In part two of this series, the role of symptom questionnaires was discussed, as well as how to measure tear quantity by tear meniscus height and the Schirmer test. Also, tear quality measures of non-invasive tear break-up time, tear sampling and Point of Care Testing (POCT) to gauge osmolarity and presence of inflammatory markers.
The next dry eye test to discuss is the widely used fluorescein breakup time (FBUT) test, it is by its nature invasive, so typically used after the tests discussed previously. Fluorescein sodium (NaFI) is instilled to enhance visibility of the tear film under a blue-light source; however, it is important to note NaFI reduces tear film stability potentially affecting results. The DEWS II report1 recommends three natural blinks for the FBUT test to distribute the fluorescein through the tear film and allow normal tear thickness to be attained. The report states a reference value for dry eye disease (DED) diagnosis when using fluorescein, ranging from a cut-off time of less than 10 seconds to less than five seconds with smaller controlled volumes.1
A study in 20202 evaluating FBUT efficacy also advocated this approach but with the addition of a yellow (Wratten 12) filter to enhance the observation of the first change or disruption (first black spot) in the tear film. This study compared the widely used standard NaFl strip wetted with unpreserved saline and a 2.0μl of 1.0% liquid drop finding no clinically significant differences. The study also concluded the diagnostic cut-point to differentiate dry eye should be in the range of 5.3 to 6.0 seconds for optimum test sensitivity and specificity. Therefore, the FBUT test is a valid and useful screening test for dry eye, when used in conjunction with additional diagnostic tests.2
To assess ocular surface cellular damage a slit-lamp examination with vital stain(s) (one that can be used on living cells safely) is required. Pre-impregnated NaFl paper strips are preferred due to the susceptibility to contamination of NaFl solution with Pseudomonas aeruginosa.3 This can be due to either the reuse of minim drops3 or using NaFl from a vial.4
When using impregnated NaFI strips excess saline should be removed ensuring a minimal volume is instilled, then wait between one and three minutes before assessing corneal staining.5 A positive result for dry eye is > 5 corneal spots observed in either eye.6 Corneal staining typically occurs as a late-stage consequence of a chronic dry eye condition that has been progressing over an extended period. Additionally, the symptoms experienced often do not correlate with the degree of observed staining. In the author’s experience, older patients with prolonged dry eye disease, regardless of its variations frequently exhibit symptoms that are less severe than indicated from the corneal staining.
Lissamine Green stains ocular surface epithelial cells that are unprotected by mucin or glycocalyx, as well as cells that have been damaged. It has good patient tolerance, is non-toxic and has been recognized as highly sensitive and specific.7 Lissamine green staining is performed to assess the conjunctival and lid margin damage. Observation should occur between one and four minutes after instillation. Observation through a red filter potentially aids visualization and a positive score is more than nine observed spots on the conjunctiva.1
The thin leading band of the marginal conjunctiva of the upper and lower lids acts as a wiping mechanism to spread the tear film across the ocular surface during blinking. This is termed the ‘lid wiper,’ normally rich in goblet cells, and appearing to be the most sensitive conjunctival tissue of the ocular surface. Lid wiper staining with dyes such as NaFI and lissamine green, which occurs principally in DED patients has been termed lid wiper epitheliopathy (LWE) and is thought to be a consequence of increased friction.1 Lissamine green is also the preferred dye for staining the conjunctiva evident in recent research.
