Metastatic tumours to the eye are most commonly found in the uvea due to its abundant blood circulation.1 Consequently, this allows a developing tumour to utilise the uvea’s rich vascular supply to facilitate growth and dissemination.1 In the uvea, ocular metastases are predominantly detected in the post-equatorial choroid, while occurrences in other sites such as the iris and ciliary body are less frequent.2
Rarely, metastases may also affect the retina, optic disc, vitreous, and lens capsule.3 Until recently, studies concerning ocular metastases primarily concentrated on understanding histopathological findings, clinical symptoms, methods of diagnosis and treatment options for uveal metastatic conditions.4
Today, there is a growing body of extensive data with reports that delve into outcomes related to various factors such as the primary tumour site, age and sex of the patient, timing of metastasis onset, and the efficacy of imaging and biopsy techniques used for diagnosis.5-10 This review thoroughly explores these parameters while also discussing various management options, pertinent clinical features and outcomes.
How common is intraocular metastasis?
In the past, intraocular metastatic tumours were considered to be uncommon; however, improved indirect ophthalmoscopy and more recently fundus imaging have revealed a higher prevalence of metastatic disease than initially reported. Dating back to 1966, few surgeons documented a case of choroidal metastasis, primarily due to use of the direct ophthalmoscopy and lack of wide angle fundus imaging or even ultrasonography; however, it was later realised by comprehensive ophthalmic pathology reports that the incidence of choroidal metastasis was greater than suspected.11
For instance, Albert et al identified histopathologically under the microscope that choroidal metastases were present in 2% of 213 patients with known systemic cancer and systemic metastases.12 Similarly, Bloch and Gartner reported histopathologically-confirmed uveal metastatic foci in 8% of eyes from 230 patients with autopsy-proven carcinomas.13
Furthermore, Nelson and colleagues, in an autopsy investigation, observed ocular metastases in 4% of patients who had died from carcinoma.14 They estimated that approximately 22,000 cancer-related deaths in 1983 were associated with ocular metastatic disease.14 More recently, large-cohort clinical studies by Shields et al have emerged reporting specific cancers from a single organ that have metastasized to the eye from primary sources with cancer of the breast and lung, as well as skin melanoma, uveal melanoma and others.5
