Domains and learning outcomes (C110037)
• One distance learning CPD point for optometrists, dispensing opticians and contact lens opticians.
• Clinical practice
Upon completion of this CPD, ECPs will be able to describe current classification systems for keratoconus (s.5).
• Specialialty CPD – Contact lens opticians
Upon completion of this CPD, contact lens opticians will be able identify the clinical signs of keratoconus (s.5).
Compared to conditions like pellucid marginal degeneration (PMD) and keratoglobus, keratoconus is the most common corneal ectatic disorder that eye care professionals (ECPs) will likely discover, refer and manage in clinical practice.1
All ECPs will be familiar with the established descriptions attributed to this disorder.2, 3 Typically, keratoconus is characterised as a bilateral, non-inflammatory corneal disease, which results in progressive stromal thinning that leads to a steepening of the inferior-central cornea.4 Such alterations occur due to significant changes in the biomechanical properties of the cornea,5 resulting in the stromal lamellar matrix no longer following a highly regularised, orthogonal pattern.6, 7
Instead, there are discrete areas of poorly aligned collagen intermixed with collagen that remains arranged in the conventional quasiregular fashion.8 A reduction in the cornea’s internal biomechanical strength leaves it more susceptible to distortion.9 This, in turn, causes the cornea to progressively protrude forwards, such that its overall shape, on both corneal surfaces,10 becomes considerably more conical, thereby inducing unwanted irregular astigmatism.11, 12 Although keratoconus usually occurs bilaterally, it is classically asymmetrical in its nature.13
The visual difficulties associated with keratoconus can vary dramatically, as they are highly variable between patients and, in part, dependent upon the stage of the disease’s progression.13-15 Nonetheless, even in its early stages, patients will typically present with unclear distance vision that does not improve even with their optimal refraction and/or a pinhole.15-16 In more advanced cases of keratoconus, the cornea can show a markedly high degree of conical protrusion, which can make both the fitting and wearing of contact lenses extremely challenging, potentially resulting in rigid corneal contact lens intolerance.17
The keratoconic cornea can also develop scarring, which may typically be attributed to wearing flat-fitting rigid gas-permeable contact lenses18 and/or significant disease progression over time; for example, through the development of acute corneal hydrops, associated with ruptures to Descemet’s membrane.2, 19 Unfortunately, significant centralised corneal scarring coupled with a high degree of conical protrusion are risk factors for the requirement of a corneal transplant.1
As the retina, optic nerve and visual cortex typically remain unaffected by keratoconus, the irreversible reduction in visual performance compared to normal healthy eyes, can be directly attributed to a combination of induced irregular corneal astigmatism,20 corneal higher-order aberrations21 and unwanted light scattering within the cornea.22
Unlike other ectatic conditions, such as PMD and keratoglobus, keratoconus characteristically affects the inferior-central two-thirds of the cornea. However, reports of centrally, inferiorly, inferior-nasally and superiorly positioned cone apices have also been published in the literature.14, 23-25 Other studies have also proposed that the cone’s apex is commonly displaced inferior–temporally in keratoconus.26-28 Overall, clinicians should remain aware that the degree of stromal thinning and the location of the cone’s apex will be unique for each keratoconic cornea.
Detecting Keratoconus
ECPs typically find it relatively straightforward to identify moderate to severe keratoconus, due to its classical topographical appearance (outlined later in this article) and characteristic clinical signs.2-4, 19 These clinical signs include:
- Irregular keratometric mires, detectable using a manual keratometer
- A ‘scissoring’ reflex, detectable using a retinoscope
- Charleaux’s oil-droplet reflex, detectable using an ophthalmoscope
- Conical protrusion (seen with a corneal slit section), Vogt’s striae (characteristically located within the posterior stroma), Fleischer’s ring (anterior to Bowman’s layer), prominent corneal nerves, and apical scarring, all of which are detectable via a thorough slit-lamp examination of the cornea
- Reduced corneal thickness, detectable via ultrasonic pachymetry and/or tomography measurements
- Rizzuti’s phenomenon, detected using a suitable light source, where light shone from the temporal side is sharply focused at the nasal limbus
- Deformation of the lower eyelid upon infraduction (Munson’s sign)
In stark contrast, the true challenge for ECPs lies in being able to detect keratoconus when it is in its infancy, with near-normal best-corrected spectacle visual acuity and no obviously identifiable clinical slit-lamp signs. Identification of early keratoconus is critical while screening candidates for corneal refractive surgery, as keratorefractive procedures are likely to cause unpredictable refractive outcomes and may even induce post-surgical keratectasia in predisposed individuals.29
Once keratoconus has been successfully detected, the next logical step will be to classify the disease’s current ‘stage’, such that its progression can be accurately monitored. This is important as the condition is typically progressive, classically starting in the second or third decades of life, potentially progressing up until the fourth decade.2-4, 19, 30
Common Keratoconus Classification Systems
The internationally recognised Global Panel of Keratoconus and Ectatic Diseases31 is composed of an expert panel of Ophthalmologists that have:
- Experience in the management of keratoconus and ectatic diseases
- Authored scientific publications in high-impact medical journals
- Received wide recognition within the specialist medical community
The panel’s main objective was to acquire the most reliable level of evidence-based, scientific agreement from a group of expert clinicians using an iterative process (they used the Delphi model) with several rounds of structured questioning. In their 2015 study,31 the panel covered the most relevant and contentious questions regarding the definition, methods of diagnosis, and treatments for keratoconus.