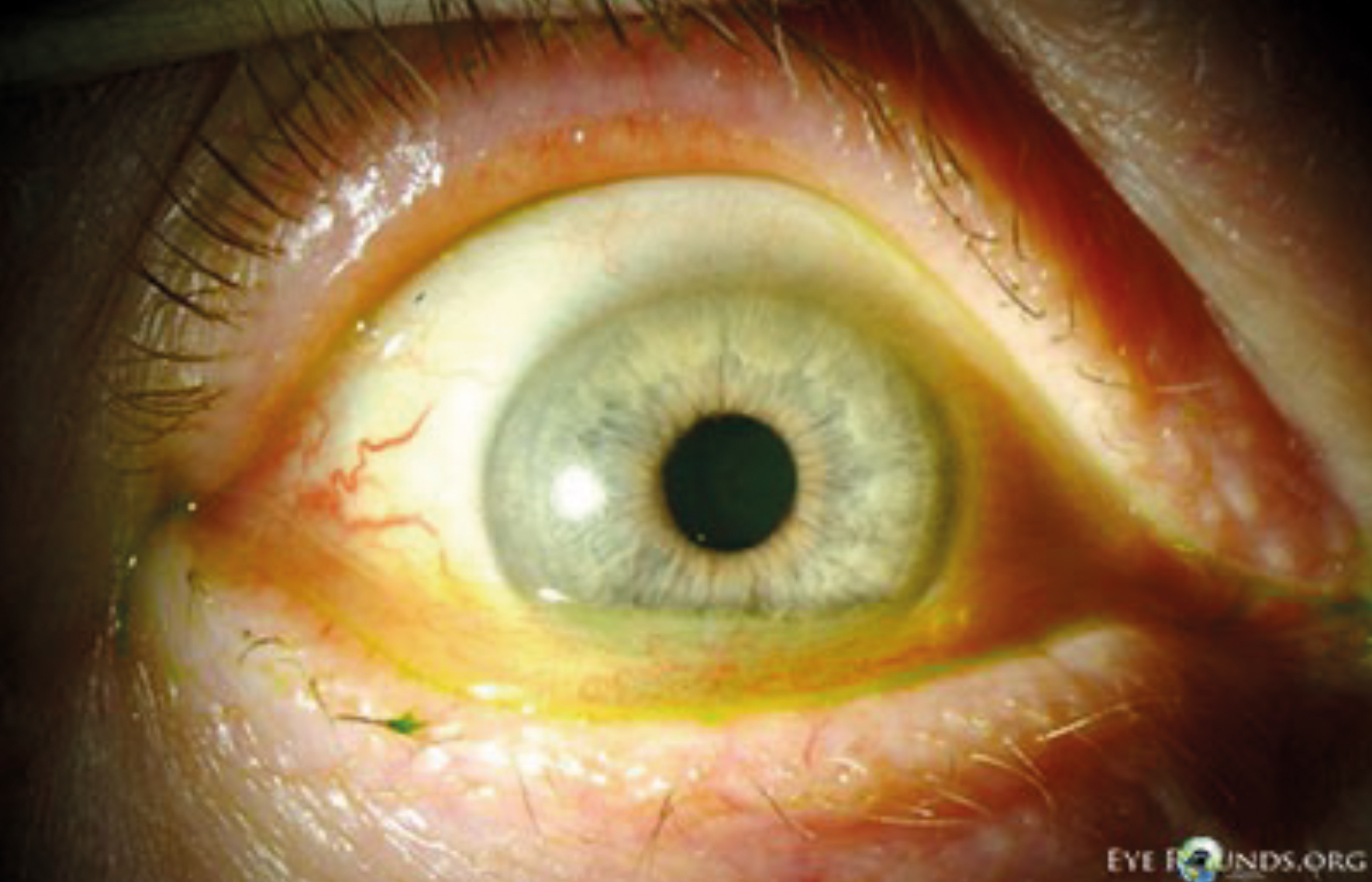
Figure 1: Conjunctivochalsis seen under slit lamp examination
(Image courtesy of eyerounds.org)
Blinking plays a crucial role in distributing the tear film across the ocular surface. This process supports optimal vision, facilitates tear film drainage, and promotes the release of meibum from the superior and inferior meibomian glands (MGs). However, when the tear film is sub-optimal, the mechanical forces exerted during blinking can potentially lead to ocular surface damage. Conjunctival folds for example, are assumed to be related to increased friction during blinks, which may arise due to inadequate mucin composition either at the ocular surface or within the tear film. These subtle, subclinical level, lid-parallel conjunctival folds (LIPCOF) should not be confused with conjunctivochalasis (CCH) as they do not appear to correlate with age (figure 1).8
In a comprehensive study of 272 dry eye patients across eleven countries, the LIPCOF test demonstrated moderate sensitivity and specificity, along with a favourable positive predictive value. These findings support the utility of LIPCOF grading as a simple, quick, and non-invasive tool for screening dry eye.9 Notably, the LIPCOF assessment becomes even more valuable when combined with NaFl application and further enhanced by lissamine green instillation.