What malignancies most often metastasize to the eye?
In one large-cohort study by Shields et al, a total of 2,214 cases of uveal metastatic tumours in 1,111 patients were reviewed, revealing an average patient age of 60 years at tumour detection in the eye.5 The majority of patients were Caucasian (88%), and a substantial portion were female (64%).5 Additionally, 82% of the tumours were found to be unilateral, and the primary tumour site was identified before the uveal metastasis in 67% of the cases and after the uveal metastasis in 18% of cases (in 16% the primary site was not found).5
In this analysis, it was found that the most common primary tumours to metastasize to the eye were from breast cancer (37%) (figure 1) and lung cancer (26%) (figure 2).5
Figure 1: Wide angle photography of the right eye (OD) (A) showing a solitary choroidal metastasis from breast cancer with overlying retinal pigment epithelial mottling. Corresponding (B) fundus autofluorescence with hyper and hypoautofluorescence and optical coherence tomography (C) showing ‘lumpy bumpy’ surface appearance, typical for metastasis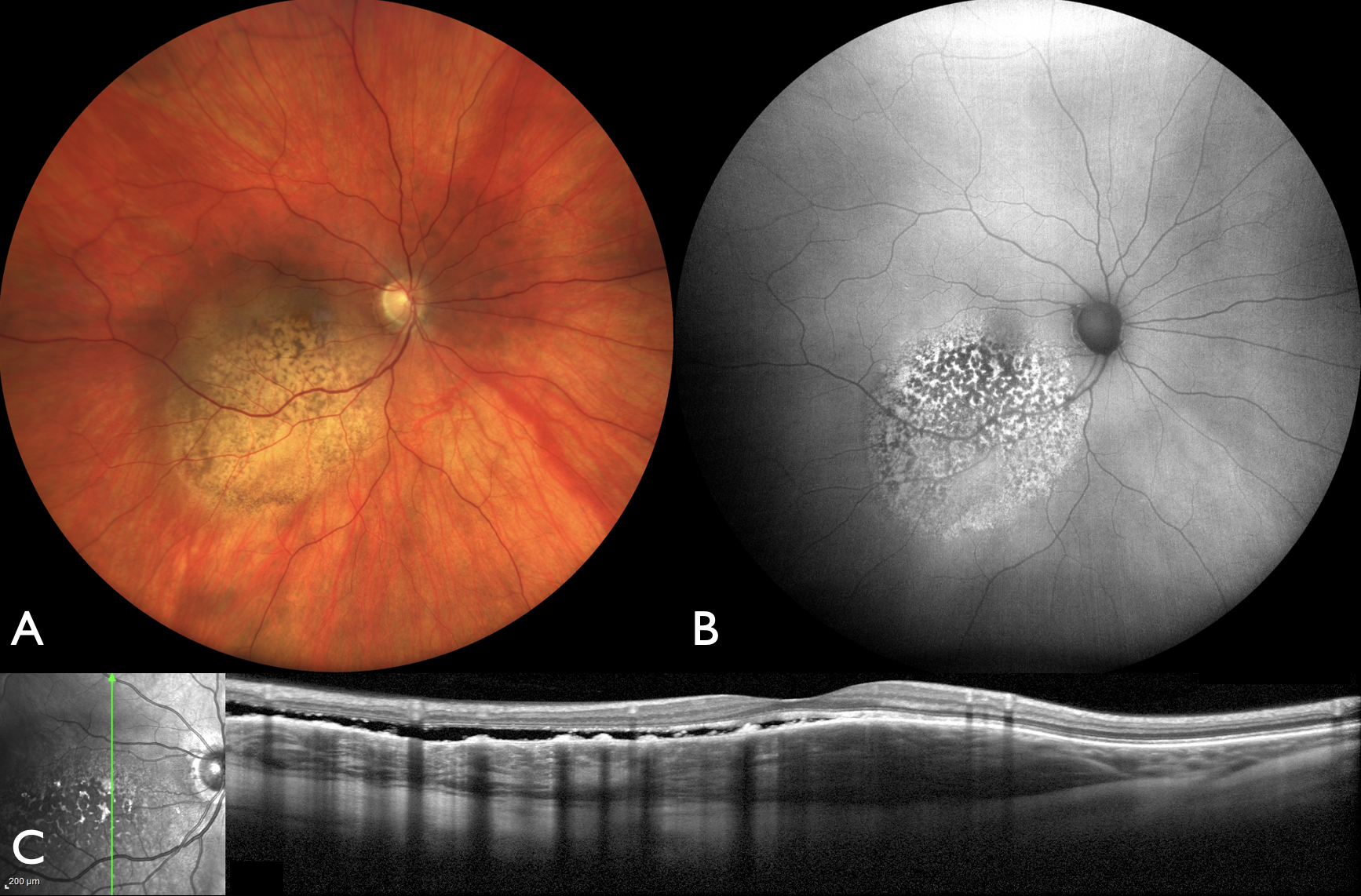
