
Fake eyelashes have been around for over 100 years. A Canadian woman Anna Taylor patented the first fake lashes in 1911. A more recent development from 2006 is that of eyelash serums, which promote the growth of natural eyelashes. While long eyelashes (be they fake or real) might be considered a charming adjunct to a person’s beauty, do they have any side effects and are any lashes long enough to hide the weeping, red eyes that may lie beneath them?
Many of my female students wear fake eyelashes and for the most part they have few complaints, other than when clumps of their lashes come out when practicing lid eversions on each other. However, one student told me about one of her more negative experiences of getting fake eyelashes applied. She said her eyes began to sting while the procedure was still in progress, but she continued to have the lashes applied as she needed them for a social event the following evening.
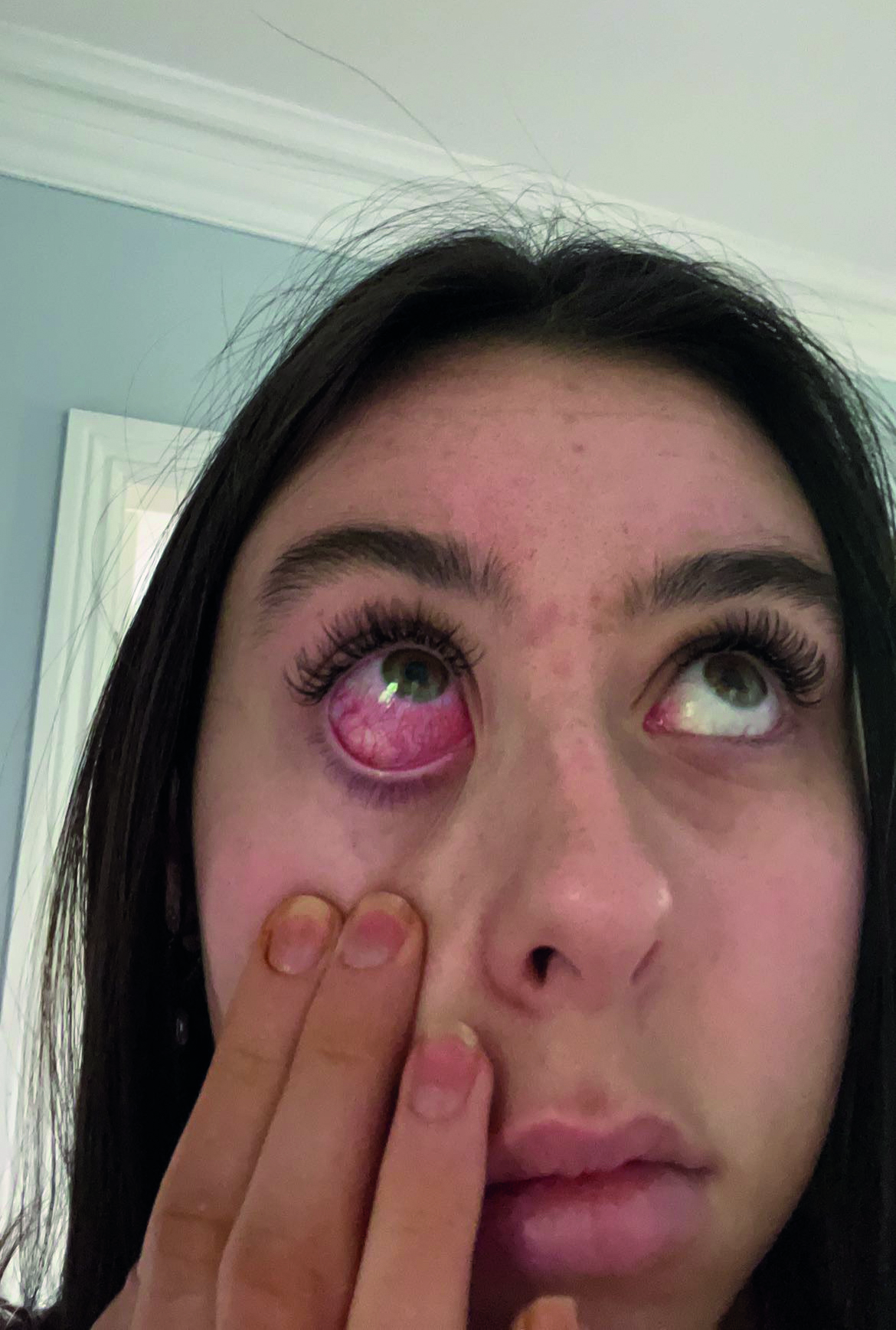
By the time the procedure was finished one eye was stinging and had marked conjunctival hyperaemia. By the next morning the hyperaemia was still present and she went back to the beauticians to have the lashes removed. The stinging had subsided at this stage, but it took approximately two days for the hyperaemia to fully resolve, see figure 1.