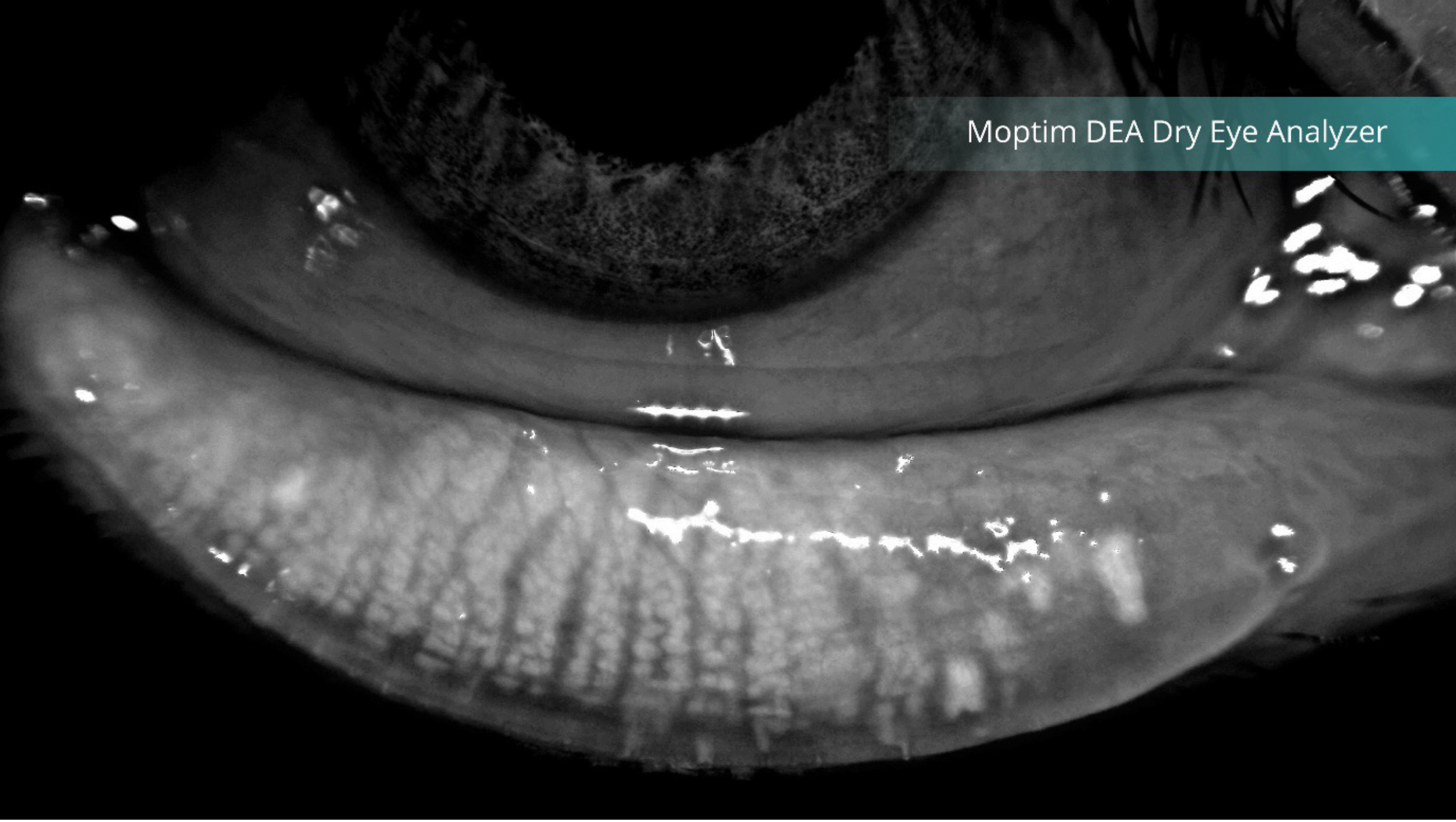
Figure 2: Meibography showing the meibomian glands
(Image courtesy of Grafton Optical)
As discussed earlier the FBUT test, and in the preceding article exploring the non-invasive tear breakup time (NIBUT) test these metrics both predominantly reflect the lipid layer of the tear film, originating from the meibomian glands in the inferior and superior lids. Advances in imaging technology found in a number of instruments now allows visualization of these glands using infra-red illumination, see figure 2.