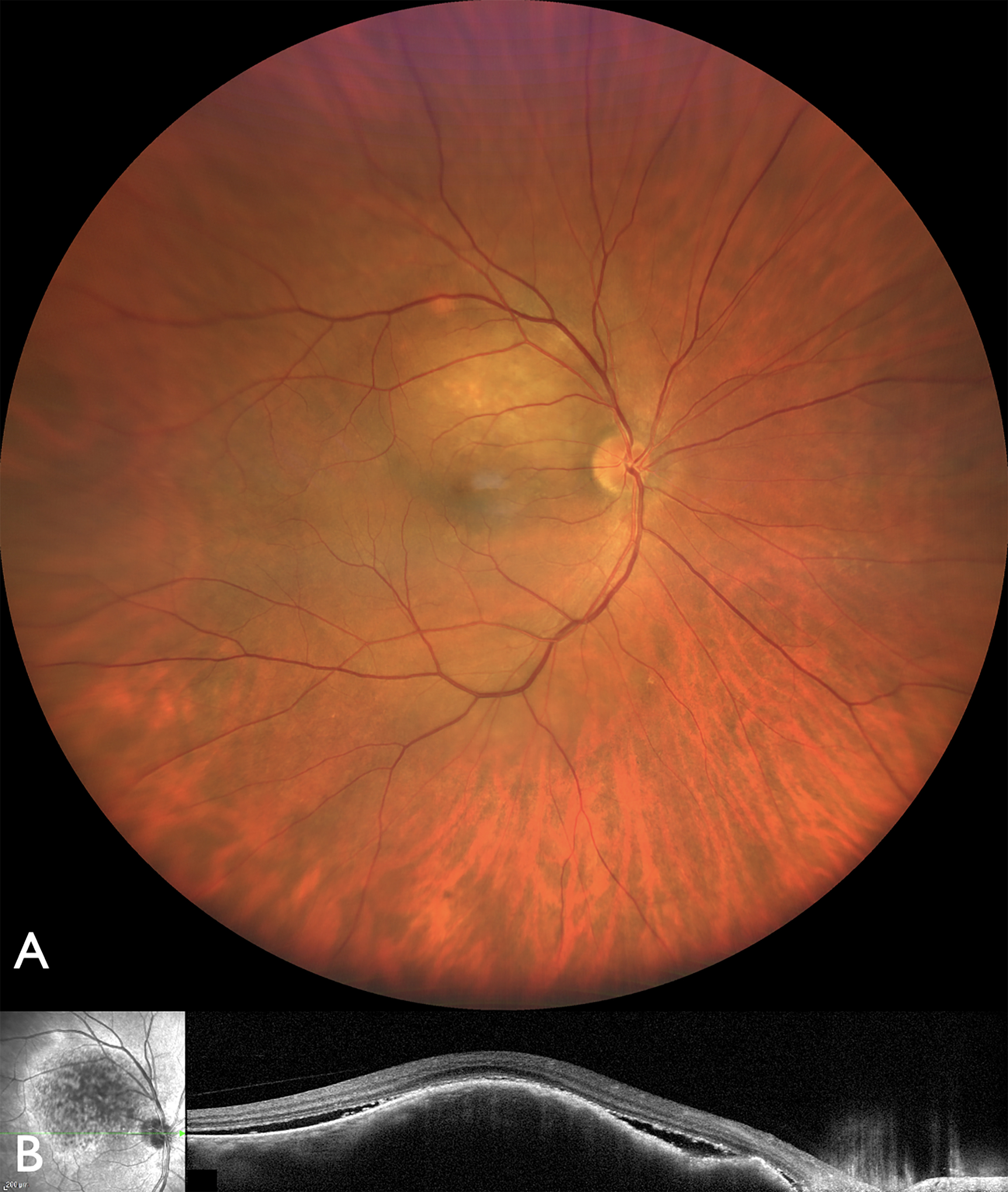
Figure 2: Wide angle photography of the right eye (OD) (A) demonstrating a nonpigmented choroidal metastasis from lung cancer. (B) Optical coherence tomography showing an elevated lesion with subretinal fluid
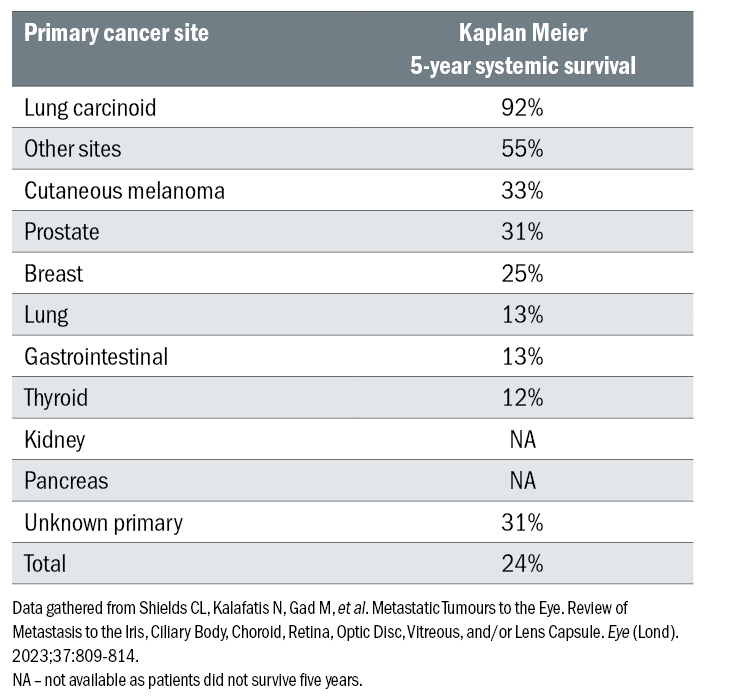
Other primary malignancies included cancer of the kidney (4%), gastrointestinal tract (4%), cutaneous melanoma (2%), lung carcinoid (2%), prostate (2%), thyroid (1%), pancreas (1%), and other unknown sites (3%) (table 1).5 The origin of the primary tumour was also documented to influence survival outcomes.
Table 1: Primary cancer sites among 1,111 consecutive patients diagnosed with uveal metastasis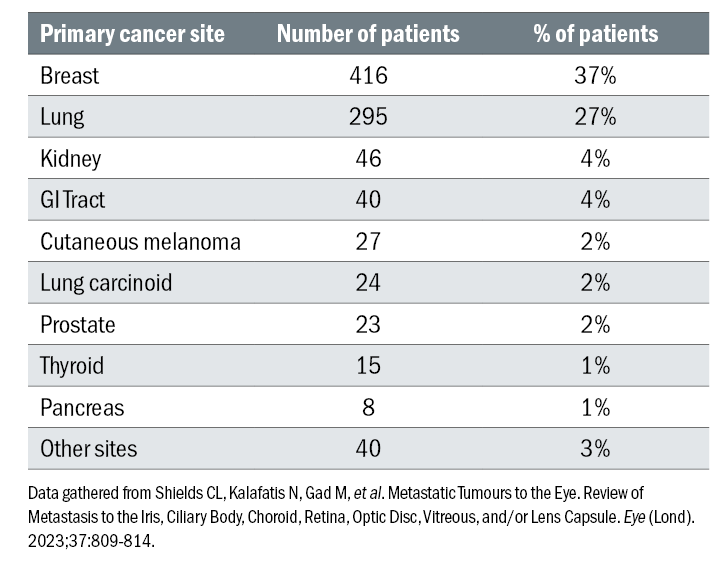
Metastases originating from cancer of the pancreas exhibited the poorest survival rate, with a mean survival duration of 4.2 months, while metastases originating from lung carcinoid showed the highest survival rate of 92% at 5 years (table 2).5
Table 2: Systemic survival at five years for patients with uveal metastasis based on primary cancer site
What age groups demonstrate intraocular metastasis?
