Domains and learning outcomes (C109889)
• One distance learning CPD point for optometrists, dispensing opticians and contact lens opticians.
Communication
(2.1) After successful completion of this CPD practitioners will be able to explain to the key components of artificial tears and justify the necessity for assessment, diagnosis and management of dry eye disease (DED) by a registered eye care professional, using professional judgment to adapt language and communication approach accordingly.
Clinical practice
(5.3) After successful completion of this CPD practitioners will be able to analyse how specific components in artificial tears impact different layers of the tear film and apply this to their clinical practice.
Specialty CPD – contact lens optician
(5.3) After successful completion of this CPD contact lens opticians will be able to analyse how specific components in artificial tears impact different layers of the tear film and apply this to the management of patients with dry eye disease to enhance patient care.
In this fourth article on dry eye disease (DED) it is important to start where almost every person with an ocular surface condition begins: eye drops. As highlighted in the first article of this series, many of these products are unfortunately obtained from almost anywhere but an eye care practice, where they would have received professional and informed guidance. In reality, at this point they are not patients but consumers of some, maybe multiple brands of ‘artificial tears’ from the shelves of the pharmacy, supermarkets or the internet.
These products often feature attractive packaging with an image of an eye and use phrases such as ‘for dry, tired eyes,’ ‘for irritation and redness relief,’ ‘for complete dry eye care,’ with some claiming they are ‘cooling’ or ‘soothe,’ while more extreme claims found online include terms like ‘healing,’ ‘FDA compliant,’ ‘third party tested.’ It is not surprising that the consumer, and often well-meaning pharmacy staff or general practitioner (GP) remain bewildered as the symptoms are only slightly reduced and then only temporarily. The current situation of self-diagnosis and self-selection of dry eye drops is a significant concern for eye care professionals (ECPs) and unfortunately a lucrative opportunity for marketers.
Despite this, we cannot blame the consumer as they only become a patient after walking through the ECP’s practice door and are appropriately assessed, diagnosed and recommended treatment. It is human nature to seek quick, easy and, preferably, inexpensive solutions to problems and for the average person with a self-diagnosed problem, DED is perceived as simple lack of tear quantity, rather like topping up car engine oil by adding more fluid to the system.