Figure 1: Hyperaemia reaction after use of fake lashes
Fake eyelashes are made from various different materials. The cheapest are made from polyester, the most expensive are made from mink fur, and silk is also used. Fake lashes may be glued in place individually lash by lash or a whole lash line held together on a thin weave may be glued in place. The adhesive that holds the lashes in place normally contains cyanoacrylates, ammonia and latex. These formulations are formaldehyde-emitting.
Any of the materials used either to make the lashes or the adhesive can cause an allergic reaction, but the most common allergen is formaldehyde. A 2012 Japanese study investigated 107 women who attended an ophthalmologist following fake eyelash complications.1
Sixty-four women had keratoconjunctivitis where some adhesive had leaked onto the ocular surface. Forty-two women had allergic blepharitis (with four of those having a combination of keratoconjunctivitis and blepharitis). The authors concluded that the formaldehyde emitted by the adhesive was the most likely causative agent in all cases.
A Nigerian study surveyed 310 fake eyelash wearers aged 16 to 52.2 They found that the most common symptom experienced by wearers was itching (45.8%) but respondents also noticed hyperaemia, pain (including burning sensations and foreign body sensations), heavy eyelids, loss of lashes, a shadow in the visual field, epiphora and a chalazion.
In a case report from Iran, ophthalmologists found apparent bilateral superior scotomas in a patient on visual field testing.3 They were initially baffled until one of them wondered if it could be the patient’s fake eyelashes causing the scotoma. A repeat test with the fake lashes removed showed a normal visual field again.
In an Optometry Times blog, an optometrist based in Arizona described a severe case of demodex blepharitis, which she believes was initiated or exacerbated by the fact that the patient had not been cleaning her eyelids or lashes, for fear that she might damage her very expensive eyelash extensions.4
A case report from Sweden described a patient who developed eczema and contact dermatitis around her eyes, from a preservative found in the gel pack used to tape down the lower eyelashes while eyelash extensions are being applied.5 The patient needed topical corticosteroids and antihistamines to eventually get the allergic reaction under control.
In the case of this patient, she was found to be allergic to methylisothiazolinone (MI). She had been using a cosmetic cream containing MI for several months before having eyelash extensions applied but had never had an allergic reaction to it. Nonetheless patch testing found MI to be the causative allergen contained within the gel pack.
Patients assume that allergic reactions can only come from the use of something new on their skin. In order for an allergic reaction to occur the body must have an initial exposure to the potential allergen. At this point, the sensitisation process occurs and the body makes specific antibodies against the allergen. Re-exposure to the allergen leads to the elicitation phase where the effects of the allergic reaction are seen.
However, many chemicals implicated in contact dermatitis are very weak sensitisers and it can take weeks, months or even years of repeated exposure before a severe allergic reaction occurs. This explains why the Swedish patient ‘became allergic’ to something to which she was seemingly never allergic before.
Eyelash serums are a relatively recent phenomenon. Ophthalmologists have long been aware of the side-effect of eyelash thickening and lengthening in patients using prostaglandin analogue (PGA) glaucoma medication. In 2006, the American ophthalmologist Michael Brikenhoff was the first person to formulate a PGA serum specifically to improve eyelash appearance.
His wife was undergoing treatment for breast cancer and her lashes and brows were weak and falling out as a result of the treatment. The serum he formulated is now manufactured by an Allergan subsidiary and sold as Latisse. It contains 0.03% bimatoprost. It is FDA approved for people with inadequate lashes and is available on prescription. Although it seems to be very easy to obtain it without a prescription.6
There are other lash serums available without prescription, but they have lower concentrations of bimatoprost, and other PGAs used such as latanoprost do not seem to be as effective at promoting lash growth. The anti-glaucoma medication Lumigan used to have the same formulation as Latisse with 0.03% bimatoprost, but since 2015 the concentration of bimatoprost has been reduced to 0.01%.