Intraocular metastases demonstrate variations related to age, being very uncommon in children and young adults but much more prevalent among middle-aged, older-aged and senior-aged individuals.6 In a large cohort analysis on uveal metastasis based on age at presentation, uveal metastases were most frequently observed in older aged adults, particularly those aged 61 to 80 years, accounting for the highest proportion at 46% of cases.6
Other age groups included middle aged adults (41 to 60 years), constituting 42% of patients and senior aged adults (81 to 100 years) representing 4%.6 Notably, the incidence of intraocular metastases was rare among young adults (21 to 40 years) accounting for only 7%, and children (0 to 20 years) comprising < 1% of cases.6 In all age groups except children, the primary cancers that most frequently metastasized to the uvea were either from primary breast or lung cancer.6 However, among children, metastases typically originated from rare sarcomas.6
According to the Kaplan-Meier survival estimates, the prognosis for individuals with uveal metastases varied across different age groups.6 Children had the lowest survival with rates of 33% at one year and 0% at five years.6 Survival rates improved progressively with age, as young adults showed rates of 48% at one year and 23% at five years, middle-aged adults with rates of 60% at one year and 29% at five years, and older adults with rates of 62% at one year and 25% at five years.6 Remarkably, senior adults exhibited the most favourable life prognosis, with survival rates of 76% at one year and 40% at five years.6
Is there a difference in survival if the patient is female or male?
Among 1,111 consecutive patients diagnosed with uveal metastasis, Welch et al found the slight majority were females, comprising 64% of cases7 (figure 3).
Figure 3: Iris metastases from breast cancer on slit lamp photography (A), and anterior segment optical coherence tomography (B) showing the cross section of the mass with thickened irregular surface
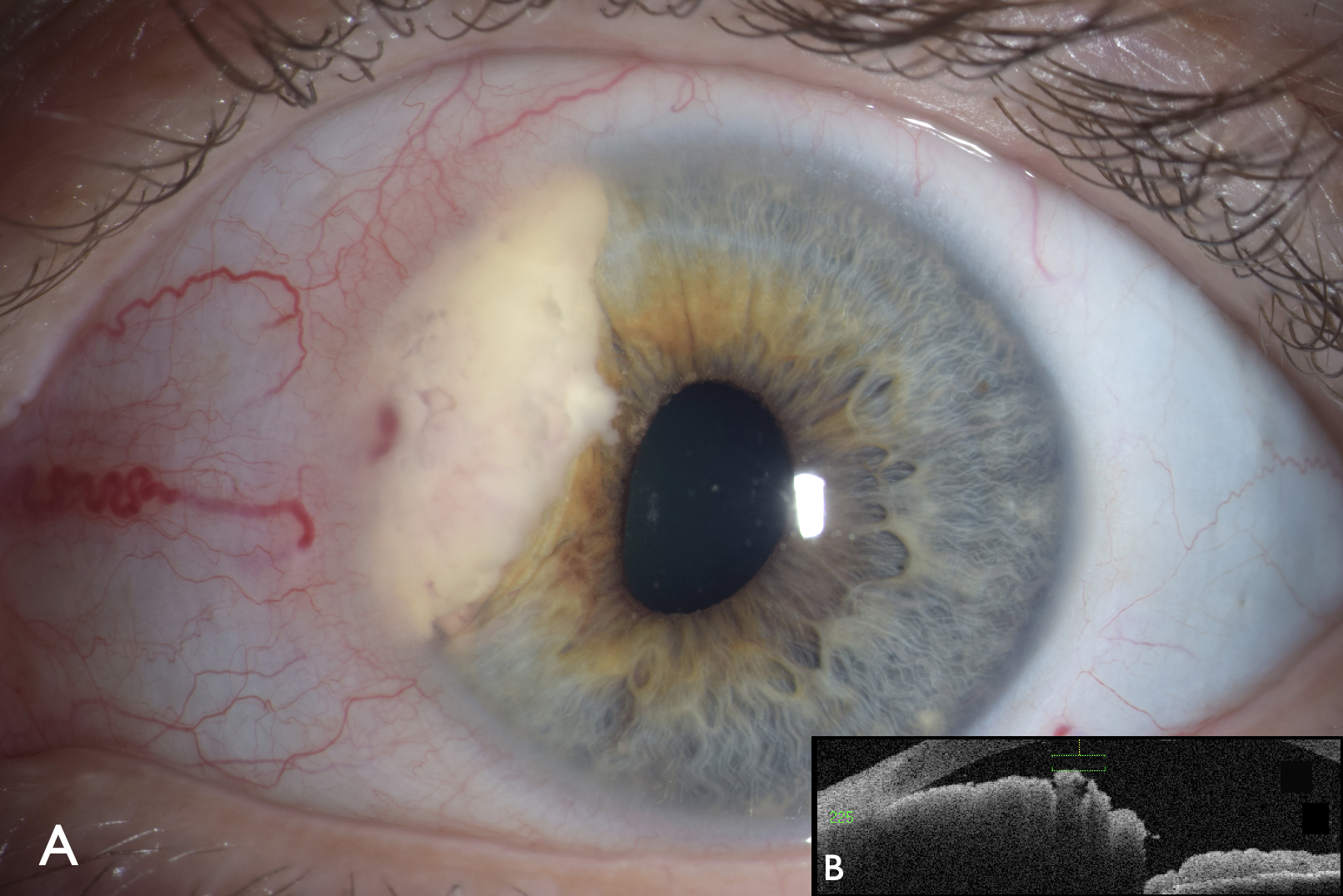
A comparison between female and male patients revealed several notable differences.7 Female patients were diagnosed at a younger age on average compared to male patients (mean age 58 years versus (vs) 63 years, p < 0.001).7 Additionally, female patients exhibited more often bilateral disease (21% vs 11%, p < 0.001) and demonstrated greater mean number of metastases per eye (1.8 vs 1.6 tumours per eye, p = 0.04).7