Historical perspectives and advancements in dry eye treatment
For a long time, even within the eye care community, it was believed that dry eye resulted from the eyes’ inability to produce sufficient tears, primarily from the lacrimal gland. The most basic solution was to apply saline to the eye. If a more sophisticated approach was needed, an eye dropper could be used but this approach was largely ineffectual and increased the risk of injury and/or infection. In the minds of many consumers the perception of dry eye treatment has not significantly evolved so it is up to ECPs to educate patients on the complexities of DED and the importance of a treatment tailored to their individual needs.
Advancements in understanding of the tear film have revealed that it is far more complicated, sophisticated and interesting than just a single layer of saline on the ocular surface. The tear film performs several vital functions:
- Protecting the ocular surface by washing away foreign bodies and unwanted microbes
- Lubricating and hydrating the ocular surface
- Supplying nutrition and oxygen to the eye
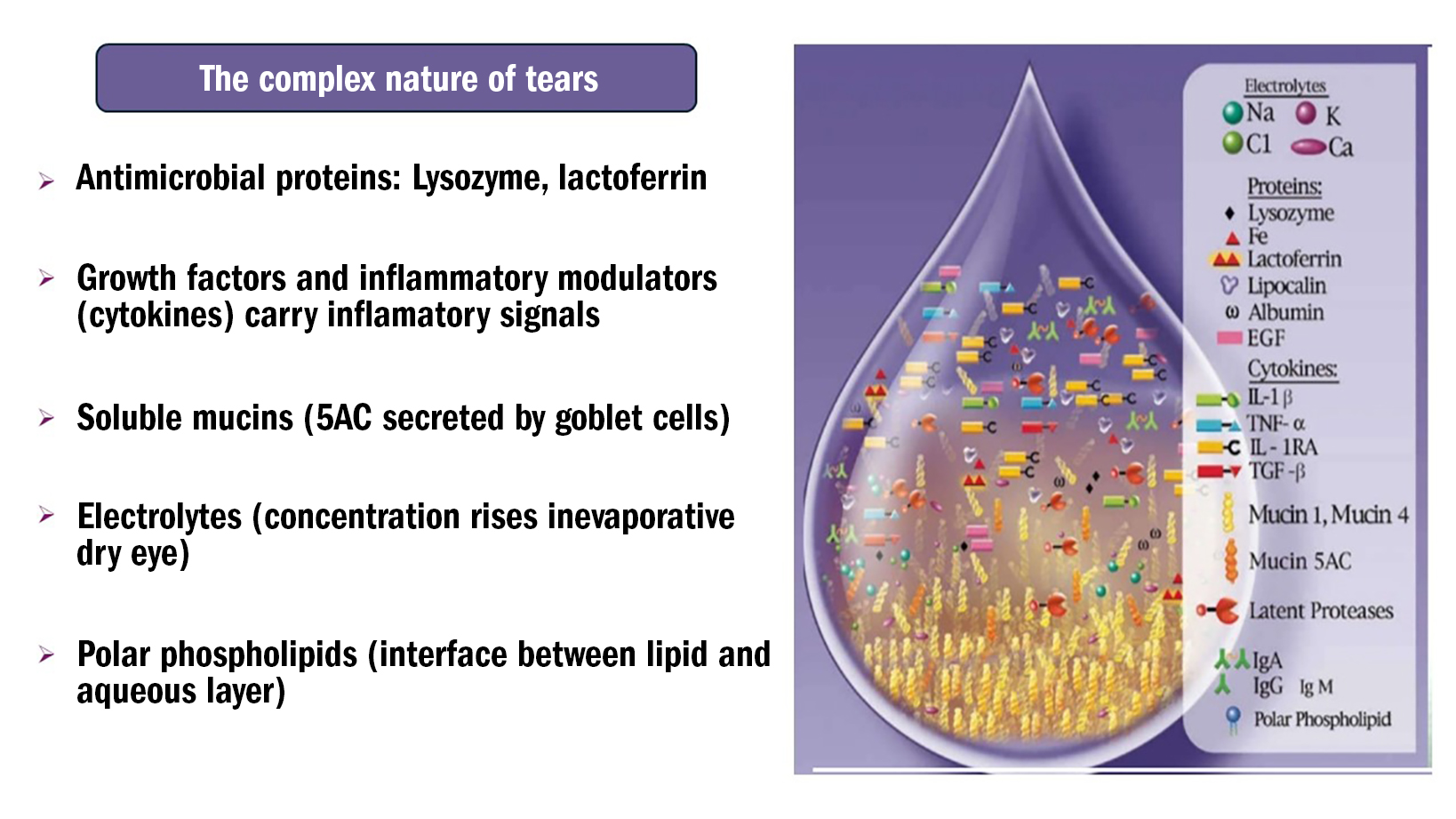
This multi-functional layer is an extremely complex fluid containing thousands of molecules with various roles and origins. Research has identified nearly 2,000 tear proteins in humans,1 and at least 90 small molecule metabolites2 (see figure 1).
Figure 1: Tear constituents
Finally, it is essential to note that the tear film forms a smooth consistent interface between the eye and the external environment, exhibiting the largest dioptric effect of all the eye’s media. As discussed in the first part of this series, the interface between tears and the air is the single most effective refractive surface of the eye. Therefore, even a minor disruption can adversely affect a patient’s appreciation of an accurate refraction, and the expertise involved in dispensing spectacles or prescribing contact lenses.3-5
Tear film structure
Lipid layer
On a simple basis, the tear film can be conceptualised as a three-layer structure (figure 2) the lipid layer, the aqueous layer and the mucin layer. The outermost layer of the three, which interfaces with the air, is a thin oily (lipid) layer. The lipids in this layer have a hydrophilic head orientated towards the aqueous below and a hydrophobic tail pointing towards the air. This crucial layer, secreted primarily by the meibomian glands within the lids, inhibits evaporation of the main body of the tears – the aqueous layer. The hydrophilic heads of the lipid layer, pointing down into the aqueous layer form the interface between this vital evaporative retarding layer and the middle layer of the tear film.
