Patients presenting to our clinics are often taking a wide variety of prescribed medications or have been through advised medical procedures. However, these days in increasing abundance, and perhaps thanks in part to Google, patients are choosing to take medications or undergo procedures of their own volition.
The recently published TFOS elective medications and procedures report attempts to review these patient choices to understand the impact that they can have on the ocular surface and the underlying mechanisms. The word ‘elective’ refers to medications and procedures undertaken by choice or with a lower grade of prioritisation.
Patients usually use elective medications or undergo elective procedures to treat pathologic conditions or for cosmetic enhancement, impacting their lifestyle positively and thus, improving their quality of life. However, those interventions can affect the homeostasis of the tear film and ocular surface. Consequently, they generate signs and symptoms that could impair the patient’s quality of life.
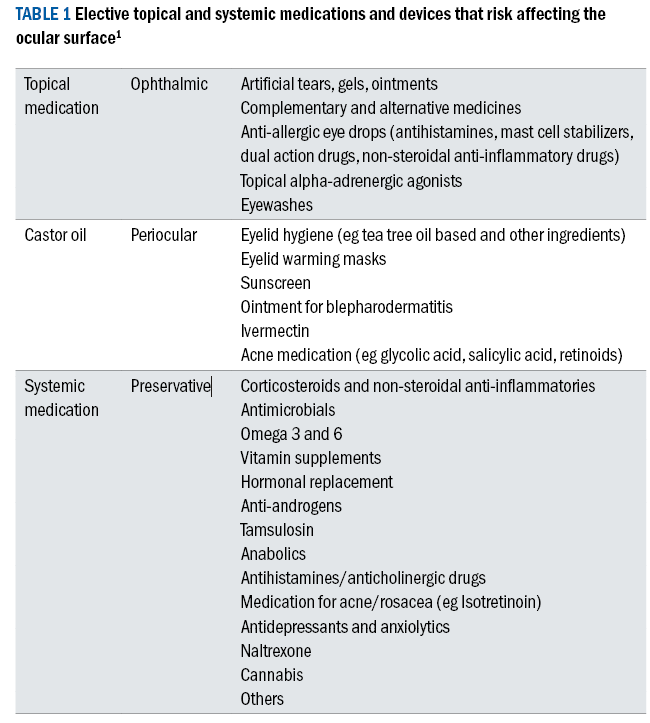
Table 1 shows a summary of the most common elective medications that have risk implications for the ocular surface.
There has also been a shift in attitude as patients become more cosmetically aware, and a consequent increase in uptake of elective procedures, in particular those that target the periocular region such as eyelid lifts. The full impact on the ocular surface of these procedures is still fairly unknown.
Table 1 and 2 offer a summary of the typical medications and procedures that have the potential to adversely affect the ocular surface.
Table 2: Ophthalmic and non-ophthalmic surgical and non-surgical procedures and devices that risk affecting the ocular surface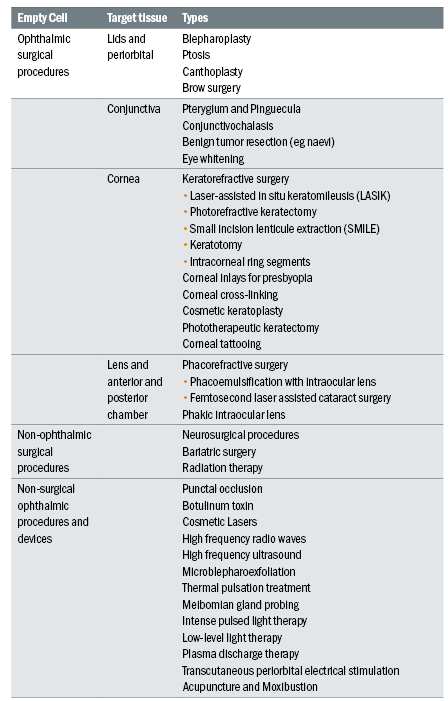
Topical Medications
Various topical ocular medications and formulations may cause problems at the ocular surface. Mechanisms of damage include ocular toxicity from preservatives, ingredients and excipients as well as the pH and tonicity of the formulations.