The lower concentration was found to be as effective as the higher one but with fewer adverse effects.7,8 The concentration of Latisse remains at 0.03%. Latisse also contains the preservative benzalkonium chloride. There are also many PGA-containing lash serums that are not FDA approved.
The growth cycle of eyelashes appears to be different to other body hair. Eyelash follicles have prostanoid receptors and when bimatoprost interacts with these receptors it stimulates the progression of the lash cycle from the dormant phase to the growth phase and it prolongs the growth phase leading to longer lashes. In addition, bimatoprost increases the size of the dermal bulb (lash bulb) and it is this that makes the lashes thicker.
A large industry-run multicentre trial of Latisse took place in 2012.9 Subjects were randomised into the treatment group where they used 0.03% bimatoprost for 16 weeks (n = 137) or the placebo group (n = 141). There were more adverse events in the treatment group and these consisted of; conjunctival hyperaemia, eye pruritus, pinguecula, eye irritation, dry eye, erythema of eyelid, upper respiratory tract infection and skin hyperpigmentation.
All adverse events resolved before the end of the trial. Four subjects in the treatment group dropped out because of; eczema, dry eye, eye inflammation and contact dermatitis. The researchers concluded that overall, the benefit to risk safety profile was positive, ie the benefits (for patients with hypotrichosis) outweigh the risks.
However, in a 2021 review of the safety of PGA lash serums, the authors advise that published studies on the use of these serums is dominated by industry-sponsored clinical trials and that the designs of these trials are such that there may be gaps in our understanding of the true safety profile of these products.10
In particular, they feel that drug application discomfort and periorbitopathy have been underreported and that the risk of increased iris pigmentation needs to be better investigated. Periocular hyperpigmentation secondary to bimatoprost use for glaucoma appears to occur because bimatoprost induces increased melanin production.11
Periorbital volume loss is a well-established side effect of the use of PGA glaucoma medication, but it is not listed as a side effect of PGA-containing lash serums. In 2022, an informal randomised control study investigated whether PGA derivatives being applied along the lash line caused a loss of periorbital fat.12 The trial used ‘before and after’ photos readily available on separate PGA lash serum manufacturer websites.
Two control groups were also employed. The control groups were photos of subjects who were using non-PGA lash serums and subjects wearing false eyelashes. While the authors acknowledge that the photos could have been digitally altered, they still concluded that PGA lash serum users experience periorbital volume loss consistent with topical PGA use. There is a natural reduction in periorbital fat with ageing and therefore this PGA-induced loss of fat has the unfortunate effect of making users appear older. The effect does seem to reverse on cessation of serum use.
A 38-year-old woman attended our contact lens clinic to ascertain if she could return to contact lens wear. She had been seen a month previously and at the time the optometrist noted that she had conjunctival hyperaemia and she was complaining of contact lens discomfort. The optometrist suspected that the problem was due to the patient’s use of a PGA-containing lash serum and the patient was advised to cease using the lash serum and cease wearing contact lenses until the signs and symptoms abated.
When we saw her, the eyes were quiet and she had no obvious adverse ocular signs. We advised her that she could return to contact lens wear. She asked us about the red appearance on her eyelids. Nothing was obviously visible to the naked eye, but a careful slit lamp examination of her upper eyelid revealed a thick line of marked telangiectasia just above her lashes in exactly the area where she had previously been applying the lash serum.
Telangiectasia is not a reported side effect of PGAs, but PGAs used in glaucoma medications do have known side effects of iris and skin hyperpigmentation.13 These effects do not appear to have been widely reported or investigated with the use of lash serums. The pigmentation changes in the iris do not appear to be reversible, whereas the changes in the lashes and skin may be reversible some time after cessation of use, but the truth is we just do not know for sure what the effect will be or for how long it will last (post-treatment) on any one individual.
There are non-PGA-containing lash serums. However, without the PGA, the serums cannot increase the length of eyelashes, but the manufacturers claim that the serums can strengthen and condition lashes. For those who do not want to use lash serums or lash extensions there is also the option of a lash lift. This is where the natural lashes are glued to a curling tong. This glue is less harsh than that used for lash extensions.
An activating solution is applied to the lashes, followed by a neutralising solution to hold the curl in place. Everything is then washed off with saline solution. The whole process takes about an hour and the effect can last from three-and-a-half to five weeks. The advantage of a lash lift over fake lashes is that the face can be washed as normal with a lash lift. As always any of the products used can cause allergic reactions on the skin and do cause irritation if they get into the eyes.