Figure 2: Three layer structure of the tear film
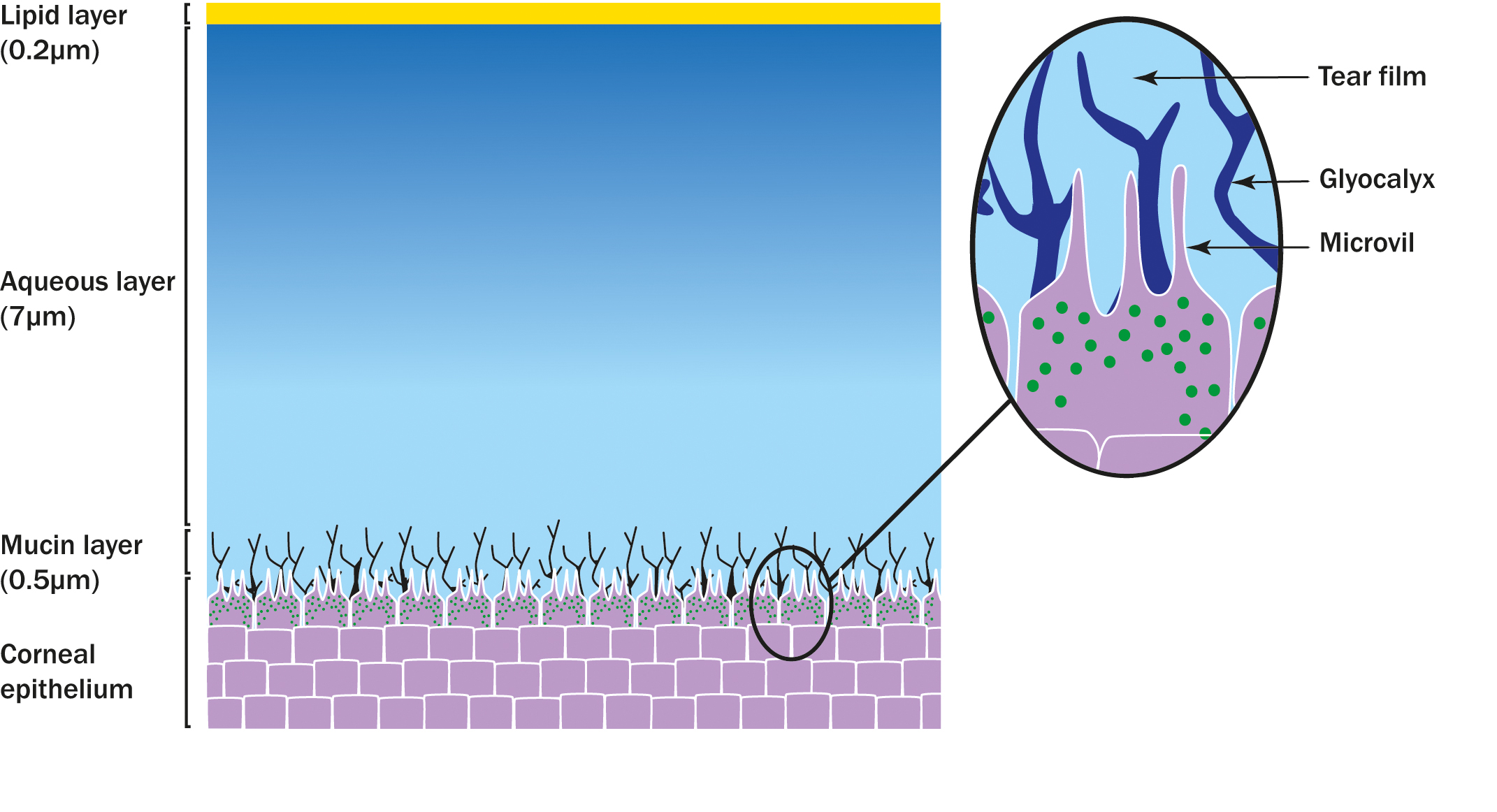
Aqueous layer
The aqueous layer is the thickest of the three layers, and contains antimicrobial proteins such as lysozyme and lactoferrin, growth factors and small, short-lived proteins that are released by one cell to regulate the function of another cell (cytokines). Additionally, this layer includes soluble mucins secreted by goblet cells in the conjunctiva, electrolytes and other components.
