ophthalmology, 123(2), pp. 316–323. Available at: http://www.aaojournal.org/article/S016164201501146X/fulltext (Accessed 21 November 2023).
- Seth, I. Bulloch, G. Tan, A. Thornell, E. Agarwal, S. (2022) ‘Incidence of Pseudophakic Cystoid Macular Oedema Post-Cataract Surgery in Illawarra Shoalhaven Local Health District, Australia,’ Biomedicine Hub, 7(1), pp. 1–10. Available at: https://www.karger.com/Article/FullText/521053 (Accessed 21 November 2023).
- Taipale, C. Holmström, E. Ilveskoski, L. Tuuminen, R. (2019) ‘Incidence of pseudophakic cystoid macular edema in eyes with and without pupil expansion device,’ Acta Ophthalmologica, 97(7), pp. 688–694. Available at: https://onlinelibrary.wiley.com/doi/full/10.1111/aos.14007 (Accessed 21 November 2023).
- Gulkilik, G. Kocabora, S. Taskapili, M. Engin, G. (2006) ‘Cystoid macular edema after phacoemulsification: risk factors and effect on visual acuity,’ Canadian Journal of Ophthalmology, 41(6), pp. 699–703. Available at: https://pubmed.ncbi.nlm.nih.gov/17224950/ (Accessed 21 November 2023).
- Erikitola, O. Siempis, T. Foot, B. Lockington, D. (2020) ‘The incidence and management of persistent cystoid macular oedema following uncomplicated cataract surgery—a Scottish Ophthalmological Surveillance Unit study,’ Eye, 35(2), pp. 584–591. Available at: https://www.nature.com/articles/s41433-020-0908-y (Accessed 21 November 2023).
- Terveen, D. Berdahl, J. Dhariwal, M. Meng, Q. (2022) ‘Real-World Cataract Surgery Complications and Secondary Interventions Incidence Rates: An Analysis of US Medicare Claims Database,’ Journal of Ophthalmology, 2022: 8653476. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9007656/ (Accessed 21 November 2023).
- Yüksel, B. Karti, Ö. Kusbeci, T. (2017) ‘Topical nepafenac for prevention of post-cataract surgery macular edema in diabetic patients: patient selection and perspectives,’ Clinical Ophthalmology, 11, pp. 2183–2190. Available at: https://www.dovepress.com/topical-nepafenac-for-prevention-of-post-cataract-surgery-macular-edem-peer-reviewed-fulltext-article-OPTH (Accessed 21 November 2023).
- Ugarte, M. (2021) ‘Pseudophakic Cystoid Macular Oedema,’ Cataract Surgery, Cham: Springer International Publishing, pp. 173–89. Available at: http://link.springer.com/10.1007/978-3-030-38234-6_10 (Accessed 21 November 2023).
- Kim, S. Bressler, N. (2009) ‘Optical coherence tomography and cataract surgery,’ Current Opinion in Ophthalmology, 20(1), pp. 46–51. Available at: https://journals.lww.com/co-ophthalmology/Abstract/2009/01000/Optical_coherence_tomography_and_cataract_surgery.10.aspx (Accessed 21 November 2023).
- Jindal, A. Ctori, I, Fidalgo, B. Dabasia, P. Balaskas, K. Lawrenson, J. (2019) ‘Impact of optical coherence tomography on diagnostic decision-making by UK community optometrists: a clinical vignette study,’ Ophthalmic and Physiological Optics, 39(3), pp. 205–215. Available at: https://onlinelibrary.wiley.com/doi/full/10.1111/opo.12613 (Accessed 21 November 2023).
- Henderson, B. Kim, J. Ament, C. Ferrufino-Ponce, Z. Grabowska, A. Cremers, S. (2007) ‘Clinical pseudophakic cystoid macular edema,’ Journal of Cataract & Refractive Surgery, 33(9), pp. 1550–1558. Available at: https://journals.lww.com/02158034-200709000-00019 (Accessed 21 November 2023).
- Kessel, L. Tendal, B. Jørgensen, K. Erngaard, D. Flesner, P. Andresen, J. Hjortdal, J. (2014) ‘Post-cataract prevention of inflammation and macular edema by steroid and nonsteroidal anti-inflammatory eye drops: A systematic review,’ Ophthalmology, 121(10), pp. 1915–1924. Available at: http://www.aaojournal.org/article/S0161642014003893/fulltext (Accessed 21 November 2023).
- Juthani, V. Clearfield, E. Chuck, R. (2017) ‘Non-steroidal anti-inflammatory drugs versus corticosteroids for controlling inflammation after uncomplicated cataract surgery,’ The Cochrane Database of Systematic Reviews, vol. 7, 7 CD010516. Available at: https://pubmed.ncbi.nlm.nih.gov/28670710/ (Accessed 21 November 2023).
- Zhang, R. Dong, L. Yang, Q. Liu, Y. Li, H. Zhou, W. Wu, H. Li, Y. Yu, C. Wei, W. (2022) ‘Prophylactic interventions for preventing macular edema after cataract surgery in patients with diabetes: A Bayesian network meta-analysis of randomized controlled trials,’ EClinicalMedicine, 49, 101463. Available at: https://www.thelancet.com/journals/eclinm/article/PIIS2589-5370(22)00193-6/fulltext (Accessed 21 November 2023).
- Heier, J. Topping, T. Baumann, W. Dirks, M. Chern, S. (2000) ‘Ketorolac versus prednisolone versus combination therapy in the treatment of acute pseudophakic cystoid macular edema,’ Ophthalmology, 107(11), pp. 2034–2038. Available at: https://www.aaojournal.org/article/S0161-6420(00)00365-1/fulltext (Accessed 3 November 2023).
- Meek, K. Knupp, C. (2015) ‘Corneal structure and transparency,’ Progress in Retinal and Eye Research, 49, pp. 1-16. Available at: https://pubmed.ncbi.nlm.nih.gov/26145225/ (Accessed 21 November 2023).
- Sharma, N. Singhal, D. Nair, S. Sahay, P. Sreeshankar, S. Maharana, P. (2017) ‘Corneal edema after phacoemulsification,’ Indian Journal of Ophthalmology, 65(12), pp. 1381-1389. Available at: https://journals.lww.com/ijo/Fulltext/2017/65120/Corneal_edema_after_phacoemulsification.17.aspx (Accessed 21 November 2023).
- Hassell, J. Birk, D. (2010) ‘The molecular basis of corneal transparency,’ Experimental Eye Research, 91(3), pp. 326-335. Available at: https://pubmed.ncbi.nlm.nih.gov/20599432/ (Accessed 21 November 2023).
- Tragakis, M. Economidis, I. Athanassiades, P. Pollalis, S. (1977) ‘Corneal thickness after cataract surgery,’ Transactions of the ophthalmological societies of the United Kingdom, 97(1), pp. 114-116. Available at: https://pubmed.ncbi.nlm.nih.gov/271374/ (Accessed 21 November 2023).
- Díez-Ajenjo, M. Luque-Cobija, M. Peris-Martínez, C. Ortí-Navarro, S. García-Domene, M. (2022) ‘Refractive changes and visual quality in patients with corneal edema after cataract surgery,’ BMC Ophthalmology, 22(1), pp. 242. Available at: https://bmcophthalmol.biomedcentral.com/articles/10.1186/s12886-022-02452-5 (Accessed 21 November 2023).