It is thought that meibomian gland (MG) structure and function deteriorate with age in a healthy population although the effects remain predominantly asymptomatic.10 Meibography (the non-invasive imaging technique for assessing the meibomian glands) can show MG ‘drop-out,’ however mild changes are observed in meibomian quantity and quality may not raise significant concern. A compensatory response to MG changes by basal tear production may occur, but it is only when these changes coincide with disrupted tear function that ocular surface signs and/or symptoms manifest.10 Despite this reassuring hypothesis, the author contends that maintaining a complete set of healthy MGs is preferable.
Figure 3: Viscous toothpaste consistency meibum
Plugged MGs are a common feature in evaporative dry eye disease (EDE). In such cases the glands retain their structural integrity and functionality, but instead of a translucent, free-flowing secretion, a more viscous ‘toothpaste’ consistency is observed (figure 3). These glands may be ‘capped,’ and the abnormal meibum consistency can be expressed upon applying pressure with a fingertip or cotton bud. The force used for diagnostic expression (1.25 g/mm2) mimics the natural forces of a deliberate blink.11 Importantly, there exists a substantial distinction between MG expressibility and secretory activity – freely expressed meibum does not indicate normal secretion.12 It is crucial to recognise we are specifically discussing diagnostic expression here; for complete evacuation of obstructed gland content, a force of 80 g/mm2 is required.13 However, only a small minority of patients (approximately 7%) can tolerate the discomfort associated with the pressure required for full therapeutic expression.13
The upper most layer of the tear film consists of a thin, consistent, free-flowing lipid layer that serves as a barrier against excessive evaporation. Conversely, a thicker, viscous, inconsistent, globular layer dosa not effectively retard evaporation.
Upon detailed slit lamp examination of the MG orifices telangiectasia (spider veins, small, dilated blood vessels) may be seen along the lid margin. Research indicates that changes in Free Fatty Acid (FFA) composition in the meibum are associated with the severity of telangiectasia and plugging of gland orifices in meibomian gland dysfunction (MGD).14