The aqueous layer primarily originates in the lacrimal gland, with a smaller contribution from the accessory lacrimal glands within the conjunctiva. Historically, most dry eye drops, previously called ‘artificial tears’, simply bulked up this layer temporarily.
Mucin layer
As we move further down the aqueous layer towards the ocular surface and away from the lipids, the quantity and nature of mucins found therein changes from free floating and soluble towards a more ‘gel-like’ mixture of mucin and glycoprotein called the glycocalyx. This glycocalyx interlocks with micro-protrusions from the ocular surface epithelial cells (microvilli), similar to how Lego building blocks interlock. These mucins originate from the sensitive goblet cells in the conjunctiva.
Evolution of artificial tears
Following the well-meaning but misguided remedies of saline solutions, the evolution of dry eye therapy continued with the introduction of commercial preparations of physiological saline. These solutions were formulated with specific salts and minerals (electrolytes) to more closely resemble human tears in terms of pH balance and osmolarity. This made them more compatible with human tears and the eye, and nearly all contained preservatives to reduce the risk of drop contamination while in the bottle.
Early additions to these formulations included components known as demulcents, which form a soothing, protective film on a mucous membrane surface. Examples would be hydroxypropyl methylcellulose (HPMC/hypromellose) and carboxymethylcellulose (CMC). These agents also help the drop to stay in contact with the existing tears and ocular surface for longer.6
Dry eye drops, such as multi-dose preserved hypromellose (HPMC), were, and are still often, the first line therapy chosen by the GP. These drops offer basic efficacy with protection against microbial contamination and are often the lowest cost option. Additionally, other demulcents such as polyvinyl alcohol (PVA), polyethylene glycol, propylene glycol and other polymers were combined together or with HPMC or CMC in the search of the ideal formulation.
This search goes on, with almost endless and subtle variations. These basic dry eye drops remain available and, for various non-clinical reasons, are the most widely prescribed by the NHS. However, our knowledge, understanding and ability to develop superior products that undoubtedly benefit the patient more have advanced.
Approximately 25 years ago, an interesting viscoelastic agent called sodium hyaluronate/hyaluronic acid (HA) was found to have properties that allow it to adhere it to cell surfaces and bind to water. On the eye, this holds the tear film in contact with the ocular surface. Originally derived from the comb structures of roosters, it is now synthesized in laboratories. Stepping up the viscosity ladder are light gels with carbomer the active ingredient, this agent swells on the ocular surface, absorbing, thickening and retaining the tears.
The latest developments in this field have produced non-blurring carbomer gels for daytime use. Other ocular surface agents that protect and prevent drying are the emollients, such as mineral oils and paraffin. These are generally found in preparations that would fall into the thick gel or even thicker ointment categories. These have a very high viscosity and long residence time but reduce optical clarity meaning they are generally reserved for severe DED and/or night-time use. One example is Xailin Night (VISUfarma).
Therefore, these polymers exhibit varying levels of viscosity, which resist flowing off the ocular surface. The high viscosity prolongs contact with the ocular surface longer (increased residence time) but reduces optical clarity, necessitating a balance. An ideal polymer would possess viscoelasticity, achieving different viscosities during blinking, sufficient to increase its residence time but decreasing immediately after the blink to increase optical clarity. Sodium hyaluronate (HA) fortunately exhibits this property along with many other beneficial features. It is available in different molecular weights, it is claimed that the higher molecular weight of HA has clinical benefits, even when the concentration is the same when compared to other products.
In recent study comparing three products, HydraMed, Evolve HA and Hylo Forte, (all containing 0.2% hyaluronic acid) Hylo Forte demonstrated increased ocular retention time, long lasting comfort, and superior clinical effectiveness.7 Hycosan Extra has the same formulation as Hylo Forte (Scope) figure 4.
Figure 4: Hycosan Extra
So far, we have examined components in dry eye drops that anchor to the surface epithelial cells and which also link to the basement tear layer of mucins, as well as those that thicken the aqueous layer. These components also contribute additional fluid to the ocular surface, however, we need to consider supporting the crucial evaporative retarding lipid layer. Agents such as phospholipids, mineral oils and plant oils more commonly found in sprays but are becoming available in conventional bottle delivery systems address this need.