In 2021, a case of bilateral preseptal cellulitis was reported in a 55-year-old woman who had had eyelash lifts.14 The authors believe that the presence of blepharitis and/or meibomitis combined with the solutions used during the treatment probably triggered the preseptal cellulitis.
Lash tinting is the application of a permanent dye to the lashes and sometimes also the eyebrows, usually to darken them. It is particularly popular among women with light coloured lashes. Until July 2022, the FDA had not approved any dyes for use on the lashes or eyebrows because the agency said ‘permanent eyelash and eyebrow tints and dyes have been known to cause serious eye injuries, including blindness’.15
Regardless, lash tints were available and many lash technicians in the USA were using them, because there was some uncertainty about their actual legality. After much lobbying on the part of the cosmetics industry, the FDA have approved silver nitrate as a colour-additive in professional-use only lash and brow tints.
Although the FDA makes it clear these tints should not be sold directly to the consumer, inevitably home-use lash tint kits are for sale direct to the consumer on the internet. Lash tints contain p-phenylenediamine (PPD), which has been implicated in allergic contact dermatitis and chemical burns.
In lash tint users, allergies to PPD have resulted in severe and persistent blepharo-conjunctivitis often with a purulent discharge, the development of xanthelasma palpebrarum lesions, centrofacial oedema and dermatitis, exudation of the eyelids and itching.16-20
A 2022 case report published in the Journal of Burn Care and Research describes how a 50-year-old woman was referred into a burns unit following a professional eyebrow tinting.21 She had to be treated for partial thickness chemical burns with an overlying superficial infection. Forty-nine days after the eyebrow tint her skin was fully healed and she suffered no permanent brow hair loss.
In 2006, a case series of three patients who had developed ocular argyrosis from the long-term self-application of eyelash tint was published.22 Ocular argyrosis is the permanent deposition of silver within tissues in the eye. In this case series the patients had silver deposits in the cornea and conjunctiva secondary to the long-term use of a lash tint.
The authors stated that the tint the patients were using was recommended only for use by professionals and they noted ocular argyrosis has never been reported in patients who have had their lashes tinted professionally. This is because lash technicians can prevent the tint from getting into or on, any part of the eyes or lids apart from the lashes. It is much more difficult to prevent tint from going where it is not supposed to when the tint is self-applied.
Permanent eyeliner or cosmetic blepharopigmentation is the tattooing of a dark line near the base of the eyelashes to make the lashes look fuller and more striking. Pigment is inserted into the superficial dermis of the eyelid using a tattoo needle. The pigment is relatively inert, but the procedure has reported side effects of allergic reaction and pigment deposition in other tissues. There have been several case reports of granulomatous inflammatory responses to blepharopigmentation.23
This reaction leaves the patient with raised lumps on their eyelids and has to be aggressively treated with steroids, including by injection. This reaction can be very delayed following the procedure. In one case report the patient developed bleeding ulcerations on top of eyelid lesions 10 years after the original tattooing.24
Microblading is a semi-permanent cosmetic procedure carried out for people with thin and sparse eyebrows. The blade used is made up of a row of tiny needles. These create tiny cuts into which pigment is deposited. These thin pigmented scratches look like fine eyebrow hairs. The effect is not permanent and it fades after one to three years. There have been several case reports of granulomatous plaques developing in the eyebrows secondary to microblading.25
In one case, the plaques were found to be signs of cutaneous sarcoidosis in a patient who had no other signs of sarcoidosis.26 Some plaques have developed as late as six years after the treatment.27 In one case report of two patients who developed sarcoidosis plaques secondary to microblading, the authors suggest that the microblading may have triggered the development of cutaneous and systemic sarcoidosis.27
A beauty trend that started circa 2022 is brow lamination. This is a treatment to make the eyebrows stick up vertically. It is available in the form of home lamination kits or it can be done by a professional. A chemical is applied initially to soften the eyebrow hairs. They are then combed into the desired shape and another fixing chemical is placed on them.
Finally, a third neutralising chemical is applied. While there are no peer-reviewed studies, randomised clinical trials or case reports available on the side effects of brow lamination, there are numerous TikTok videos showing erythema and oedema secondary to allergic reactions from the treatment and severe eyebrow hair loss in some cases (where presumably one or more chemicals was left in place for too long).