For example, topical alpha-adrenergic agonists used in the management of ocular allergies, but also frequently chronically (ab)used as eye-whiteners, may lead to ocular surface damage over time.
Preservatives
Many topical medications contain preservatives, and these days their toxicity is widely understood among practitioners. They typically act at the ocular surface through various mechanisms, exerting allergic, toxic and immune-inflammatory effects, or by chemical interactions with different components of the ocular surface.
Possible targets of preservative toxicity are the tear film, either by disrupting the lipid layer through detergent type effects, by reducing aqueous secretion or adversely affecting the conjunctival goblet cells, conjunctival and corneal epithelia, the corneal nerves through neurotoxic effects, or the eyelids at the skin or the meibomian glands.
Experimental data demonstrate an increase in tear film osmolarity and direct pro-inflammatory effects of benzalkonium chloride, with release of inflammatory cytokines and increased expression of chemokine receptors, as well as a significantly increased infiltration of dendritic inflammatory cells into the central cornea.
Studies consistently show improvement in reported symptoms and signs of ocular surface disease when patients are switched from preserved to non-preserved preparations.
Ophthalmic products
Certain products are more likely to induce issues on the ocular surface. Artificial tears can contain a variety of components, with a meta-analysis showing a superior efficacy of hyaluronic acid containing drops. Common side effects generally can be blurred vision or stinging on instillation.
Lipid containing drops more commonly cause burning on initial application in up to 24% of patients. Manuka honey-based eye drops and gels have been shown to improve MG expressibility and reduce bacterial colony counts but are known to commonly sting on application.
Allergy
Antihistamines are effective in ocular allergy, with emedastine and cetirizine known in particular to commonly cause burning and stinging. Mast cell stabilisers require multiple daily doses and have a delayed onset of action compared to antihistamines.
Stinging on instillation, dysgeusia, itching, burning, foreign body sensation, conjunctival chemosis and dry eye have been reported after the use of the mast cell stabilisers sodium cromoglycate and nedocromil sodium. Dual-acting anti-allergy drugs provide a combined mast cell stabiliser and antihistaminic function (selective H1 receptor antagonist), thus providing improved symptom control than single-action drugs.
Topical ketotifen and olopatadine did not induce short-term drying and did not worsen signs and symptoms of mild-to-moderate dry eye disease in clinical studies. Non-steroidal anti-inflammatories are rarely used in the treatment of ocular allergy due to their local side effects on instillation.
These include conjunctival hyperaemia, burning, stinging due to inherent properties of the free compounds, which alone can adversely impact the unprotected mucous membrane. Corneal melt is the most serious side effect of topical non-steroidal anti-inflammatories and occurs usually in patients whose cornea is compromised by ocular surgery, diabetes or autoimmune disease.
Topical alpha agonists are often used as first line treatment due to their over-the-counter availability. They improve hyperaemia, but have little to no effect on itching, and have a short duration of action. First generation ocular topical anticongestants include phenylephrine (selective alpha 1 agonist).
Second generation alpha-adrenergic receptor agonists are naphazoline (a mixed α1/α2 receptor agonist with a binding affinity of 2:1 for α2:α1 receptors) and oxymetazoline. Brimonidine is a third-generation adrenergic receptor agonist with noticeably increased binding affinity for α2 receptors relative to α1 receptors (1000:1), licensed for lowering IOP, for facial rosacea in gel form and an ocular solution for reduction of redness, and can last for up to eight hours.
Common side effects of sustained use of mixed α1/α2 adrenergic agonists include allergy, rebound hyperemia, chronic toxic follicular conjunctivitis, (eczematoid) blepharoconjunctivitis and tachyphylaxis.
Periocular products
These may include products for eyelid hygiene, eyelid warm compresses, sunscreens and creams or ointments for eyelid inflammation, acne or rosacea. Some may be recommended by healthcare practitioners, while others are chosen by the patient with no formal consultation.
Products used peri-ocularly have the potential to interact with the ocular surface facilitated by the action of the blink or simply due to the proximity of the relevant anatomical structures. Many of the products applied to the periocular area contain a combination of chemical, organic or synthetic compounds, preservatives, buffers, surfactants, fragrances, dyes and other excipients.