In terms of overall survival, Welch et al found that female patients exhibited a more favourable prognosis compared to males, (median survival 20 months vs 13 months, p = 0.03).7 This difference in survival was likely attributed to variations in the underlying primary cancer as female patients were more likely demonstrated primary breast cancer whereas male patients were more likely to have underlying lung cancer.7
Kaplan-Meier survival estimates at five years further demonstrated superior survival rates among females ( 31% vs 21%, p < 0.001).7 When examining specific primary cancers, for example for those with lung cancers, the survival favourability persisted in the female patient population ( 24% vs 9%, p = 0.009).7
How do we image uveal metastasis?
Various methodologies have been shown to effectively capture images of uveal metastases. Among the common techniques employed are wide angle fundus photography, ultrasonography, fluorescein angiography and optical coherence tomography. Wide angle fundus photography is an excellent way to image intraocular tumours and confirm the laterality and multifocality as well as the size and location of each tumour.
All patients are imaged with wide-angle fundus photography. This is used for each appointment so that the physician can confirm tumour response to therapy. Ultrasonography is used to measure the thickness of tumour and the intralesional echogenic quality. This technology utilises sound waves to capture the anatomy of intrinsic structures and has been shown to be a reliable imaging technique.
Ultrasound depicts most metastatic tumours as dome-shaped or placoid (flat, mesa-like) as well as echodense (with dense ultrasound signal within the tumour). Fluorescein angiography is a method of showing the blood flow to each and every tumour in the poster segment of the eye, and is important in demonstrating tumour size, configuration, and location, as well as tumour related leakage in the overlying retinal pigment epithelium and retina that can lead to retinal detachment. This technology uses a special fluorescent dye that follows the flow of blood into the eye and provides extensive information on tumour vascularity.
Wide-angle fundus imaging, ultrasonography and fluorescein angiography are all helpful in distinguishing between choroidal melanoma and metastasis. On wide-angle imaging choroidal melanoma appears non-pigmented, lightly pigmented or darkly pigmented whereas choroidal metastasis appear non-pigmented. On ultrasonography, choroidal melanoma appears echolucent whereas choroidal metastasis appears echodense.