The Sun newspaper ran an article with illustrative photographs showing what appears to have been a relatively severe allergic response to brow lamination.28 What advice can the eye care professional offer patients who ask about cosmetic procedures involving the lids, lashes and brows?
You can try advising patients just to accept themselves for who they are and that it is the beauty inside that counts, but assuming this falls on deaf ears then this subsequent advice may be helpful.
- Always have a patch test 24 hours before any cosmetic procedure to rule out the possibility of an allergic reaction.
- Have the procedure done by a professional rather than attempting to do it yourself. A professional is in a better position to make sure the chemicals only go where they are supposed to and that they will not end up in other parts of the eye or adnexa. Try to use a professional that has come recommended or that has been given good reviews. (If they do not offer a patch test before the procedure, they may not have the customer’s best interests at heart).
- Clear up any inflammation, eg blepharitis, dermatitis, meibomitis, etc, before commencing any cosmetic procedure. This will reduce the risk of any secondary infection.
- Use non-PGA-based lash serums. Although their effects on lash growth are likely to be less dramatic than with those containing PGA, the side effects will be equally less dramatic.
- If wearing fake eyelashes, clean and brush the lashes daily with specialist non-oil-based cleaners to avoid the build-up of debris, the proliferation of demodex and any subsequent blepharitis.
Eye care professionals should also be alert to the fact that periocular and ocular changes in a patient could be secondary to a cosmetic procedure carried out many years previously and so as always, a very careful history should be elicited from the patient.
- Claire McDonnell is an optometrist and lecturer at Technological University Dublin. She is also a member of BUCCLE (British and Irish University and College Contact Lens Educators). Her research area is specialist contact lenses. She has presented in the UK, Ireland and Europe on contact lenses and optometric education.
Disclosure statement
The author has no financial interest in any of the treatments or products mentioned in this article.
References
- Amano Y, Sugimoto Y, Sugita M. Ocular disorders due to eyelash extensions. Cornea. 2012 Feb;31(2):121-5.
- Abah ER, Oladigbolu KK, Rafindadi AL, Audu O. Eyelash extension use among female students in a Tertiary Institution in Nigeria: A study of kaduna polytechnic, Kaduna. Niger J Clin Pract. 2017 Dec;20(12):1639-1643
- Yadgari M, Shahriari M. Error in Measurement of Visual Field Caused by False Eyelashes: A Case Report. Journal of Ophthalmic and Optometric Sciences. 2019;3(4): 47-50.
- Coats, J. Blog: a case of Demodex infestation with eyelash extensions. Optometry Times. April 24th 2019. Available at https://www.optometrytimes.com/view/blog-case-demodex-infestation-eyelash-extensions Accessed 10th April 2024
- Dahlin J, Hindsén M, Persson C, Isaksson M. What lash stylists and dermatologists should know! Contact Dermatitis. 2016 Nov;75(5):317-319.
- Saint Louis, C. Long lashes without prescription, but with risks. The New York Times. May 1, 2010. Available at https://www.nytimes.com/2010/05/02/health/02latisse.html accessed 12th April 2024.
- Allergan. Healthcare professional product information: Lumigan 0.01%. Available at https://www.lumigan.com/HCP/ Accessed 19th April 2024.
- Katz LJ, Cohen JS, Batoosingh AL, Felix C, Shu V, Schiffman RM. Twelve-month, randomized, controlled trial of bimatoprost 0.01%, 0.0125%, and 0.03% in patients with glaucoma or ocular hypertension. Am J Ophthalmol. 2010 Apr;149(4):661-671.e1.
- Smith S, Fagien S, Whitcup SM, Ledon F, Somogyi C, Weng E, Beddingfield FC 3rd. Eyelash growth in subjects treated with bimatoprost: a multicenter, randomized, double-masked, vehicle-controlled, parallel-group study. J Am Acad Dermatol. 2012 May;66(5):801-6.
- Steinsapir KD, Steinsapir SMG. Revisiting the Safety of Prostaglandin Analog Eyelash Growth Products. Dermatol Surg. 2021 May 1;47(5):658-665.