These products can affect the eyelids, eyelid margins, tear film and ocular surface epithelium. Eyelid microbiota, obstruction of eyelid margin gland orifices, tear film pH, tear osmolarity, tear stability, ocular surface integrity can be altered. This may lead to temporary and local irritation, allergic contact reactions and dry eye disease.
Eyelid hygiene
The therapeutic objective of eyelid hygiene products is to reduce the bioburden along the lid margins to help curb inflammatory responses.
The pathophysiology of blepharitis is not yet completely understood, root causes include microbial (bacterial, fungal, viral or parasitic) infections, immunological conditions (dermatitis, Stevens-Johnson syndrome, graft versus host disease), eyelid tumours, trauma (chemical, thermal, radiation) and toxins (medicamentosa).
Studies have highlighted the antimicrobial properties of several eyelid hygiene products including those containing coconut oil, 4-terpineol, hypochlorous acid, extracts from Abelmoschus esculentus (okra), Manuka honey and aloe vera.
Eyelid warm compresses
Eyelid warm compresses are globally part of the first line recommendations in the management of evaporative dry eye and meibomian gland dysfunction. The therapeutic objective is to soften the meibum in the glands so that it can be expressed more easily during a blink.
The melting point of normal meibum ranges between 32 and 40°C, and it is typically liquid at body temperature, whereas it melts at 3°C higher in abnormal secretions meaning it may be solid in the meibomian glands, at body temperature. Warm compresses have been shown to improve symptoms of dry eye disease, increase tear film lipid layer thickness, reduce tear evaporation, improve tear film stability, improve meibomian gland obstruction, improve contact lens comfort and reduce partial blink rates.
There is no consensus or evidence as to which type of heat delivery option is more efficacious or preferred by patients. Eye masks may or may not be washable or have an antimicrobial cover, rendering them prone to contamination from cosmetics and after repeated use.
However, heating for 30s in an 800W microwave oven did significantly reduce the load of applied Staphylococcus A, Pseudomonas A and Streptococcus A on flaxseed-filled masks, but not to sterility.
External eyelid creams
Suncream nanoparticles of titanium dioxide and zinc oxide, commonly found in sunscreen products, have been found to be cytotoxic to corneal cells in animal models and affect dry eye cases more than controls.
Ivermectin is a broad spectrum antiparasitic used in dermatology (rosacea, head lice), tropical medicine (river fever), and ophthalmology (demodicosis). A meta-analysis on the efficacy of local versus systemic treatments for demodex, revealed no difference for mite counts, eradication rate or symptom improvement. Another meta-analysis on the effectiveness of interventions for demodex, found that pharmacological interventions were superior to thermal, mechanical or light therapy.
Corticosteroids have well-documented ocular adverse effects and have been associated with glaucoma and cataract. Topical use for inflammatory conditions of the periocular area (blepharodermatitis) have been reported to cause elevated intraocular pressures and reduced visual function as well as allergies (to the corticosteroid or preservatives), decreased wound healing and increased susceptibility to infections.
Retinol/retinoid therapy can be used to treat acne as well as wrinkles. It is well known that systemic retinoid therapy affects the ocular surface. Dry eye disease in patients treated with retinoids, whether topical or systemic, seems to be due to decreased meibomian gland function and consequently increased tear film evaporation and osmolarity.
Systemic medications
Systemic therapies such as anti-inflammatories, immunomodulatory and antimicrobial drugs have been used to treat dry eye disease. However, elective systemic therapies can also have deleterious effects on ocular surface health via different mechanisms, including their impact on meibomian glands, lacrimal glands and goblet cells, which may result in dry eye disease.
The effect can result from deposition but also through affecting innervation or regulation of blood vessels that lead to reduced functioning. Some contribute to amplifying the immune system and increasing inflammatory markers that contribute to the degeneration of ocular surface health. They can also lead to reduced sensitivity or increased pain of the ocular surface. Extensive use of oral corticosteroids might lead to corticosteroid dependence and rebound of the ocular surface inflammation when tapered, causing conjunctival hyperaemia and dry eye disease symptoms.