On fluorescein angiography, choroidal melanoma will show a bright ‘double circulation’ from both retinal and choroidal vessels whereas this is less likely see with choroidal metastasis. Additionally, indocyanine green angiography can be used to detect choroidal tumours using a cyanine dye, typically depicting choroidal melanoma as hyperfluorescent whereas choroidal metastasis appears hypofluorescent.
When a patient has a small choroidal metastasis (<5mm thickness), optical coherence tomography (OCT) is useful in depicting the mass within the choroid and the overlying subretinal fluid.8 This is also important in differentiating choroidal melanoma from metastasis as melanoma tends to have a smooth contour to its surface whereas metastatic tumours show a lumpy bumpy surface.8
When is fine needle aspiration biopsy necessary?
Fine needle aspiration biopsy (FNAB) serves as a valuable technique for confirming uveal metastasis when clinical evaluation and imaging are insufficient for diagnosis.9 A study that utilised FNAB in 140 intraocular malignancies with adequate cytologic material found that the sensitivity and specificity rates were 100% and 98%, respectively.9
A thin, 27-gauge needle is inserted into the pars plana through the vitreous into the tumour apex posteriorly in the eye and cells are aspirated for cytologic analysis. Special stains are applied to the cells to grade the uptake and differentiate melanoma from metastatic carcinoma. Following FNAB, an accurate diagnosis is established and then a proper treatment pathway is discussed and implemented.
How are uveal metastases managed?
The primary objective of therapeutic interventions for uveal metastases is to mitigate and reduce tumour burden and impede its advancement. Our personal goals are to first, save the life of the patient, save the eye of the patient, and then protect their vision. This allows them maintenance of the organ and hopefully function of the organ. Several techniques have been shown to effectively manage uveal metastases including external beam radiotherapy (EBRT), proton beam radiotherapy, and plaque radiotherapy.
These methods utilise high-energy radiation to target and destroy cancer cells in a precise and accurate manner. Systemic chemotherapy, systemic targeted medication and hormonal therapies can be effective. Recent data has been published, showing that photodynamic therapy can be affective, and this is an excellent technique that uses a special dye and a laser to target small choroidal metastasis.10 Vision loss is uncommon with this technique.10 On the horizon, there will be newer methods of nanoparticle therapy for management of uveal metastasis.16
Summary
Several prior studies have indicated that ocular metastases are most commonly identified within the uvea, particularly the choroid. Less frequent locations include the ciliary body and iris, with rare occurrences in the retina, optic disc, vitreous, and lens capsule. Extensive literature exists regarding the primary tumour of origin, clinical presentation, diagnostic characteristics and treatment approaches, often involving systemic chemotherapy for disseminated metastases or radiotherapy for localised tumours.
In recent years, there has been a notable surge in research aimed at elucidating the variations in outcomes associated with the primary tumour site, age and sex in individuals with ocular metastases. Considerable attention has been directed towards understanding imaging technologies, refining treatment modalities and enhancing diagnostic methods, particularly in scenarios where the diagnosis of uveal metastasis poses significant challenges.
Upon establishing a prognosis, diverse strategies are employed to mitigate the burden of metastatic lesions. Some therapeutic interventions adopt a broader approach, using systemic chemotherapy to treat and eradicate cancerous cells throughout the body. Conversely, other modalities adopt a more targeted strategy, such as high-energy radiotherapy or targeted chemotherapy. It is imperative to acknowledge that each therapeutic avenue carries its unique set of advantages and limitations, necessitating a comprehensive evaluation to determine the most appropriate course.
- Dr Carol L Shields serves as the director of the Ocular Oncology Service at Wills Eye Hospital of Thomas Jefferson University in Philadelphia, PA USA.
- Madison Woods is a second year medical student at Sidney Kimmel Medical College at Thomas Jefferson University. She is affiliated with the Ocular Oncology Service, Wills Eye Hospital, Philadelphia, PA.