- Vaiciuliene, R. Rylskyte, N. Baguzyte, G. Jasinskas, V. (2022) ‘Risk factors for fluctuations in corneal endothelial cell density (Review),’ Experimental and Therapeutic Medicine, 23(2), pp. 129. Available at: https://pubmed.ncbi.nlm.nih.gov/34970352/ (Accessed 21 November 2023).
- Pricopie, S. Istrate, S. Voinea, L. Leasu, C. Paun, V. Radu, C. (2017) ‘Pseudophakic bullous keratopathy,’ Romanian Journal of Ophthalmology, 61(2), pp. 90-94. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5710027/ (Accessed 21 November 2023).
- Knezović, I. Dekaris, I. Gabrić, N. Cerovski, J. Barisic, A. Bosnar, D. Rastegorac, P Parac, A (2006) ‘Therapeutic efficacy of 5% NaCl hypertonic solution in patients with bullous keratopathy,’ Collegium Antropologicum, 30(2), pp. 405-408. Available at: https://pubmed.ncbi.nlm.nih.gov/16848159/ (Accessed 21 November 2023).
- Claesson, M. Armitage, W. Stenevi, U. (2009) ‘Corneal oedema after cataract surgery: predisposing factors and corneal graft outcome,’ Acta Ophthalmologica, 87(2), pp. 154-159. Available at: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1755-3768.2008.01180.x (Accessed 21 November 2023).
- Oku, H. Mori, K. Watanabe, M. Aoki, T. Wakimasu, K. Yamamura, K. Yamasaki, T. Yoshii, K. Sotozono, C. Kinoshita, S. (2022) ‘Risk factors for intraocular pressure elevation during the early period post cataract surgery,’ Japanese Journal of Ophthalmology, 66, pp. 373–378. Available at: https://link.springer.com/article/10.1007/s10384-022-00918-z (Accessed 21 November 2023).
- Annam, K. Chen, A. Lee, I. Paul, A. Rivera, J. Greenberg, P. (2018) ‘Risk Factors for Early Intraocular Pressure Elevation After Cataract Surgery in a Cohort of United States Veterans,’ Military Medicine, 183(9-10):e427-e433. Available at: https://pubmed.ncbi.nlm.nih.gov/29425312/ (Accessed 21 November 2023).
- Levkovitch-Verbin, H. Habot-Wilner, Z. Burla, N. Melamed, S. Goldenfeld, M. Bar-Sela, S. Sachs, D. (2008) ‘Intraocular pressure elevation within the first 24 hours after cataract surgery in patients with glaucoma or exfoliation syndrome,’ Ophthalmology, 115(1), pp. 104–108. Available at: https://pubmed.ncbi.nlm.nih.gov/17561259/ (Accessed 21 November 2023).
- Zhu, X. Qi, J. He, W. Zhang, S. Zhang, K. Lu, Q. Lu, Y. (2020) ‘Early transient intraocular pressure spike after cataract surgery in highly myopic cataract eyes and associated risk factors,’ British Journal of Ophthalmology, 104(8), pp. 1137–1141. Available at: https://pubmed.ncbi.nlm.nih.gov/31704699/ (Accessed 21 November 2023).
- O’Brien, P. Ho, S. Fitzpatrick, P. Power, W. (2007) ‘Risk factors for a postoperative intraocular pressure spike after phacoemulsification,’ Canadian Journal of Ophthalmology, 42(1), pp. 51–55. Available at: https://pubmed.ncbi.nlm.nih.gov/17361241/ (Accessed 21 November 2023).
- Næser, K. Thim, K. Hansen, T. Degn, T. Madsen, S. Skov, J. (1986) ‘Intraocular pressure in the first days after implantation of posterior chamber lenses with the use of sodium hyaluronate (Healon),’ Acta Ophthalmologica, 64(3), pp. 330–337. Available at: https://pubmed.ncbi.nlm.nih.gov/3529803/ (Accessed 21 November 2023).
- Yang, X. Liu, Z. Fan, Z. Grzybowski, A. Wang, N. (2020) ‘A narrative review of intraoperative floppy iris syndrome: an update 2020,’ Annals of Translational Medicine, 8(22), pp. 1546. Available at: https://pubmed.ncbi.nlm.nih.gov/33313291/ (Accessed 21 November 2023).
- Borazan, M. Karalezli, A. Akman, A. Akova, Y. (2007) ‘Effect of antiglaucoma agents on postoperative intraocular pressure after cataract surgery with Viscoat,’ Journal of Cataract & Refractive Surgery, 33(11), pp. 1941–1945. Available at: https://pubmed.ncbi.nlm.nih.gov/17964402/ (Accessed 21 November 2023).
- Hayashi, K. Yoshida, M. Sato, T. Manabe, S. (2019) ‘Effect of Topical Hypotensive Medications for Preventing Intraocular Pressure Increase after Cataract Surgery in Eyes with Glaucoma,’ American Journal of Ophthalmology, 205, pp. 91–98. Available at: https://pubmed.ncbi.nlm.nih.gov/30902694/ (Accessed 21 November 2023).
- Chang, D. Masket, S. Miller, K. Braga-Mele, R. Little, B. Mamalis, N. Oetting, T. Packer, M. (2009) ‘Complications of sulcus placement of single-piece acrylic intraocular lenses: recommendations for backup IOL implantation following posterior capsule rupture,’ Journal of Cataract & Refractive Surgery, 35(8), pp. 1445–1458. Available at: https://pubmed.ncbi.nlm.nih.gov/19631134/ (Accessed 21 November 2023).
- Porteous, A. Crawley, L. (2018) ‘Case report of secondary pigment dispersion glaucoma, recurrent uveitis and cystoid macular oedema following inadvertent implantation of an intraocular lens into the ciliary sulcus following cataract surgery,’ BMC Ophthalmology, 18(S1), pp. 219. Available at: https://bmcophthalmol.biomedcentral.com/articles/10.1186/s12886-018-0858-3 (Accessed 21 November 2023).
- Turalba, A. Cakiner-Egilmez, T. Payal, A. Gonzalez-Gonzalez, L. Chomsky, A. Vollman, D. Baze, E. Lawrence, M. Daly, M. (2017) ‘Outcomes after cataract surgery in eyes with pseudoexfoliation: Results from the Veterans Affairs Ophthalmic Surgery Outcomes Data Project,’ Canadian Journal of Ophthalmology, 52(1), pp. 61–68. Available at: https://www.canadianjournalofophthalmology.ca/article/S0008-4182(16)30010-2/fulltext (Accessed 21 November 2023).
- Fu, T. Zhou, X. Liu, K. (2019) ‘The effect of persistent high intraocular pressure on cataract surgery in eyes with acute primary closed angle glaucoma,’ International Eye Science, 19(2), pp. 194–199. Available at: https://pesquisa.bvsalud.org/portal/resource/pt/wpr-712995 (Accessed 21 November 2023).
- Pleyer, U. Ursell, P. Rama, P. (2013) ‘Intraocular Pressure Effects of Common Topical Steroids for Post-Cataract Inflammation: Are They All the Same?,’ Ophthalmology and Therapy, 2(2), pp. 55–72. Available at: https://pubmed.ncbi.nlm.nih.gov/25135807/ (Accessed 21 November 2023).