Antibiotics can be the causative agents of Stevens-Johnson Syndrome. In a large multinational study on drug use and onset of Stevens-Johnson Syndrome, the use of trimethoprim-sulfamethoxazole and other sulfonamide antibiotics, aminopenicillins, quinolones and cephalosporins were significant risk factors for developing Stevens-Johnson Syndrome and potentially severe ocular surface alterations.
HRT – as elaborated in the TFOS DEWS II epidemiology Report, estrogen replacement therapy after menopause has been associated with increased incidence of dry eye disease in a study done of 25,665 women. It is hypothesised that estrogen causes dry eye disease through inducing regression of lacrimal glands, reduced metabolic function and consequent tear output.
Isotretinoin is a well-known risk factor for the development of meibomian gland dysfunction due to its reduction of proliferation and differentiation of glandular epithelial cells. This can lead to subsequent glandular atrophy, keratinisation of ducts, acinar cell degeneration and peri-acinar cell fibrosis.
The use of antidepressants and anti-anxiety medications has been strongly associated with prevalence of dry eye disease. The possible mechanisms for the effects of antidepressants on dry eye disease may be due to their anticholinergic effects. Another plausible theory is that their production of increased serotonin and inflammatory mediators leads to sensitisation of corneal nerve endings and reduced pain thresholds.
Elective procedures
Surgical procedures aimed at rejuvenating the periorbital area often comprise treatment to both the upper and lower eyelid as well as the brow. Periocular surgery aims for an aesthetic outcome while maintaining functional eyelids and a healthy ocular surface.
Periocular cosmetic surgery may also affect the ocular surface and tear film depending on the location, since the lids lubricate and protect, depending on technique, amount of tissue removed. Lacrimal gland injury can also occur during upper blepharoplasty. Conjunctival chemosis can develop in the early or intermediate postoperative period after blepharoplasty due to incomplete eyelid closure and conjunctival exposure.
Botox can be administered peri-ocularly for a wide variety of clinical disorders, predominantly blepharospasm, but can also be used for protective ptosis. There currently seem to be no reported permanent serious adverse events, but with reversible issues such as ptosis being a risk of between 1-5%.
Ablative laser surgery for resurfacing is designed to improve skin tone by increasing collagen and remodelling through healing. The laser can cause thermal injuries to the cornea.
Refractive surgical procedures have grown over the past decades. Techniques and success rates continue to improve, however ocular surface disease signs and symptoms remain common, particularly in the early postoperative period, and is the main reason for patients being dissatisfied after Lasik surgery.
Most of these patients recover, but a small percentage develop chronic issues or develop neuropathic pain. In Lasik, reduction of corneal sensitivity is a universal phenomenon due to the amputation of the corneal nerves, in creating a flap, and after photorefractive keratectomy due to the ablation of nerves sprouting in the superficial stroma.
After Lasik there is a marked reduction in the subbasal nerve plexus density and pattern, which may take more than five years to recover to standard values as evaluated with in vivo confocal microscopy.
Reduced corneal nerve sensory function reduces feedback to the lacrimal gland and basal tear secretion. The anterior cornea of patients who undergo small incision lenticule extraction refractive surgery (Smile) receive only a 2-3mm incision in the superficial cornea. Therefore, it induces less denervation and less reduction of corneal sensation than Lasik in the postoperative period (see figure 1).
Figure 1: The potential impact of ocular surgery on the corneal nerve network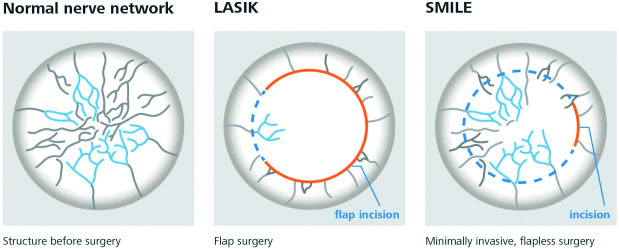
Regarding more standard cataract surgery, signs and symptoms of dry eye disease are common in the early postoperative period with a peak at about one week after surgery. Incidence of dry eye disease post-cataract surgery has been reported from 8% to 42% of patients seven days postoperatively.
Improvement is evident usually at three months postoperatively, although some studies report longer rehabilitation time of up to six months or more. Surgical transection of the corneal nerves by the corneal incision is a mechanism common to corneal surgical techniques and a causative factor of postoperative dry eye disease.