A recent formulation such as Advanced Triple Action Eye Drops (Eye Doctor Ltd) contains Sacha Inchi oil, this oil plant, native to the tropical Amazon rainforests of South America, also contains omega-3 fatty acid (which is thought to have anti-inflammatory properties) and a form of vitamin E (antioxidant). Also, Systane Balance (Alcon) contains Mineral oil (np) Dimyristoyl phosphatidylglycerol (p) and Soothe XP (Bausch & Lomb) contains mineral oils (np).
Figure 5: Eye drops that support the lipid layer
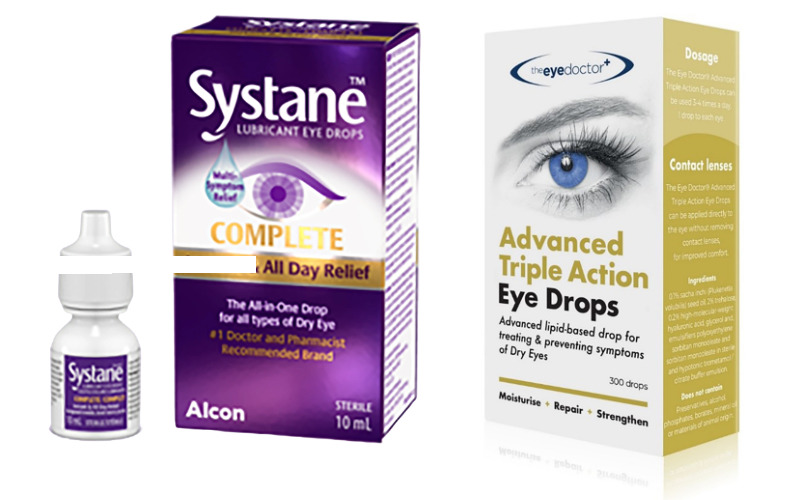
It is well established that the primary cause of most DED is the reduced efficiency of the lipid layer in preventing evaporation of the underlying aqueous layer of the tear film. As highlighted in the first article, around 86% of DED patients have meibomian gland dysfunction,8 which is a leading cause of evaporative dry eye (EDE). Therefore, supporting the tear’s lipid layer appears a logical approach.
A recently published paper had a primary focus on treatment alternatives utilising lipids, concluded that in placebo-controlled trials lipid-containing formulations increased lipid layer thickness (LLT) and improved tear break-up time for the treatment of evaporative dry eyes.9
In EDE, the aqueous layer loses water to the atmosphere while the existing electrolytes-potassium, chloride, bicarbonate, sodium, calcium and magnesium remain in the fluid, becoming more concentrated. The concentration of molecules in a fluid is called osmolarity, and a normal physiological concentration being iso-osmolar or isotonic. An increase in concentration, or a hypertonic tear film, incites ocular surface inflammation, which is largely responsible for dry eye symptoms and creates a vicious cycle of inflammation (figure 6).10,11

The sensitive conjunctival goblet cells, responsible for mucin production, are adversely affected by this inflammation. Consequently, inefficiency of the lipid layer leads to aqueous evaporation and a higher concentration of electrolytes, which leads to inflammation, which in turn leads to mucin production being adversely affected and so the vicious cycle continues.
As described earlier, the base mucin layer being likened to interlocking Lego, by removing blocks from this foundation destabilises the structure. To counteract this hypertonicity, many dry eye drops are formulated to be hypotonic, so when they mix with the existing hypertonic film, they help achieve an isotonic tear film. Specific electrolytes in these products, such as, potassium, chloride, bicarbonate, sodium, calcium and magnesium, help them resemble human tears.
Perhaps, the most important electrolytes are potassium, which protects against epithelial oedema, and bicarbonate that promotes epithelial repair following damage. Products containing bicarbonate must be packaged to prevent air contact, which converts it into carbon dioxide and permeates through normal plastic packaging.