Slit-lamp examination of the lid margin may also show signs of inflammation, including the presence of scurf, crusts, and flakes. These findings indicate the presence of anterior blepharitis, which affects the eyelid skin at the base of the eyelashes and the eyelash follicles. Posterior blepharitis or MGD can exist on its own, but importantly, anterior blepharitis often leads to MGD and EDE, making it a matter of ‘when’ rather than ‘if.’ Patients with longstanding chronic blepharitis may present with hypertrophy of the lid margin, scars, madarosis (loss of lashes), trichiasis (misdirected lashes), and poliosis (whitening of the lashes).15
In a 2009 study16 conducted in the USA, ophthalmologists (n = 120) and optometrists (n = 84) reported that blepharitis is commonly found encountered in clinical practice affecting 37% and 47% of their patients, respectively.
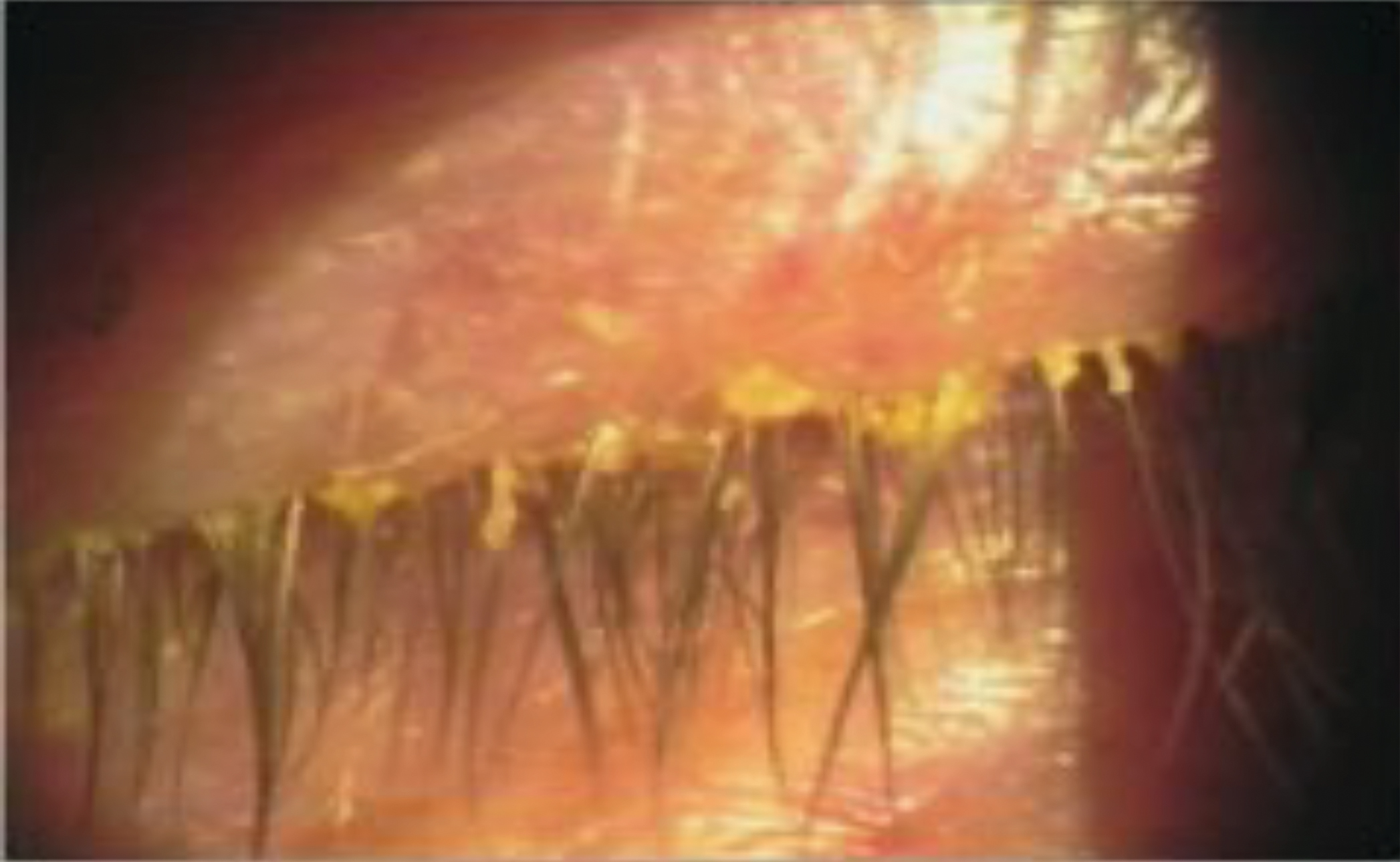
Figure 4: Staphylococcal blepharitis
Anterior blepharitis can manifest in staphylococcal and/or seborrheic forms, with or without demodex infestation. Staphylococcal blepharitis is characterized by scaling, crusting, and erythema of the eyelid margin with collarette formation at the base of the cilia (not to be confused with cylindrical dandruff (figure 4). Additionally, a soapy appearance of the inferior tear prism (saponification) may be observed. Chronic inflammation can lead to the development of ulcerative blepharitis, loss of eyelashes and corneal involvement, including punctate epithelial erosions, marginal infiltrates, peripheral corneal defects, and corneal neovascularization. Seborrheic blepharitis conversely presents with greasy scaling of the anterior eyelid and is frequently associated with seborrheic dermatitis of the eyebrows and scalp. Regardless of the specific form both will result in tear film instability, even in the absence of MGD.17
Demodex infestation is an underdiagnosed horribly fascinating form of anterior blepharitis, involving two distinct mite species: Demodex folliculorum, which resides in the lash follicle, and the smaller Demodex brevis, found within the MGs. Although typically inhabitants of human skin, these mites can provoke sensitivity reactions and, in some cases, proliferate excessively.