- Armaly, M. (1968) ‘Genetic factors related to glaucoma,’ Annals of the New York Academy of Sciences, 151(2), pp. 861–874. Available at: https://nyaspubs.onlinelibrary.wiley.com/doi/abs/10.1111/j.1749-6632.1968.tb48270.x (Accessed 21 November 2023).
- Becker, B. (1965) ‘Intraocular pressure response to topical corticosteroids,’ Invest Ophthalmol, 4, pp. 198–205. Available at: https://pubmed.ncbi.nlm.nih.gov/14283013/ (Accessed 21 November 2023).
- Tripathi, R. Parapuram, S. Tripathi, B. Zhong, Y. Chalam, K. (1999) ‘Corticosteroids and Glaucoma Risk,’ Drugs & Aging, 15(6), pp.439–50. Available at: https://link.springer.com/article/10.2165/00002512-199915060-00004 (Accessed 21 November 2023).
- Razeghinejad, M. Katz, L. (2012) ‘Steroid-induced iatrogenic glaucoma,’ Ophthalmic Research, 47(2), pp. 66-80. Available at: https://pubmed.ncbi.nlm.nih.gov/21757964/ (Accessed 21 November 2023).
- Bartlett, J. Woolley, T. Adams, C. (1993) ‘Identification of high intraocular pressure responders to topical ophthalmic corticosteroids,’ Journal of Ocular Pharmacology and Therapeutics, 9(1), pp. 35–45. Available at: https://pubmed.ncbi.nlm.nih.gov/8463731/ (Accessed 21 November 2023).
- Chan, E. Mahroo, O. Spalton, D. (2010) ‘Complications of cataract surgery,’ Clinical and Experimental Optometry, 93(6), pp. 379–389. Available at: https://pubmed.ncbi.nlm.nih.gov/20735786/ (Accessed 21 November 2023).
- Bhangra, J. Babar, N. Patel, K. Dutton, C. Price, L. Hardman-Lea, S. (2022) ‘Cataract post-op complications: A community optometrist’s guide,’ Optician Online, 12/08/2022, pp. 17-25. Available at: https://www.opticianonline.net/cpd-archive/6396 (Accessed: 24 November 2023).
- Raj, S. Vasavada, A. Johar, S. Vasavada, V. Vasavada, V. (2007) ‘Post-operative capsular opacification: a review,’ International Journal of Biomedical Science, 3(4), pp. 237–250. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3614664/ (Accessed: 24 November 2023).
- Donachie, P. Barnes, B. Olaitan, M. Sparrow, J. Buchan, J. (2022) ‘The Royal College of Ophthalmologists’ National Ophthalmology Database study of cataract surgery: Report 9, Risk factors for posterior capsule opacification,’ Eye, 2022. Available at: https://www.nature.com/articles/s41433-022-02204-1 (Accessed: 24 November 2023).
- Aslam, T. Dhillon, B. Werghi, N. Taguri, A. Wadood, A. (2002) ‘Systems of analysis of posterior capsule opacification,’ The British Journal of Ophthalmology, 86(10), pp. 1181–1186. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1771330/ (Accessed: 24 November 2023).
- Kruger, A. Schauersberger, J. Abela, C. Schild, G. Amon, M. (2000) ‘Two year results: sharp versus rounded optic edges on silicone lenses,’ Journal of Cataract and Refractive Surgery, 26(4), pp. 566–570. Available at: https://www.sciencedirect.com/science/article/abs/pii/S0886335000003230 (Accessed: 24 November 2023).
- Sellman, T. Lindstrom, R. (1988) ‘Effect of a plano-convex posterior chamber lens on capsular opacification from Elschnig pearl formation,’ Journal of Cataract and Refractive Surgery, 14(1), pp. 68–72. Available at: https://pubmed.ncbi.nlm.nih.gov/3339551/ (Accessed: 24 November 2023).
- Gupta, G. Jain, A. Malhotra, C. (2019) ‘Anterior capsular phimosis,’ British Medical Journal, 367:l5387. Available at: https://www.bmj.com/content/367/bmj.l5387 (Accessed: 24 November 2023).
- Rotsos, T. Moschos, M. (2008) ‘Cystoid macular edema,’ Clinical Ophthalmology, 2(4), pp. 919–930. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2699812/ (Accessed: 24 November 2023).
- Bessant, D. Sullivan, P. Aylward, G. (1998) ‘The management of dislocated lens material after phacoemulsification,’ Eye, 12(4), pp. 641–645. Available at: https://pubmed.ncbi.nlm.nih.gov/9850257/ (Accessed: 18 November 2023).
- Syed, Z. Moayedi, J. Mohamedi, M. Tashter, J. Anthony, T. Celiker, C. Khazen, G. Melki, S. (2015) ‘Cataract surgery outcomes at a UK independent sector treatment centre,’ British Journal of Ophthalmology, 99(11), pp. 1460–1465. Available at: https://bjo.bmj.com/content/99/11/1460 (Accessed: 18 November 2023).
- Salabati, M. Mahmoudzadeh, R. Wakabayashi, T. Hinkle, J. Ho, A. (2022) ‘Indications for surgical management of retained lens fragments,’ Current Opinion in Ophthalmology, 33(1), pp. 15–20. Available at: https://pubmed.ncbi.nlm.nih.gov/34743089/ (Accessed: 18 November 2023).
- Odugbo, O. Babalola, O. Morgan, R. (2010) ‘Outcome of cataract surgeries in Plateau State, Nigeria,’ Highland Medical Research Journal, 8(1), pp. 28-38. Available at: https://www.ajol.info/index.php/hmrj/article/view/52874 (Accessed: 18 November 2023).
- Tranos, P. Dervenis, N. Vakalis, A. Asteriadis, S. Stavrakas, P. Konstas, A. (2016) ‘Current Perspectives of Prophylaxis and Management of Acute Infective Endophthalmitis,’ Advances in Therapy, 33(5), pp. 727–746. Available at: https://pubmed.ncbi.nlm.nih.gov/26935830/ (Accessed: 18 November 2023).
- Fastenberg, D. Schwartz, P. Shakin, J. Golub, B. (1991) ‘Management of dislocated nuclear fragments after phacoemulsification,’ American Journal of Ophthalmology, 112(5), pp. 535–539. Available at: https://pubmed.ncbi.nlm.nih.gov/1951590/ (Accessed: 18 November 2023).
- Kim, J. Flynn, H. Smiddy, W. Murray, T. Rubsamen, P. Davis, J. Nicholson, D. (1994) ‘Retained lens fragments after phacoemulsification,’ Ophthalmology, 101(11), pp. 1827–1832. Available at: https://pubmed.ncbi.nlm.nih.gov/7800364/ (Accessed: 18 November 2023).
- Irvine, W. Flynn, H. Murray, T. Rubsamen, P. (1992) ‘Retained lens fragments after phacoemulsification manifesting as marked intraocular inflammation with hypopyon,’ American Journal of Ophthalmology, 114(5), pp. 610–614. Available at: https://pubmed.ncbi.nlm.nih.gov/1443024/ (Accessed: 18 November 2023).