Dry eye drops have significantly advanced, with increasingly sophisticated formulations that alter the response of cells and ocular surface tissues. Under hyperosmolar conditions, the most common form of DED, ocular surface epithelial cells lose water to the more concentrated tears above increasing their electrolyte concentration (continuing another vicious cycle), and potentially leading to cellular damage. Adding specific solutes to dry eye products such as osmo- or bio-protectants, like erythritol, levocarnitine and trehalose, can mitigate this.10 These components provide osmoprotection by restoring osmotic balance at the ocular surface and bioprotection by protecting proteins and cellular membranes from inactivation or denaturation caused by desiccation and oxidative stress.
As previously discussed, inflammation is widely recognised as a core mechanism in dry eye disease.15, 16 Leptospermum spp (Manuka) honey is a natural therapy that has antimicrobial,17 antioxidant and anti-inflammatory properties.18, 19 In a randomised control trial, formulated eye drops with Leptospermum spp honey were effective for reducing tear film evaporation rate and reducing ocular symptoms related to dry eye compared with conventional eye drops and, as a result, it was thought eye drops formulated with honey should be considered in the management of dry eye.20
Preservatives
All eye drops have the potential to become contaminated by microbes that could subsequently lead to eye infections. Therefore, historically, a key component of all forms of eye drops has been preservatives to resist, or at least delay, this potential contamination. The two main approaches to preservatives have been in the form of detergents and oxidants. The most widely used detergents are benzalkonium chloride (BAK) and polyquaternium-1 (Polyquad). BAK makes cell membranes more fragile, inducing inflammation, oxidative stress and programmed cell death (apoptosis).
While this is beneficial for eliminating harmful micro-organisms, it also adversely affects conjunctival and corneal epithelial cells.11 Even in very low concentrations, BAK has been shown to be toxic to the ocular surface, including causing loss of conjunctival goblet cells.11 In the author’s opinion this is not acceptable in eye drops.
Polyquad, another detergent, also makes cell membranes more fragile and has a greater affinity for bacterial cells while repelling epithelial cells. Although these characteristics are advantageous, Polyquad has also been shown to cause superficial epithelial damage, mainly by decreasing conjunctival goblet cell numbers.13 The sensitive conjunctival goblet cells, responsible for mucin production, are not only affected by inflammation but are also adversely impacted by preservatives.
An alternative approach to the detergents is oxidative or ‘vanishing’ preservatives, such as a stabilised oxychloro complex and sodium perborate. These preservatives degrade in the tear film, which theoretically minimises ocular toxicity. However, as with detergent preservatives, patients with DED may not have sufficient tear volume to break down this detergent and may experience irritation.11
Given the importance of keeping the drops free from contamination while minimising the negative effects of preservatives, one approach is to physically keep the product free from contamination. Unit/single dose or single day vials can be preservative free as they are designed to (and must) be discarded immediately after use or on the day of use.
Additionally, more drop bottles are now being designed with specially engineered valves and/or micro-filters that allow the drop fluid out but prevent micro-organisms from entering. The design of the drop container is crucial, especially for patients, many of whom are older with restricted finger pressure and dexterity to express a drop. Most manufacturers are acutely aware of this and design containers accordingly. The act of instilling eye drops safely and effectively is not intuitive, compliance aids such as Ezidrops (Ezidrops Ltd) may facilitate safe and effective instillation (figure 7).
Figure 7: Ezidrops applicator (Image: EziDrops Ltd)

Some patients prefer a spray alternative, which may be less prone to contamination as they do not come into direct contact with the patient’s skin. However, consideration must be given to the possibility of the spray transferring microorganisms from the lids and lashes as it runs down onto the eye. Some are designed for use on closed eyes, while others instruct keeping the eyes open. Generally, these are preservative free, although they do not necessarily offer all the optional ingredients discussed earlier.
Summary
Topical dry eye drops should be included as part of a home treatment protocol for DED. While they are a vital component, they are not the sole solution, as will be discussed in future articles. The vast majority of products discussed here are available without legal restriction and thus advice, guidance and selling them to patients is entirely within the dispensing optician’s scope of practice. Knowledge and awareness of a wide range of eye drops is valuable to the practice, both clinically and commercially. A handful of products are historically labelled ‘P’ medicines, and these can only be dispensed to the public under the supervision of a pharmacist or optometrist for relevant P medicines.