Lacking internal digestive mechanisms, demodex mites excrete proteases and lipases for the external digestion of healthy epithelial cells and meibum on the eyelid. This poses a risk to the MGs and the meibum they produce. Furthermore, the absence of excretory organs in these mites leads to the regurgitation of undigested material, often combined with the epithelial cells, resulting in the formation of collarettes, commonly referred to as cylindrical dandruff (CD).18 These lash-cuffing collarettes, unique to Demodex, may contain, lipids, keratin, Demodex eggs and dead Demodex mites. The presence of these collarettes is a pathognomonic sign of Demodex blepharitis. Notably, in a study of eyelashes with and without CD, 100% of lashes exhibiting CD were found to have Demodex mites.18 Additionally, recent research utilising PCR testing confirmed the presence of mite DNA, in all eyes with CD.18
Large-scale recent studies conducted both in and outside the USA have shown prevalence rates ranging from 41% to 70% among typical patients presenting at optometry and ophthalmology clinics for a diverse range of reasons, including, annual exam, cataracts, dry eye, and glaucoma. Remarkably, these patients were identified as having demodex infestation based on the presence of CD, even though it was not their primary complaint and related symptoms were not always evident (figure 5).18
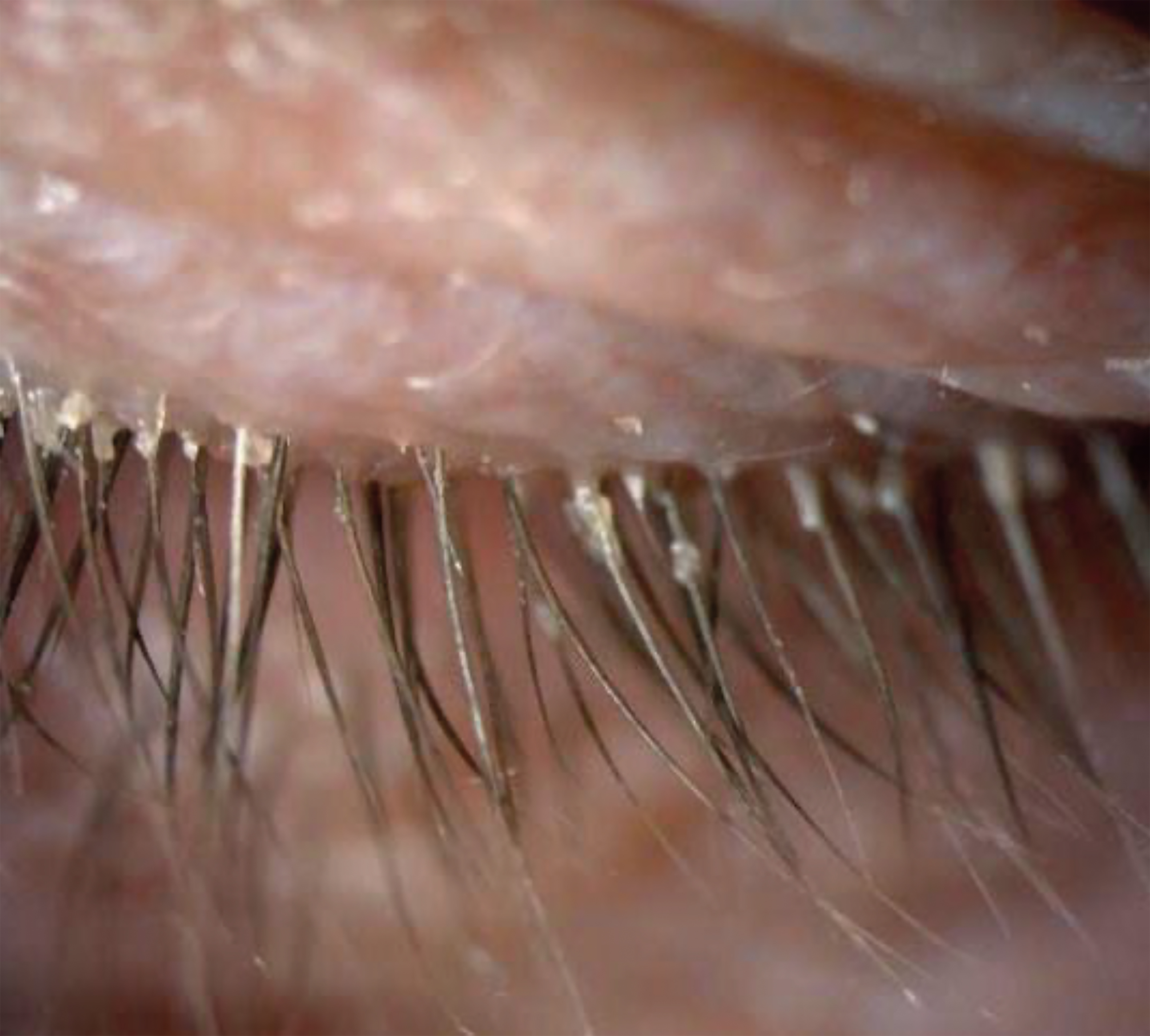
Figure 5: Cyclical dandruff (CD) from Demodex infestation
Given the multitude of available tests, outlined in this, and the preceding article, it is completely understandable for readers to ask, which is the most accurate single test for definitively diagnosing dry eye? The succinct response is that no single test suffices. Ocular surface staining (corneal and conjunctival) using NaFl stands out as the most accurate individual test, but it is essential to recognise that staining can result from various aetiologies.