- Rofagha, S. Bhisitkul, R. (2011) ‘Management of retained lens fragments in complicated cataract surgery,’ Current Opinion in Ophthalmology, 22(2), pp. 137–140. Available at: https://pubmed.ncbi.nlm.nih.gov/21191293/ (Accessed: 18 November 2023).
- Gedde, S. Karp, C. Budenz, D. (1998) ‘Retained Nuclear Fragment in the Anterior Segment,’ Archives of Ophthalmology, 116(11), pp. 1532–1533. Available at: https://jamanetwork.com/journals/jamaophthalmology/fullarticle/264070 (Accessed: 18 November 2023).
- Mimouni, M. Schaap-Fogler, M. Polkinghorne, P. Rabina, G. Ehrlich, R. (2021) ‘Prognostic Factors for Low Visual Acuity after Cataract Surgery with Vitreous Loss,’ Journal of Ophthalmology, 2021: 6691904. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8225442/ (Accessed: 18 November 2023).
- Ng, D. Rowe, N. Francis, I. Kappagoda, M. Haylen, M. Schumacher, R. Alexamder, S. Boytell, K. Lee, B. (1998) ‘Intraoperative complications of 1000 phacoemulsification procedures: A prospective study,’ Journal of Cataract & Refractive Surgery, 24(10), pp. 1390–1395. Available at: https://pubmed.ncbi.nlm.nih.gov/9795858/ (Accessed: 18 November 2023).
- Fontana, L. Coassin, M. Iovieno, A. Moramarco, A. Cimino, L. (2017) ‘Cataract surgery in patients with pseudoexfoliation syndrome: Current updates,’ Clinical Ophthalmology, 11, pp. 1377–1383. Available at: https://pubmed.ncbi.nlm.nih.gov/28814824/ (Accessed: 18 November 2023).
- Li, E. Wu, W. Jhanji, V. (2011) ‘Pupillary block glaucoma secondary to vitreous prolapse after Nd:YAG capsulotomy,’ Clinical and Experimental Optometry, 94(4), pp. 383–384. Available at: https://pubmed.ncbi.nlm.nih.gov/21599749/ (Accessed: 18 November 2023).
- Kim, T. Kang, H. Kim, C. Koh, H. Kim, S. Kim, M. (2021) ‘Delayed vitreous prolapse after cataract surgery: clinical features and surgical outcomes,’ Scientific Reports, 11, 16107. Available at: https://www.nature.com/articles/s41598-021-95527-0 (Accessed: 18 November 2023).
- Bali, E. Haider, H. Tassignon, M. (2019) ‘IOL Dislocation and the Diving BIL’ In: Tassignon, M. Dhubhghaill, S. Os, L. editors. Innovative Implantation Technique – Bag-in-the-lens Cataract Surgery. Springer Cham; pp. 191–195.
- Chakrabarti, M. Chakrabarti, A. (2020) ‘Dislocated Intraocular Lens’ In: Chakrabarti, M., Chakrabarti, A. (eds) Posterior Segment Complications of Cataract Surgery. Springer, Singapore; 2020. pp. 139–173.
- Lee, G. Lim, D. Chi, S. Kim, S. Han, J. Shin, D. Chung, T. (2021) ‘Incidence and characteristics of intraocular lens dislocation after phacoemulsification: An eight-year, nationwide, population-based study,’ Journal of Clinical Medicine, 10(17), 3830. Available at: https://pubmed.ncbi.nlm.nih.gov/34501279/ (Accessed: 18 November 2023).
- Lee, G. Lim, D. Chi, S. Kim, S. Shin, D. Chung, T. (2020) ‘Risk Factors for Intraocular Lens Dislocation After Phacoemulsification: A Nationwide Population-Based Cohort Study’, American Journal of Ophthalmology, 214, pp. 86–96. Available at: https://www.ajo.com/article/S0002-9394(20)30112-4/fulltext (Accessed: 18 November 2023).
- Riedl, J. Rings, S. Schuster, A. Vossmerbaeumer, U. (2022) ‘Intraocular lens dislocation: manifestation, ocular and systemic risk factors,’ International Ophthalmology, 43(4), pp. 1317-1324. Available at: https://pubmed.ncbi.nlm.nih.gov/36149618/ (Accessed: 18 November 2023).
- Krėpštė, L. Kuzmienė, L. Miliauskas, A. Janulevičienė, I. (2013) ‘Possible Predisposing Factors for Late Intraocular Lens Dislocation After Routine Cataract Surgery,’ Medicina, 49(5), pp. 229-234. Available at: https://pubmed.ncbi.nlm.nih.gov/24247919/ (Accessed: 18 November 2023).
- Singh, K. (2020) ‘Commentary: Management of dislocated and subluxated intraocular lens,’ Indian Journal of Ophthalmology, 68(6), pp. 1150. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7508128/ (Accessed: 18 November 2023).
- Ali, N. Little, B. (2010) ‘Causes of cataract surgery malpractice claims in England 1995–2008,’ British Journal of Ophthalmology, 95, pp. 490-492. Available at: https://bjo.bmj.com/content/95/4/490 (Accessed: 18 November 2023).
- Brogan, K. Diaper, C. Rotchford, A. (2019) ‘Cataract surgery refractive outcomes: representative standards in a National Health Service setting,’ British Journal of Ophthalmology, 103(4), pp. 539-543. Available at: https://pubmed.ncbi.nlm.nih.gov/29907629/ (Accessed: 18 November 2023).
- Rementería-Capelo, L. García-Pérez, J. Gros-Otero, J. Moran, A. Sanchez-Pina, J. Contreras, I. (2020) ‘Visual and Refractive Outcomes of Cataract Surgeries Performed in One Year in a Private Practice Setting: Review of 2714 Procedures,’ Journal of Ophthalmology, Apr(14), 2421816. Available at: https://pubmed.ncbi.nlm.nih.gov/32377414/ (Accessed 20 October 2023).
- Moshirfar, M. Ziari, M. Ronquillo, Y. (2023) ‘Cataract surgery considerations in patients with prior history of keratoconus and ectasia,’ Current Opinion in Ophthalmology, 34(1), pp. 41-47. Available at: https://pubmed.ncbi.nlm.nih.gov/36165405/ (Accessed: 18 November 2023).
- Hamilton, D. Hardten, D. (2003) ‘Cataract surgery in patients with prior refractive surgery,’ Current Opinion in Ophthalmology, 14(1), pp. 44-53. Available at: https://pubmed.ncbi.nlm.nih.gov/12544810/ (Accessed: 18 November 2023).
- Charlesworth, E. Alderson, A. Fylan, F. Armstrong, R. Chandra, A. Elliott, D. (2022) ‘Investigating target refraction advice provided to cataract surgery patients by UK optometrists and ophthalmologists,’ Ophthalmic and Physiological Optics, 42(3), pp. 440-453. Available at: https://pubmed.ncbi.nlm.nih.gov/35179791/ (Accessed: 18 November 2023).
- Wee, D. ‘Astigmatism,’ In: Riaz, K. Vicente, G. Wee, D. editors. Optics for the New Millennium An Absolute Review Textbook. Springer Cham; 2022. pp. 153–166.