Several elective dry eye treatment procedures are now available such as IPL, low level light therapy, gland probing, thermal
pulsation, microblepharoexfoliation and quantum molecular resonance. The safety profile of these treatments is generally good and they offer a diverse range of options for the clinician.
Neuropathic corneal pain
Neuropathic corneal pain has been identified as a complication of laser vision correction surgeries, cataract surgery and glaucoma surgery. Neuropathic corneal pain is caused by injury or disease affecting the corneal somatosensory pathways.
It is characterised by non-specific ocular symptoms, including pain, discomfort, burning, grittiness, irritation, dryness, light sensitivity, hyperalgesia (enhanced pain response to infra-threshold noxious stimuli) and allodynia (pain caused by non-noxious stimuli), which occur out of proportion to what could be expected from the ocular surface findings.
Peripheral nerve damage and inflammation may also result in increased nerve sensitivity and augment peripheral pain. Over time, the central neurons may become more highly responsive to stimuli, in addition to spontaneous discharges, suggesting central sensitisation. Peripheral and central sensitisation result in development of chronic, persistent symptoms.
Understanding these underlying mechanisms is important to the potential management of these patients. Damage to the nerves alters the afferent stimuli of the neural reflux arc and reduces tear production, which can activate inflammatory cascades with a loop effect that can result in neuroinflammation, with corneal nerve regeneration impairment and development of micro-neuromas adding to neuropathic pain.
To test this in clinic, it is possible to instil 5% sodium chloride drops and assess comfort scores (visual analogue scales are useful for this, see figure 2) after 20 seconds – an exaggerated pain response indicates probable functional changes in the corneal nerves.
Figure 2: Example visual analogue scale (VAS), based upon Haas et al 201614
Another test for corneal nerve function; differentiating peripheral versus central sensitisation is using topical proxymetacaine 0.5% anaesthetic: Patients evaluate their discomfort immediately after instillation on the visual analogue scale, they then wait 90 seconds and re-evaluate the pain level.
- Total pain relief = peripheral sensitisation
- Partial pain relief = mixed sensitisation
- Unchanged (or exaggerated) pain relief = central sensitisation
Current specific treatment strategies are very limited and include controlling the inflammation and suppressing central mechanisms of pain. Neurotrophic keratopathy can occur with persistent corneal nerve damage from many origins. Corneal nerves are involved in corneal sensation, but also regulate corneal epithelial growth, migration, and collagen production.
Impairment therefore affects epithelial homeostasis, reducing the ability of the corneal epithelium to maintain its integrity. This can result in persistent epithelial defects and can progress to stromal melt and perforation if untreated.
Management strategies
Obviously, these will depend on the causative issues, but thoroughly addressing any dry eye disease and controlling inflammation prior to any ocular surgery (and immediately after if required) is not to be underestimated, and also can help significantly with successful, stable and accurate outcomes.
When topical or systemic drugs are a suspected contributory factor, a subtraction strategy can be helpful, but oftentimes it is hard to identify the culprit.
Conclusions
Patients often undergo non-urgent elective procedures or use topical or systemic medications to improve their quality of life or cosmetic appearance, but sometimes these interventions are followed by ocular surface disease that paradoxically negatively affects their quality of life.
As clinicians we are well equipped to help and advise patients when issues arise as long as we embrace the opportunity to support them with awareness of the potential causes and arm ourselves with the skills and knowledge to help restore and improve their ocular surface.
- Sarah Farrant is a director at Earlam and Christopher Optometrists, an optometrist and dry eye specialist and acts as a consultant and key opinion leader for a number of national and global companies. Farrant has a great passion for her work, which is reflected in the wide variety of different interests she has within the profession. She has undertaken a number of additional qualifications and is a leading national expert in therapeutics and dry eye management and represents the UK as an Ambassador for TFOS. She is currently president elect for the BCLA and sat for 12 years on the executive and council boards for the College of Optometrists. She has been instrumental in a number of local and national initiatives to improve access to education amongst the profession and access to eye care for patients. She is also a keen lecturer and regularly teaches other optometrists both nationally and internationally.