- Harrison Fellheimer is a second year medical student at Sidney Kimmel Medical College at Thomas Jefferson University. He is affiliated with the Ocular Oncology Service, Wills Eye Hospital, Philadelphia, PA
- Thomas Catapano is a fourth year medical student at St. George’s University and research intern with the Ocular Oncology Service, Wills Eye Hospital, Philadelphia, PA
No financial disclosures relevant to this topic.
References
- Arepalli S, Kaliki S, Shields CL. Choroidal metastases: origin, features, and therapy. Indian J Ophthalmol. 2015;63:122–7.
- Konstantinidis L, Damato B. Intraocular metastases–a review. Asia Pac J Ophthalmol. 2017;6:208–14.
- Shields JA, Shields CL. Metastatic tumors to the uvea retina and optic disc. In: Shields JA, Shields CL, editors. Intraocular tumors. an atlas and textbook. 3rd ed. Philadelphia, PA: Lippincott Wolters Kluwers; 2016, pp. 213–45.
- Cohen VML. Ocular metastases. Eye. 2013;27:137–41.
- Shields CL, Welch RJ, Malik K, Acaba-Berrocal LA, Selzer EB, Newman JH, et al. Uveal metastasis: clinical features and survival outcome of 2214 tumors in 1111 patients based on primary tumor origin. Middle East Afr J Ophthalmol. 2018;25:81–90.
- Shields CL, Acaba-Berrocal LA, Selzer EB, Mayro EL, Newman JH, Malik K, et al. Uveal metastasis based on patient age in 1,111 patients: Comparison of clinical features and outcomes per age category. Retina. 2020;40:204–13.
- Welch RJ, Malik K, Considine SP, Acaba-Berrocal L, Selzer E, Newman J, et al. Uveal metastasis based on patient sex in 2214 tumors of 1111 patients. A comparison of female versus male clinical features and outcomes. Asia Pac J Ophthalmol. 2019;8:298–303.
- Al-Dahmash SA, Shields CL, Kaliki S, Johnson T, Shields JA. Enhanced depth imaging optical coherence tomography of choroidal metastasis in 14 eyes. Retina. 2014;34:1588–93.
- Shields JA, Shields CL, Ehya H, Eagle RC, Jr, DePotter P. Fine needle aspiration biopsy of suspected intraocular tumors. The 1992 Urwick Lecture. Ophthalmology. 1993;100:1677–84.
- Shields CL, Khoo CTL, Mazloumi M, Mashayekhi A, Shields JA. Photodynamic therapy for choroidal metastasis. Tumor control and visual outcomes in 58 cases. The 2019 Burnier International Ocular Pathology Society Lecture. Ophthalmol Ret 2020;4:310–319.
- Duke-Elder S. System of ophthalmology, vol 9: Diseases of the uveal tract. St Louis: CV Mosby; 1966, 917.
- Albert DM, Rubenstein RA, Scheie HG. Tumors metastasis to the eye. I. Incidence in 213 adult patients with generalized malignancy. Am J Ophthalmol. 1967;63:723–726.
- Bloch RS, Gartner S. The incidence of ocular metastatic carcinoma. Arch Ophthalmol. 1971;85:673–5.
- Nelson CC, Hertzberg BS, Klintworth GK. A histopathologic study of 716 unselected eyes in patients with cancer at the time of death. Am J Ophthalmol. 1983;95:788–93.
- Francis J, Berry D, Abramson DH, et al. Intravitreous cutaneous metastatic melanonma in the era of checkpoint inhibition. Ophthalmology. 2020;127:240–8.
- Shields CL, Lim LS, Dalvin LA, Shields JA. Small choroidal melanoma: detection with multimodal imaging and management with plaque radiotherapy or AU-011 nanoparticle therapy. Curr Opin Ophthalmol. 2019;30(3):206-214.