- Kapur R, Osmanovic S, Toyran S, Edward DP. Bimatoprost-induced periocular skin hyperpigmentation: histopathological study. Arch Ophthalmol. 2005 Nov;123(11):1541-6
- Jamison A, Okafor L, Ullrich K, Schiedler V, Malhotra R. Do Prostaglandin Analogue Lash Lengtheners Cause Eyelid Fat and Volume Loss?, Aesthetic Surgery Journal, Volume 42, Issue 11, November 2022, Pages 1241–1249
- Wirta D, Vandenburgh AM, Weng E, Whitcup SM, Kurstjens S, Beddingfield FC 3rd. Long-term safety evaluation of bimatoprost ophthalmic solution 0.03%: a pooled analysis of six double-masked, randomized, active-controlled clinical trials. Clin Ophthalmol. 2011;5:759-65.
- Mangan MS, Imamoglu S. Preseptal cellulitis associated with cosmetic eyelash lifting procedure. J Cosmet Dermatol. 2021 Jun;20(6):1846-1848. doi: 10.1111/jocd.14142. Epub 2021 Apr 16. https://www.fda.gov/cosmetics/cosmetic-products/eye-cosmetic-safety
- Ali L, Foulds JS, Abdul Ghaffar S. Severe eyelid allergic contact dermatitis secondary to eyelash tint: two case reports. Contact Dermatitis. 2017 Jul;77(1):59-60.
- Hansson C, Thorneby-Andersson K. Allergic contact dermatitis from 2-chloro-p-phenylenediamine in a cream dye for eyelashes and eyebrows. Contact Dermatitis. 2001 Oct;45(4):235-6.
- Teixeira M, de Wachter L, Ronsyn E, Goossens A. Contact allergy to para-phenylenediamine in a permanent eyelash dye. Contact Dermatitis. 2006 Aug;55(2):92-4.
- Bhat J, Smith AG. Xanthelasma palpebrarum following allergic contact dermatitis from para-phenylenediamine in a black eyelash-tinting product. Contact Dermatitis. 2003 Dec;49(6):311.
- Vogel TA, Coenraads PJ, Schuttelaar ML. Allergic contact dermatitis presenting as severe and persistent blepharoconjunctivitis and centrofacial oedema after dyeing of eyelashes. Contact Dermatitis. 2014 Nov;71(5):304-6.
- Brondeel KC, Duggan RP, Kirk K, Palackic A, Branski LK. 728 Chemical Burns and Eyebrow Tinting: An Unusual Case. J Burn Care Res. 2022 Mar 23;43(Suppl 1):S170.
- Gallardo MJ, Randleman JB, Price KM, Johnson DA, Acosta S, Grossniklaus HE, Stulting RD. Ocular argyrosis after long-term self-application of eyelash tint. Am J Ophthalmol. 2006 Jan;141(1):198-200.
- Vagefi MR, Dragan L, Hughes SM, Klippenstein KA, Seiff SR, Woog JJ. Adverse reactions to permanent eyeliner tattoo. Ophthalmic Plast Reconstr Surg. 2006 Jan-Feb;22(1):48-51.
- Bussel, II, Dhaliwal, DK. Cosmetic eyeliner tattoo as a risk factor for ocular surface disease. Ophthalmology Times. Jan 1st 2018. Available at https://www.ophthalmologytimes.com/view/cosmetic-eyeliner-tattoo-risk-factor-ocular-surface-disease. Accessed 10th May 2024.
- Ibraheim MK, Desai M, Tawfik M, Elsensohn A, Furukawa B. Microblading-Induced Granulomatous Reaction: Case Report and Review of the Literature. Am J Dermatopathol. 2023 Jul 1;45(7):487-491.
- Spurr A, Hanna N, Colantonio S. Cutaneous sarcoidosis in eyebrows cosmetically pigmented with microblading method: A case report and review of the literature. SAGE Open Med Case Rep. 2022 Aug 11;10:2050313X221117720.
- Merzel Šabović EK, Starbek Zorko M, Hosta V, Žgavec B, Bajuk V. Microblading reaction as a manifestation of systemic sarcoidosis: two case reports and a review of the literature. J Med Case Rep. 2024 Apr 24;18(1):221.
- Kulniece, K. Low brow. I was desperate for perfect brows so got them laminated, my massive allergic reaction shows why you NEED a patch test. The Sun newspaper. 17th Feb 2022. Available at https://www.thesun.ie/fabulous/8378237/eyebrow-lamination-brutal-reaction-swelling/ accessed 15th May 2024.