One further eye drop that could be utilised as part of a strategy to regain ocular surface homeostasis, but is rarely used, is topical cyclosporine. Cyclosporine has potent immunosuppressive properties, which reduce ocular surface inflammation to break the dry eye vicious circle.
A systematic review and meta-analysis found that using these eye drops twice daily significantly improved both objective and subjective outcomes in dry eye syndrome (DES) patients.14 This topical prescription only medicine eye drug has been specifically reserved for specialist ophthalmologists up until now, perhaps partly because of its cost to the NHS of £72 for a month’s supply. It is thought that treatment, alongside other necessary dry eye treatments would extend over several months.
As has been stressed in previous articles, meticulous record keeping regarding all advice and dispensed products should be maintained to ensure continuity of patient care.
Ideal dry eye drops characteristics
A topical dry eye drop treatment would ideally have the following components and characterises within its formulation:
- First and foremost, it must be safe with no adverse systemic or topical effects. Recent cases of devastating infections directly attributed to contaminated eye drops in various countries (including the USA and the UK) have resulted in, or contributed to, permanent sight loss, eye enucleations and deaths.
- The bottle/delivery system should be effective in countering contamination, while at the same time not containing components (preservatives) that give rise to toxic adverse responses.
- The drop should be delivered sterile out of the bottle/delivery system for a long period of time during normal usage and that should be indicated to the user.
- A long shelf-life indicates it can be stored unopened while remaining free from contamination and/or formulation degradation.
- The bottle/delivery system needs to have the ability to be manipulated safely and effectively when instilling a drop/gel/ointment, considering the user may not be dexterous (rheumatoid arthritis is a risk factor for dry eyes), may belong to an older age group, and/or may have poor near vision if presbyopic/hyperopic (it is almost impossible to instil eye preparations with spectacles on). Compliance aids, such as EziDrop (EziDrops Ltd) and the AutoSqueeze Drop Dispenser can help ensure the safety and effectiveness of instillation.
- Hyaluronic acid (HA) is now a basic foundation for dry eye drops, for all the reasons listed and is ubiquitous, certainly in any relatively modern formulation.
- Recognising the vast majority of dry eye conditions involve significant evaporation, resulting in a hypertonic tear film, a hypotonic formulation could potentially restore the balance towards isotonicity.
- Considering the suboptimal lipid layer due to meibomian gland dysfunction is a major contributor to EDE incorporating lipid support within the formulation could be beneficial.
- Osmo- or bio-protectants will provide cellular protection from the inflammatory environment of a hypertonic tear film.
- The formulation should ensure a prolonged residence time on the ocular surface without adverse effects, such as blurring of vision.
- Andrew D Price FBDO(Hons)CL MBCLA, CEO of ADP Consultancy and ADP-EyeCare is a clinician providing contact lens and dry eye clinics, a principal investigator in clinical trials, professional services consultant, educator and author. He received certification in ophthalmic assisting during work in ophthalmology practice in the USA and has worked in laser vision and ophthalmology clinics in the UK and USA. He has published and presented over 300 lectures and workshops, in the UK and abroad and was involved in developing Eye Drops Database. Andrew is also a past ABDO National Clinical Committee and Optometry Wales Board member and has been a guest lecturer for ABDO and Anglia Ruskin University.
- Further information on dry eye topical products may be found in the British National Formulary (bnf.org), on the MIMS website (mims.co.uk) and in its publications, and at the author’s professional website (free to access) eyedropsdatabase.co.uk.
References
- Zhou L, Zhao SZ, Koh SK, Chen L, Vaz C, Tanavde V, Li XR, Beuerman RW. In-depth analysis of the human tear proteome. J. Proteomics. 2012 Jul. 16;75(13):3877-85.
- Chen L, Zhou L, Chan EC, Neo J, Beuerman RW. Characterization of the human tear metabolome by LC-MS/MS. J. Proteome Res. 2011 Oct. 7;10(10):4876-82.