- Sathish, S. (2021) ‘Correction of corneal astigmatism and stability of toric intraocular lenses,’ Journal of Cataract & Refractive Surgery, 47(11), pp. 1385-1386. Available at: https://pubmed.ncbi.nlm.nih.gov/34675146/ (Accessed: 18 November 2023).
- Elsayed, M. Ahmad, K. Al-Abdullah, A. Malik, R. Khandekar, R. Martinez-Osorio, H. Mura, M. Schatz, P. (2019) ‘Incidence of Intraocular Lens Exchange after Cataract Surgery,’ Scientific Reports, 9:12877. Available at: https://www.nature.com/articles/s41598-019-49030-2 (Accessed: 18 November 2023).
- Moshirfar, M. Thomson, A. Thomson, R. Martheswaran, T. McCabe, S. (2021) ‘Refractive enhancements for residual refractive error after cataract surgery,’ Current Opinion in Ophthalmology, 32(1), pp. 54-61. Available at: https://pubmed.ncbi.nlm.nih.gov/33122488/ (Accessed: 18 November 2023).
- Naylor, G. (1993) ‘Iris prolapse; Who? When? Why?’ Eye, 7(3), pp. 465–467. Available at: https://www.nature.com/articles/eye199394 (Accessed: 18 November 2023).
- Francis, P. Morris, R. (1997) ‘Post-operative iris prolapse following phacoemulsification and extracapsular cataract surgery,’ Eye, 11(1), pp. 87-90. Available at: https://pubmed.ncbi.nlm.nih.gov/9246283/ (Accessed: 18 November 2023).
- Tabandeh, H. Thompson, G. Kon, C. Bolton, T. (1995) ‘Phenylephrine and pilocarpine in the treatment of post-operative irido-corneal adhesion,’ Eye, 9(4), pp. 452-455. Available at: https://pubmed.ncbi.nlm.nih.gov/7498565/ (Accessed: 18 November 2023).
- Ernest, P. Kiessling, L. Lavery, K. (1991) ‘Relative strength of cataract incisions in cadaver eyes,’ Journal of Cataract & Refractive Surgery, 17, pp. 668–671. Available at: https://pubmed.ncbi.nlm.nih.gov/1955983/ (Accessed: 18 November 2023).
- Chang, P. Chang, S. (2015) ‘Rapid anterior capsular phimosis after cataract surgery in a patient with chronic angle closure glaucoma,’ Taiwan Journal of Ophthalmology, 5(4), pp. 192–194. Available at: https://pubmed.ncbi.nlm.nih.gov/29018698/ (Accessed: 24 November 2023).
- Toto, L. Viggiano, P. Vecchiarino, L. Evangelista, F. Borrelli, E. Mastropasqua, L. (2019) ‘Anterior capsule contraction syndrome: a successful multimodal therapeutic approach,’ International Journal of Ophthalmology, 12(8), pp. 1356-1358. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6694047/ (Accessed 27 November 2023).
- Narnaware, S. Bawankule, P. (2019) ‘Anterior capsular phimosis,’ Indian Journal of Ophthalmology, 67(9), pp. 1476. Available at: https://journals.lww.com/ijo/fulltext/2019/67090/anterior_capsular_phimosis.25.aspx (Accessed: 24 November 2023).
- Wilde, C. Ross, A. Awad, M. Chen, H. Dua, H. (2018) ‘Management of anterior capsular contraction syndrome: pitfall of circular capsulotomy technique with the neodymium YAG laser,’ Eye, 32, pp. 1546-1548. Available at: https://www.nature.com/articles/s41433-018-0125-0 (Accessed: 24 November 2023).
- Naik, M. Sethi, H. Mehta, A. (2020) ‘Capsular bag phimosis,’ American Journal of Ophthalmology Case Reports, 20:100999. Available at: https://doi.org/10.1016/j.ajoc.2020.100999 (Accessed: 24 November 2023).
- Ding, Z. Zeng, J. (2012) ‘Clinical study on Hypotony following blunt ocular trauma,’ International Journal of Ophthalmology, 5(6), pp. 771-773. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3530823/ (Accessed: 18 November 2023).
- Wang, Q. Thau, A. Levin, A. Lee, D. (2019) ‘Ocular hypotony: A comprehensive review,’ Survey of Ophthalmology, 64(5), pp. 619-638. Available at: https://pubmed.ncbi.nlm.nih.gov/31029581/ (Accessed: 18 November 2023).
- Fine, H. Biscette, O. Chang, S. Schiff, W. (2007) ‘Ocular hypotony: a review,’ Comprehensive Ophthalmology Update, 8(1), pp. 29-37. Available at: https://pubmed.ncbi.nlm.nih.gov/17394757/ (Accessed: 18 November 2023).
- Samuelson, T. Liaboe, C. (2021) ‘Management of Hypotony related maculopathy after combined phacoemulsification and trabeculectomy: January consultation #1,’ Journal of Cataract & Refractive Surgery, 47(1), pp. 130. Available at: https://pubmed.ncbi.nlm.nih.gov/33901082/ (Accessed: 18 November 2023).
- Camp, A. Weinreb, R. (2020) ‘Hypotony Keratopathy following Trabeculectomy,’ Journal of Glaucoma, 29(2), pp. 77-80. Available at: https://pubmed.ncbi.nlm.nih.gov/31876870/ (Accessed: 18 November 2023).
- Livny, E. Mimouni, M. Sourkin, N. Bhar, I. Rootman, D. Nahum, Y. (2021) ‘Descemet membrane endothelial keratopathy in eyes with chronic ocular Hypotony following glaucoma surgery,’ American Journal of Ophthalmology, 230, pp. 256-263. Available at: https://pubmed.ncbi.nlm.nih.gov/33991516/ (Accessed: 18 November 2023).
- Thomas, M. Vajaranant, T. Aref, A. (2015) ‘Hypotony Maculopathy: Clinical presentation and Therapeutic methods,’ Ophthalmology and Therapy, 4(2), pp. 79-88. Available at: https://pubmed.ncbi.nlm.nih.gov/26253854/ (Accessed: 18 November 2023).
- Albano de Guimaraes, J. Teixeira, G. Silva, T. Moura, F. (2021) ‘Optic disc Edema and posterior globe flattening secondary to ocular hypotony: Case report and discussion regarding pathophysiology and clinical findings,’ Journal of Neuroophthalmology, 41(2), pp. 220-222. Available at: https://pubmed.ncbi.nlm.nih.gov/33105416/ (Accessed: 18 November 2023).
- Sahoo, N. Balijepalli, P. Singh, S. Jhingan, M. Senthil, S. Chhablani, J. (2018) ‘Retina and glaucoma: surgical complications,’ International Journal of Retina and Vitreous, 4, pp. 29. Available at: https://pubmed.ncbi.nlm.nih.gov/30202602/ (Accessed: 18 November 2023).
- Costa, J. Alio, J. (2019) ‘Significant hyperopic shift in a patient with extreme myopia following severe hypotonia caused by glaucoma filtering surgery,’ European Journal of Ophthalmology, 29(1), pp. 6-9. Available at: https://pubmed.ncbi.nlm.nih.gov/30175614/ (Accessed: 18 November 2023).