The inaccurate reliance on a single diagnostic test has been widely recognised for many years, so the TFOS DEWS II workshop19 recommended a diagnostic based criterion using the presence of symptoms alongside at least one positive sign (such as osmolarity, tear break up time, or ocular surface staining). However, questions remain, which symptom questionnaire OSDI or DEQ-5 and which objective test should be included? The next logical question, even before deciding on the answer to this is, would it help if I added more than one test?
The dilemma of single tests versus symptoms and single test versus symptoms and multiple tests is addressed in a recently published paper by Eric Papas (2023).20 Diagnosing dry eye: Which tests are most accurate? (reviewing this paper is recommended by the author). An assumption of dry eye prevalence was taken as 11.6%, the study highlights that test sensitivity and specificity probabilities are dependent on the prevalence of the disease within a population, but the prevalence figure is not vital to the accuracy when comparing one test with another.
The inconvenient truth highlighted in his paper is that when comparing a single symptom questionnaire (such as OSDI) to a single objective test, (corneal staining with NaFl), the latter is twice as accurate as the OSDI, albeit with a probability of 0.28 (28 times out of 100).20 Even when as recommended by the TFOS DEWS II workshop,1 symptoms (specifically the better performing DEQ-5 questionnaire) are combined with corneal staining, it still only has a resulting probability of 0.42 (42 times out of 100). It is important to recall that the original TFOS DEWS II workshop recommendation of ‘at least one positive sign’, a qualifier often omitted when citing the criterion.
Therefore, to enhance diagnostic efficacy, eye care practitioners need to explore combinations of multiple tests, the best performing combination of tests in Papas (2023)20 was conjunctival + corneal staining + osmolarity + FBUT/NIBUT, alongside the DEQ-5, giving a probability of 0.90 (90 times out of 100).
In summary, following a systematic evidence-based approach is essential when determining a dry eye diagnosis, and a combination of tests clearly improves diagnostic accuracy. As eye care practitioners, the aim is to deliver the best patient care, this article hopefully provides a rationale taken from current research that can be applied in routine clinical practice.
- Andrew D Price FBDO(Hons)CL MBCLA, CEO of ADP Consultancy and ADP-EyeCare is a clinician providing contact lens and dry eye clinics, a principal investigator in clinical trials, professional services consultant, educator and author. He received certification in ophthalmic assisting during work in ophthalmology practice in the USA and has worked in laser vision and ophthalmology clinics in the UK and USA. He has published and presented over 300 lectures and workshops, in the UK and abroad and was involved in developing Eye Drops Database. Andrew is also a past ABDO National Clinical Committee and Optometry Wales Board member and has been a guest lecturer for ABDO and Anglia Ruskin University.
References
- Wolffsohn JS, Arita R, Chalmers R, Djalilian A, et al. TFOS DEWS II Diagnostic Methodology report. Ocular Surface. 2017; 15(3): 539-574. https://doi.org/10.1016/j.jtos.2017.05.001
- Paugh j, Tse J, Nguyen A, Sasai A, et al. Efficacy of the Fluorescein Tear Breakup Time (TBUT) Test in Dry Eye. Cornea. 2020 January; 39(1): 92–98. doi:10.1097/ICO.0000000000002148
- Rautenbach P, Wilson A, Gouws P. The reuse of ophthalmic Minims: an unacceptable cross infection risk? Eye (Lond). 2010;24(1):50-2.
- Norn MS, Thomsen VF. CONTAMINATION OF EYE DROPS USED FOR VITAL STAINING. Acta Ophthalmologica. 1967;45(5):650-7.