- Montes-Mico R, Cervino A, Ferrer-Blasco T, Garcia-Lazaro S, Madrid-Costa D. The tear film and the optical quality of the eye. The Ocular Surface. 2010; 8(4):185-192. https://doi. org/10.1016/S1542-0124(12)70233-1
- Montés-Micó R, Alió JL, Muñoz G, Pérez-Santonja JJ, Charman WN. Postblink changes in total and corneal ocular aberrations. Ophthalmology. 2004;111(4):758–67. https://doi. org/10.1016/j.ophtha.2003.06.027
- D’Souza S, Annavajjhala S, Thakur P, Mullick R, Tejal SJ, Shetty N. Study of tear film optics and its impact on quality of vision. Indian J Ophthalmol. 2020 Dec;68(12):2899-2902. https://doi.org/10.4103/ijo.ijo_2629_20
- Wander AH, Koffler BH. Extending the duration of tear film protection in dry eye syndrome: review and retrospective case series study of the hydroxypropyl cellulose ophthalmic
- Semp, D, Dutta, D and Wolffsohn, JS, 2023. The clinical efficacy of higher molecular weight sodium hyaluronate in artificial tears: A randomised clinical trial. Investigative Ophthalmology & Visual Science, 64(8), pp.3970-3970.
- Lemp LA, Crews LA, Bron AJ, Foulkes GN, Sullivan BD. Distribution of aqueous- deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea 2012;31(5):472-8.
- Furqan A Maulvi, Ditixa T, Desai, Parthasarathi Kalaiselvan et al, Lipid-based eye drop formulations for the management of evaporative dry eyes. CLAE, Volume 47, ISSUE 3, 102154, June 2024. https://doi.org/10.1016/j.clae.2024.102154
- Baudouin C et al. Role of hyperosmolarity in the pathogenesis and management of dry eye disease: Proceedings of the OCEAN Group Meeting. The Ocular Surface 2013; 11(4) 246-258.
- International Dry Eye Workshop. Management and therapy of dry eye disease: Report of the management and therapy subcommittee of the International Dry Eye Workshop (2007). The Ocular Surface 2007; 5:163-178.
- Tressler CS, Beatty R, Lemp MA. Preservative use in topical glaucoma medications. The Ocular Surface 2011; 9:140-158.
- Lopez B, Ubel J. Quantitative evaluation of the corneal epithelial barrier effect of artificial tears and preservatives. Current Eye Research 1991; 10:645-656
- Kelvin H Wan, Li Jia Chen, Alvin L Young, Efficacy and Safety of Topical 0.05% Cyclosporine Eye Drops in the Treatment of Dry Eye Syndrome: A Systematic Review and Meta-analysis, The Ocular Surface, Volume 13, Issue 3, 2015, Pages 213-225, ISSN 1542-0124, https://doi.org/10.1016/j.jtos.2014.12.006. (https://www.sciencedirect.com/science/article/pii/S1542012415000361)
- Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf 2017; 15:276–83. doi: 10.1016/j.jtos.2017.05.008
- Wei Y, Asbell PA. The core mechanism of dry eye disease is inflammation. Eye Contact Lens 2014; 40:248–56. doi:10.1097/ICL.0000000000000042
- Albietz JM, Lenton LM Effect of antibacterial honey on the ocular flora in tear deficiency and meibomian gland disease. Cornea 2006; 25:1012–9. doi: 10.1097/01.ico.0000225716.85382.7b
- Tonks AJ, Cooper RA, Jones KP, et al Honey stimulates inflammatory cytokine production from monocytes. Cytokine 2003; 21:242–7. doi:10.1016/S1043-4666(03)00092-9
- Almasaudi SB, El-Shitany NA, Abbas AT, et al Antioxidant, anti-inflammatory, and antiulcer potential of manuka honey against gastric ulcer in rats. Oxid Med Cell. doi:10.1155/2016/3643824
- Tan J, Jia T, Liao R, Stapleton F. Effect of a formulated eye drop with Leptospermum spp honey on tear film properties. Br J Ophthalmol. 2020 Oct;104(10):1373-1377. doi: 10.1136/bjophthalmol-2019-315160. Epub 2020 Jan 16. PMID: 31949092.
- Price Andrew D. Eye Drop Problems - Part 1 (warning not for the faint hearted!). Eye Drops Database, Guest Articles. https://www.eyedropsdatabase.co.uk/guest-articles/253-eye-drop-problems-part-1