- Tripathy, K. Chawla, R. Temkar, S. Sagar, P. Kashyap, S. Pushker, N. Sharma, Y. (2018) ‘Phthsis Bulbi - a clinicopathological perspective,’ Seminars in Opthalmology, 33(6), pp. 788-803. Available at: https://pubmed.ncbi.nlm.nih.gov/29902388/ (Accessed: 18 November 2023).
- Roters, S. Szurman, P. Engels, B. Bartz-Schmidt, K. Krieglstein, G. (2002) ‘Ultrasound biomicroscopy in chronic ocular Hypotony:its impact on diagnosis and management,’ Retina, 22(5), pp. 581-588. Available at: https://pubmed.ncbi.nlm.nih.gov/12441723/ (Accessed: 18 November 2023).
- Clark, A. Moriet, N. Ng, J. Preen, D. Semmens, J. (2012) ‘Risk for retinal detachment after phacoemulsification: a whole-population study of cataract surgery outcomes,’ Archives of Ophthalmology, 130(7), pp. 882–888. Available at: https://jamanetwork.com/journals/jamaophthalmology/fullarticle/1214784 (Accessed: 18 November 2023).
- Morano, M. Khan, A. Halfpenny, C. Wisner, D. Zhang, Q. Sharpe, J. Li, A. Tomaiuolo, M. Hyman, L. Ho, A. (2022) ‘Incidence and Risk Factors for Retinal Detachments and Tears after Cataract Surgery: An Analysis of the American Academy of Ophthalmology IRIS® Registry (Intelligent Research in Sight),’ Investigative Ophthalmology & Visual Science, 63, pp. 3431. Available at: https://iovs.arvojournals.org/article.aspx?articleid=2780980 (Accessed: 18 November 2023).
- Thylefors, J. Jakobsson, G. Zetterberg, M. Sheik, R. (2022) ‘Retinal detachment after cataract surgery: a population-based study’ Acta Ophthalmologica, 100(8), pp. 1595-1599. Available at: https://pubmed.ncbi.nlm.nih.gov/35338568/ (Accessed: 18 November 2023).
- Petousis, V. Sallam, A. Haynes, R. Patel, C. Tyagi, A. Kirkpatrick, J. Johnston, R. (2016) ‘Risk factors for retinal detachment following cataract surgery: the impact of posterior capsular rupture,’ British Journal of Ophthalmology, 100(11), pp. 1461-1465. Available at: https://pubmed.ncbi.nlm.nih.gov/26858087/ (Accessed: 18 November 2023).
- Qureshi, M. Steelc, D. (2020) ‘Retinal detachment following cataract phacoemulsification—a review of the literature,’ Eye, 34(4), pp. 616–631. Available at: https://pubmed.ncbi.nlm.nih.gov/31576027/ (Accessed: 18 November 2023).
- Schwartz, S. Flynn, H. Dasc, T. Mieler, W. (2016) ‘Ocular Infection: Endophthalmitis,’ Developments in Ophthalmology, 55, pp. 176–188. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5548375/ (Accessed 19th November 2023).
- Cao, H. Zhang, L. Li, L. Lo, S. (2013) ‘Risk Factors for Acute Endophthalmitis following Cataract Surgery: A Systematic Review and Meta-Analysis,’ PloS one, 8(8):e71731. Available at: https://pubmed.ncbi.nlm.nih.gov/23990980/ (Accessed 19th November 2023).
- Durand, M. (2013) ‘Endophthalmitis,’ Clinical Microbiology and Infection, 19(3), pp. 227-234. Available at: https://pubmed.ncbi.nlm.nih.gov/23438028/ (Accessed 19th November 2023).
- The College of Optometrists (2021) ‘Clinical Management Guidelines - Endophthalmitis (post-operative) (Exogenous endophthalmitis)’. Available at: https://www.college-optometrists.org/clinical-guidance/clinical-management-guidelines/endophthalmitis_post-operative_exogenousendophthal (Accessed 19th November 2023).
- Day, A. Donachie, P. Sparrow, J. Johnston, R. (2015) ‘The Royal College of Ophthalmologists’ National Ophthalmology Database study of cataract surgery: report 1, visual outcomes and complications,’ Eye, 29(4), pp. 552-560. Available at: https://pubmed.ncbi.nlm.nih.gov/25679413/ (Accessed 19th November 2023).
- The Royal College of Ophthalmologists (2022) ‘Ophthalmic Services Guidance - Managing an outbreak of postoperative endophthalmitis.’ Available at: https://www.rcophth.ac.uk/resources-listing/managing-an-outbreak-of-postoperative-endophthalmitis-2016/ (Accessed 19th November 2023).
- Shirodkar, A. Pathengay, A. Flynn, H. Albini, T. Berrocal, A. Davis, J. Lalwani, G. Murray, T. Smiddy, W. Miller, D. (2012) ‘Delayed- versus acute-onset endophthalmitis after cataract surgery,’ American Journal of Ophthalmology, 153(3), pp. 391-398. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3381653/ (Accessed 19th November 2023).
- Endophthalmitis Vitrectomy Study Group (1995) ‘Results of the Endophthalmitis Vitrectomy Study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis,’ Archives of Ophthalmology, 113(12), pp. 1479-1496. Available at: https://pubmed.ncbi.nlm.nih.gov/7487614/ (Accessed 19th November 2023).
- Verma, L. Chakravarti, A. (2017) ‘Prevention and management of postoperative endophthalmitis: A case-based approach,’ Indian Journal of Ophthalmology, 65(12), pp. 1396-1402. Available at: https://pubmed.ncbi.nlm.nih.gov/29208820/ (Accessed 19th November 2023).
- National Institute for Health and Care Excellence (NICE) (2017) ‘Cataracts in adults: management [NG77]’. Available at: https://www.nice.org.uk/guidance/ng77 (Accessed 19th November 2023).
- Kessel, L. Flesner, P. Andresen, J. Erngaard, D. Tendal, B. Hjortdal, J. (2015) ‘Antibiotic prevention of postcataract endophthalmitis: a systematic review and meta-analysis,’ Acta Ophthalmologica, 93(4), pp. 303-317. Available at: https://pubmed.ncbi.nlm.nih.gov/25779209/ (Accessed 19th November 2023).
- Endophthalmitis Study Group, European Society of Cataract and Refractive Surgeons (2007) ‘Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factors,’ Journal of Cataract and Refractive Surgery, 33(6), pp.978-988. Available at: https://pubmed.ncbi.nlm.nih.gov/17531690/ (Accessed 19th November 2023).
- Gower, E. Lindsley, K. Tulenko, S. Nanji, A. Leyngold, I. McDonnell, P. (2017) ‘Perioperative antibiotics for prevention of acute endophthalmitis after cataract surgery,’ Cochrane Database Syst Rev., 2(2):CD006364. Available at: https://pubmed.ncbi.nlm.nih.gov/28192644/ (Accessed 19th November 2023).
- Durand, M. (2017) ‘Bacterial and Fungal Endophthalmitis,’ Clinical Microbiology Reviews, 30(3), pp. 597-613. Available at: https://pubmed.ncbi.nlm.nih.gov/28356323/ (Accessed 19th November 2023).