Further information
Further information can be accessed at tearfilm.org:
Gomes JAP, Azar DT, Baudouin C, Bitton E, Chen W, Hafezi F, Hamrah P, Hogg RE, Horwath-Winter J, Kontadakis GA, Mehta JS, Messmer EM, Perez VL, Zadok D, Willcox MDP. TFOS Lifestyle: Impact of elective medications and procedures on the ocular surface. Ocul Surf. 2023 Jul;29:331-385. doi: 10.1016/j.jtos.2023.04.011.
Acknowledgements:
José Alvaro P Gomes, Dimitri T Azar, Christophe Baudouin, Etty Bitton, Wei Chen, Farhad Hafezi, Pedram Hamrah, Ruth E Hogg, Jutta Horwath-Winter 9, Georgios A Kontadakis, Jodhbir S Mehta, Elisabeth M Messmer, Victor L Perez, David Zadok, Mark D P Willcox
References
- José Alvaro P. Gomes, Dimitri T. Azar, Christophe Baudouin, Etty Bitton, Wei Chen, Farhad Hafezi, Pedram Hamrah, Ruth E. Hogg, Jutta Horwath-Winter, Georgios A. Kontadakis, Jodhbir S. Mehta, Elisabeth M. Messmer, Victor L. Perez, David Zadok, Mark D.P. Willcox, TFOS Lifestyle: Impact of elective medications and procedures on the ocular surface, The Ocular Surface, Volume 29,2023,Pages 331-385
- ASPS national clearinghouse of plastic surgery procedural statistics 2021 https://www.plasticsurgery.org/documents/News/Statistics/2020/plastic-surgery-statistics-full-report-2020.pdf
- C. Baudouin, A. Labbe, H. Liang, A.Pauly, F. Brignole-Baudouin. Preservatives in eyedrops: the good, the bad and the ugly, Prog Retin Eye Res, 29 (2010), pp. 312-334
- N. Jaenen, C. Baudouin, P. Pouliquen, G.Manni, A. Figueiredo, T. Zeyen. Ocular symptoms and signs with preserved and preservative-free glaucoma medications. Eur J Ophthalmol, 17 (2007), pp. 341-349
- Y.J. Yang, W.Y. Lee, Y.J. Kim, Y.P. Hong. A meta-analysis of the efficacy of hyaluronic acid eye drops for the treatment of dry eye syndrome. Int J Environ Res Publ Health, 18 (2021)
- A. Feser, V. Mahler. Periorbital dermatitis: causes, differential diagnoses and therapy. J Dtsch Dermatol Ges, 8 (2010), pp. 159-166
- G. Amescua, E.K. Akpek, M. Farid, F.J.Garcia-Ferrer, A. Lin, M.K. Rhee, et al. Blepharitis preferred practice pattern(R). Ophthalmology, 126 (2019), pp. P56-P93
- R. Ahmad, H. Mehta. The ocular adverse effects of oral drugs. Aust Prescr, 44 (2021), pp. 129-136
- J.C. Roujeau, J.P. Kelly, L. Naldi, B. Rzany, R.S. Stern, T. Anderson, et al. Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N Engl J Med, 333 (1995), pp. 1600-1607
- D.A. Schaumberg, J.E. Buring, D.A.Sullivan, M.R. Dana. Hormone replacement therapy and dry eye syndrome. JAMA, 286 (2001), pp. 2114-2119
- Y.C. Liu, A.S.J. Jung, J.Y. Chin, L.W.Y. Yang, J.S. Mehta. Cross-sectional study on corneal denervation in contralateral eyes following SMILE versus LASIK. J Refract Surg, 36 (2020), pp. 653-660
- D. Jiang, X. Xiao, T. Fu, A. Mashaghi, Q.Liu, J. Hong. Transient tear film dysfunction after cataract surgery in diabetic patients. PLoS One, 11 (2016), Article e0146752
- G. Dieckmann, S. Goyal, P. Hamrah. Neuropathic corneal pain: approaches for management. Ophthalmol Times, 124 (2017), pp. S34-S47
- Haas P, Falkner-Radler C, Wimpissinger B, Malina M, Binder S. Needle size in intravitreal injections–pain evaluation of a randomized clinical trial. Acta ophthalmologica. 2016 Mar;94(2):198-202)