- Peterson RC, Wolffsohn JS, Fowler CW. Optimization of anterior eye fluorescein viewing. Am J Ophthalmol 2006; 142:572e5.
- Whitcher JP, Shiboski CH, Shiboski SH, Heidenreich AM, et al. A Simplified Quantitative Method for Assessing Keratoconjunctivitis Sicca from the Sjögren’s Syndrome International Registry. American Journal of Ophthalmology. 2010; 149(3): 405-415. ISSN 0002-9394, https://doi.org/10.1016/j.ajo.2009.09.013.
- Hamrah P, Alipour, F, Jiang, S. et al. Optimizing evaluation of Lissamine Green parameters for ocular surface staining. Eye 25, 1429–1434 (2011). https://doi.org/10.1038/eye.2011.184
- Pult H, Riede-Pult, Britta H.; Murphy, P J. The Relation Between Blinking and Conjunctival Folds and Dry Eye Symptoms. Optometry and Vision Science. 2013; 90(10): 1034-1039, October 2013. | DOI: 10.1097/OPX.0000000000000029
- Németh J, Fodor E, Lang Z, et al. Lid-parallel conjunctival folds (LIPCOF) and dry eye: a multicentre study. British Journal of Ophthalmology 2012;96:1380-1385. https://hawlina.eu/files/3814/0468/1399/sc_BJO-2012-Nemeth-LIPCOF.pdf
- Yeotikar NS, Zhu H, Markoulli M, Nichols KK, Naduvilath T, Papas EB. Functional and morphologic changes of meibomian glands in an asymptomatic adult population. Invest Ophthalmol Vis Sci. 2016;57:3996–4007. DOI:10.1167/iovs.15-18467
- Blackie CA, Korb DR, Knop E et al. Nonobvious obstructive Meibomian gland dysfunction. Cornea 2010; 29: 1333–1345. doi:10.1097/ICO.0b013e3181d4f366.
- Tomlinson A, Bron AJ, Korb DR et al. The international workshop on Meibomian gland dysfunction: report of the diagnosis subcommittee. Invest Ophthalmol Visual Sci 2011; 52: 2006–2049. doi:10.1167/iovs.10-6997f.
- Korb DR, Blackie CA. Meibomian gland therapeutic expression: quantifying the applied pressure and the limitation of resulting pain. Eye Contact Lens. 2011; 37: 298–301. doi:10.1097/ICL. 0b013e31821bc7c5.
- Arita R, Mori N, Shirakawa R, Asai K, et al. Linoleic acid content of human meibum is associated with telangiectasia and plugging of gland orifices in meibomian gland dysfunction, Experimental Eye Research. 2016; 145:359-362. https://doi.org/10.1016/j.exer.2016.02.005.
- Bernardes, TF, Bonfioli, AA. Blepharitis. Seminars in Ophthalmology. 2010; 25(3), 79–83. https://doi.org/10.3109/08820538.2010.488562
- Michael A. Lemp, Kelly K. Nichols, Blepharitis in the United States 2009: A Survey-based Perspective on Prevalence and Treatment. Ocular Surface. 2009; 7(2): S1-S14 https://doi.org/10.1016/S1542-0124(12)70620-1.
- American Academy of Ophthalmology. Blepharitis Preferred Practice Pattern. 2018. Available from: https://www.aaojournal.org/article/S0161-6420%2818%2932645-9/pdf
- Trattler W, Karpecki P, Rapoport Y, Sadri E, et al. The Prevalence of Demodex Blepharitis in US Eye Care Clinic Patients as Determined by Collarettes: A Pathognomonic Sign. Clinical Ophthalmology. 2022; 1153-1164. https://doi.org/10.2147/OPTH.S354692
- Craig JP, Nichols KK, Akpek EK, Caffery B, et al. TFOS DEWS II Definition and Classification Report. Ocular Surface. 2017; 15(3): 276-283. https://doi.org/10.1016/j.jtos.2017.05.008
- Papas EB, Diagnosing dry-eye: Which tests are most accurate? Contact Lens and Anterior Eye, Volume 46, Issue 5, 2023, 102048, ISSN 1367-0484, https://doi.org/10.1016/j.clae.2023.102048