- Wang, C. Cheang, W. Hwang, D. Lin, C. (2017) ‘Vitreous haemorrhage: a population-based study of the incidence and risk factors in Taiwan,’ International Journal of Ophthalmology, 10(3), pp. 461–466. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5360784/ (Accessed: 18 November 2023).
- Jena, S. Tripathy, K. (2023) ‘Vitreous Hemorrhage,’ In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Available at: https://www.ncbi.nlm.nih.gov/books/NBK559131 (Accessed: 18 November 2023).
- Shukla, D. Naresh, K. Kim, R. (2005) ‘Optical coherence tomography findings in valsalva retinopathy,’ American Journal of Ophthalmology, 140(1), pp. 134-136. Available at: https://pubmed.ncbi.nlm.nih.gov/16038658/ (Accessed: 18 November 2023).
- Shaikh, N. Srishti, R. Khanum, A. Thirumalesh, M. Dave, V. Arora, A. Bansal, R. Surve, A. Azad, S. Kumar, V. (2023) ‘Vitreous hemorrhage – Causes, diagnosis, and management,’ Indian Journal of Ophthalmology, 71(1), pp. 28–38. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10155538/ (Accessed: 18 November 2023).
- Spraul, C. Grossniklaus, H. (1997) ‘Vitreous haemorrhage,’ Survey of Ophthalmology, 42(1), pp. 3–9. Available at: https://www.surveyophthalmol.com/article/S0039-6257(97)84041-6/fulltext (Accessed: 18 November 2023).
- Zhang, T. Zhang, J. Sun, X. Tian, J. Shi, W. Yuan, G. (2017) ‘Early vitrectomy for dense vitreous hemorrhage in adults with non‐traumatic and non‐diabetic retinopathy,’ Journal of International Medical Research, 45, pp. 2065‐2071. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5805203/ (Accessed: 18 November 2023).
- Paris, G. Quickert, M. (1976) ‘Disinsertion of the aponeurosis of the levator palpebrae superioris muscle after cataract extraction,’ American Journal of Ophthalmology, 81(3), pp. 337-340. Available at: https://www.sciencedirect.com/science/article/abs/pii/0002939476902506 (Accessed: 24 November 2023).
- Parsa, F. Wolff, D. Parsa, N. Elahi, E. (2001) ‘Upper eyelid ptosis repair after cataract extraction and the importance of Hering’s test,’ Plastic and Reconstructive Surgery, 108(6), pp. 1527-1536. Available at: https://pubmed.ncbi.nlm.nih.gov/11711923/ (Accessed: 24 November 2023).
- Bacharach, J. Lee, W. Harrison, A. Freddo, T. (2021) ‘A review of acquired blepharoptosis: prevalence, diagnosis, and current treatment options,’ Eye, 35, pp. 2468–2481. Available at: https://www.nature.com/articles/s41433-021-01547-5 (Accessed: 24 November 2023).
- Bernadino, C. Rubin, P. (2002) ‘Ptosis after cataract surgery,’ Seminars in Ophthalmology, 17:3-4, pp. 144-148. Available at: https://www.tandfonline.com/doi/abs/10.1076/soph.17.3.144.14782 (Accessed: 24 November 2023).
- Kaplan, L. Jaffe, N. Clayman, H. (1985) ‘Ptosis and cataract surgery. A multivariant computer analysis of a prospective study,’ Ophthalmology, 92(2), pp. 237–242. Available at: https://www.sciencedirect.com/science/article/abs/pii/S0161642085340460 (Accessed: 24 November 2023).
- Loeffler, M. Solomon, L. Renaud, M. (1990) ‘Postcataract extraction ptosis: effect of the bridle suture,’ Journal of Cataract and Refractive Surgery, 16(4), pp. 501–504. Available at: https://www.sciencedirect.com/science/article/abs/pii/S0886335013808073 (Accessed: 24 November 2023).
- Altieri, M. Truscott, E. Kingston, A. Bertagno, R. Altieri, G. (2005) ‘Ptosis secondary to anterior segment surgery and its repair in a two-year follow-up study,’ Ophthalmologica, 219(3), pp. 129–135. Available at: https://www.karger.com/Article/Abstract/85244 (Accessed: 24 November 2023).
- Singh, S. Sekhar, G. Gupta, S. (1997) ‘Etiology of ptosis after cataract surgery,’ Journal of Cataract and Refractive Surgery, 23(9), pp. 1409–1413. Available at: https://pubmed.ncbi.nlm.nih.gov/9423917/ (Accessed: 24 November 2023).
- Tamaki, R. Gosho, M. Mizumoto, K. Kato, N. Zako, M. (2016) ‘Influence of upper and temporal transconjunctival sclerocorneal incision on marginal reflex distance after cataract surgery,’ BMC Ophthalmology, 16:95. Available at: https://bmcophthalmol.biomedcentral.com/articles/10.1186/s12886-016-0286-1#citeas (Accessed: 24 November 2023).
- Patel, J. Blount, M. Jones, C. (2002) ‘Surgical blepharoptosis--the bridle suture factor?’ Eye, 16(5), pp. 535–537. Available at: https://pubmed.ncbi.nlm.nih.gov/12194064/ (Accessed: 24 November 2023).
- Linberg, J. McDonald, M. Safir, A. Googe, J. (1986) ‘Ptosis following radial keratotomy. Performed using a rigid eyelid speculum,’ Ophthalmology, 93(12), pp. 1509–1512. Available at: https://www.sciencedirect.com/science/article/abs/pii/S0161642086335292 (Accessed: 24 November 2023).
- Feibel, R. Custer, P. Gordon, M. (1993) ‘Postcataract ptosis. A randomized, double-masked comparison of peribulbar and retrobulbar anesthesia,’ Ophthalmology, 100(5), pp. 660–665. Available at: https://www.sciencedirect.com/science/article/abs/pii/S0161642093315927 (Accessed: 24 November 2023).
- Hildreth, H. Silver, B. (1967) ‘Sensory Block of the Upper Eyelid,’ Archives of Ophthalmology, 77(2), pp. 230–231. Available at: https://pubmed.ncbi.nlm.nih.gov/6019018/ (Accessed: 24 November 2023).
- Kashkouli, M. Abdolalizadeh, P. Es’haghi, A. Nilforushan, N. Aghaei, H. Karimi, N. (2020) ‘Postoperative Blepharoptosis After Modern Phacoemulsification Procedure,’ American Journal of Ophthalmology, 213, pp. 17-23. Available at: https://www.sciencedirect.com/science/article/abs/pii/S0002939420300039 (Accessed: 24 November 2023).
- Ahuero, A. Hatton, M. (2010) ‘Eyelid malposition after cataract and refractive surgery,’ International Ophthalmology Clinics, 50(1), pp. 25–36. Available at: https://pubmed.ncbi.nlm.nih.gov/20057293/ (Accessed: 24 November 2023).
- Parsa, F. Roach, J. (1996) ‘A case report on spontaneous return of levator function following postcataract blepharoptosis repair,’ Annals of Plastic Surgery, 37(6), pp. 638–640. Available at: https://pubmed.ncbi.nlm.nih.gov/8988778/ (Accessed: 24 November 2023).
- Farber, S. Codner, M. (2020) ‘Evaluation and management of acquired ptosis,’ Plastic and Aesthetic Research, 7:20. Available at: https://parjournal.net/article/view/3430 (Accessed: 24 November 2023).
- Bellows, A. Chylack, L. Hutchinson, B. (1981) ‘Choroidal detachment: Clinical manifestation, therapy and mechanism of formation,’ Ophthalmology, 88(11), pp. 1107-1115. Available at: https://pubmed.ncbi.nlm.nih.gov/7335316/ (Accessed: 18 November 2023).
- Savastano, A. Rizzo, S. Savastano, M. Piccirillo, V. Forte, R. Sbordone, S. Diurna, F. Savastano, S. (2015) ‘Choroidal effusion and suprachoroidal haemorrhage during phacoemulsification: intraoperative management to prevent expulsive haemorrhage,’ European Journal of Ophthalmology, 26(4), pp. 338-341. Available at: https://journals.sagepub.com/doi/abs/10.5301/ejo.5000707 (Accessed: 18 November 2023).
- Reddy, A. Salim, S. Fekrat, S. Scott, I. (2012) ‘Choroidal Effusions,’ (2012) American Academy of Ophthalmology, EyeNet Magazine, Nov 2012, pp. 47-49. Available at: https://www.aao.org/eyenet/article/choroidal-effusions?november-2012 (Accessed: 18 November 2023).
- Chu, T. Green, R. (1999) ‘Suprachoroidal hemorrhage,’ Survey of Ophthalmology, 43, pp. 471–486. Available at: https://pubmed.ncbi.nlm.nih.gov/10416790/ (Accessed: 18 November 2023).
- Gressel, M. Parrish, R. Heuer, D. (1984) ‘Delayed nonexpulsive suprachoroidal hemorrhage,’ Archives of Ophthalmology, 102, pp. 1757–1760. Available at: https://jamanetwork.com/journals/jamaophthalmology/article-abstract/635325 (Accessed: 18 November 2023).
- Ling, R. Cole, M. James, C. Kamalarajah, S. Foot, B. Shaw, S. (2004) ‘Suprachoroidal haemorrhage complicating cataract surgery in the UK:epidemiology, clinical features, management, and outcomes,’ British Journal of Ophthalmology, 88(4), pp. 478–480. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1772112/ (Accessed: 18 November 2023).
- Davison, J (1986) ‘Acute intraoperative suprachoroidal hemorrhage in extracapsular cataract surgery,’ Journal of Cataract & Refractive Surgery, 12(6), pp. 606-622. Available at: https://pubmed.ncbi.nlm.nih.gov/3491206/ (Accessed: 18 November 2023).
- Eriksson, A. Koranyi, G. Seregard, S. Philipson, B. (1998) ‘Risk of acute suprachoroidal hemorrhage with phacoemulsification,’ Journal of Cataract and Refractive Surgery, 24(6), pp. 793-800. Available at: https://pubmed.ncbi.nlm.nih.gov/9642590/ (Accessed: 18 November 2023).
- Banta, J. Hoffman, K. Budenz, D. Ceballos, E. Greenfields, D. (2001) ‘Presumed topiramate-induced bilateral acute angle-closure glaucoma,’ American Journal of Ophthalmology, 132(1), pp. 112-114. Available at: https://pubmed.ncbi.nlm.nih.gov/11438067/ (Accessed: 18 November 2023).
- Maumenee, A. Schwartz, M. (1985) ‘Acute intraoperative choroidal effusion,’ American Journal of Ophthalmology, 100(1), pp. 147-154. Available at: https://pubmed.ncbi.nlm.nih.gov/3893137/ (Accessed: 18 November 2023).
- Chang, R. Du, Y. Peng, Z. Lu, Y. Zhu, X. (2017) ‘Acute uveal effusion during phacoemulsification with preoperative central serous chorioretinopathy: a case report,’ BMC Ophthalmology, 17(1), pp. 137. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5543589/ (Accessed: 18 November 2023).
- Meier, P. Wiedemann, P. (2000) ‘Massive suprachoroidal hemorrhage: secondary treatment and outcome,’ Graefe’s Archive for Clinical and Experimental Ophthalmology, 238(1), pp. 28-32. Available at: https://pubmed.ncbi.nlm.nih.gov/10664049/ (Accessed: 18 November 2023).
- Ruderman, J. Harbin, T. Campbell, D. (1986) ‘Postoperative suprachoroidal hemorrhage following filtration procedures,’ Archives of Ophthalmology, 104(2), pp. 201-205. Available at: https://jamanetwork.com/journals/jamaophthalmology/article-abstract/635902 (Accessed: 18 November 2023).
- Sabti, K. Lindley, S. Mansour, M. Discepola, M. (2001) ‘Uveal effusion after cataract surgery: an echographic study,’ Ophthalmology, 108(1), pp. 100–103. Available at: https://pubmed.ncbi.nlm.nih.gov/11150272/ (Accessed: 18 November 2023).
- Sami, M. Soparkar, C. Patrinely, J. Tower, R. (2007) ‘Eyelid Edema,’ Seminars in Plastic Surgery, 21(1), pp. 24–31. Available at: https://pubmed.ncbi.nlm.nih.gov/20567653/ (Accessed: 24 November 2023).
- Hoffman, R. Braga-Mele, R. Donaldson, K. Emerick, G. Henderson, B. Kahook, M. Mamalis, N. Miller, K. Realini, T. Shorstein, N. Stiverson, R. Wirostko, B. (2016) ‘Cataract surgery and nonsteroidal antiinflammatory drugs,’ Journal of Cataract and Refractive Surgery, 42(9), pp. 1368-1379. Available at: https://pubmed.ncbi.nlm.nih.gov/27697257/ (Accessed: 24 November 2023).
- Gawęcki, M. Grzybowski, A. (2016) ‘Diplopia as the Complication of Cataract Surgery’ Journal of Ophthalmology 2016, 2728712. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4779543/ (Accessed 19th November 2023).
- Kalantzis, G. Papaconstantinou, D. Karagiannis, D. Koutsandrea, C. Stavropoulou, D. Georgalas, I. (2014) ‘Post-cataract surgery diplopia: aetiology, management and prevention’ Clinical and Experimental Optometry 97(5), pp. 407-410. Available at: https://onlinelibrary.wiley.com/doi/full/10.1111/cxo.12197 (Accessed 19th November 2023).
- Nayak, H. Kersey, J. Oystreck, D. Cline, R. Lyons, C. (2008) ‘Diplopia following cataract surgery: a review of 150 patients’ Eye 22, pp. 1057–1064. Available at: https://www.nature.com/articles/6702847 (Accessed 19th November 2023).
- Alemu, H. Kumar, P. (2021) ‘Monocular Diplopia: An Optical Correction Modality’ Case Reports in Ophthalmology 12, pp 501–506. Available at: https://www.karger.com/Article/FullText/513215 (Accessed 19th November 2023).
- Shi, M. Ye, Y. Qin, A. Cheng, J. Ren, H. Chen, S. Xing, Y. (2022) ‘Etiology and Management of Binocular Diplopia after Cataract Surgery’ PREPRINT (Version 1). Available at: https://assets.researchsquare.com/files/rs-2040245/v1/16944563-3332-4a40-a0ea-b0a0da8d2dec.pdf?c=1667923174 (Accessed 19th November 2023).