As part of their work, the panel had also intended to determine a classification system for keratoconus. However, after two rounds of extensive discussions on the topic, the panel conceded that, currently, there is no clinically adequate classification system for keratoconus.
So, where might this leave optometrists, contact lens opticians and dispensing opticians? The purpose of this article is to furnish ECPs with the most recent developments in keratoconus classification systems, so that we can better understand those patient diagnosis letters that arrive from our ophthalmology colleagues.
Such correspondence might include descriptive diagnostic terms such as ‘moderate keratoconus’, ‘A-K stage 2 keratoconus’ or ‘KSS 3: affected – mild disease’. These can be rather confusing to interpret, especially because all three of these diagnostic terms could potentially be used to describe/diagnose the same keratoconic cornea, by virtue of using three different classification systems. Consequently, ECPs need to be alert to the existence and use of different systems, especially in light of the fact that, currently, there is no internationally accepted gold standard for classifying keratoconus.31
Using a contemporary and chronological approach, this article shall now describe the most commonly used classification systems throughout the world and discuss their relative strengths and weaknesses.
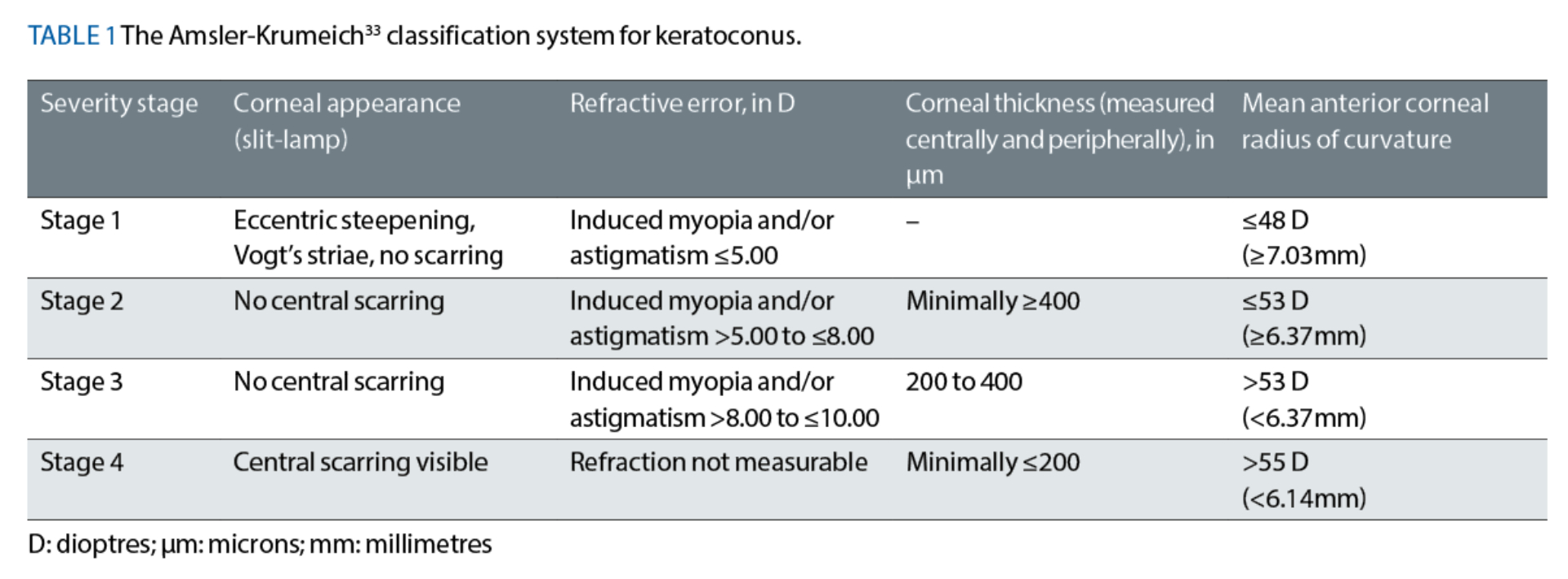
The Amsler-Krumeich (A-K) Classification System
Originally published circa 1946 by Amsler32 and updated in 1998 by Krumeich et al,33 readers may have read about and possibly even used the A-K classification system as part of their clinical practice.33 This classic system is based on four clinical features:
- Mean keratometric readings, which were measured using videokeratography-based topography (ie from simulated keratometry readings)
- Corneal thickness measurements (measured centrally and peripherally)
- Measurements of manifest refraction
- The presence or absence of corneal scarring
The A-K system has four severity stages, ranging from Stage 1 to Stage 4, where Stage 1 represents the earliest stage of the disease (see table 1). Although useful, this well-established classification system suffers from notable limitations, which include:
- Relying solely on anterior corneal surface curvature measurements only, ignoring any posterior surface irregularities
- Its inability to properly classify a cornea if its four clinical features happen to fall into different severity stages
- Monitoring of the disease’s progression across the four severity stages can be very challenging due to the issue raised in point b)
- Not considering the patient’s best-corrected visual acuity, despite accounting for the patient’s refractive error
- Not measuring the corneal thickness specifically at the cone’s apex
- Not accounting for several technologies that are currently used to detect, evaluate and monitor keratoconic patients, for example, Scheimpflug tomography-based measurements and higher-order aberration measurements.
The Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study Group’s Classification System (1996)14
Addressing the issue of monitoring keratoconus progression across different severity stages, the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) study group formulated a much simpler system which focused on ‘manual’ keratometry measurements.14 Keratoconic patients were subsequently classified as having:
- ‘Mild’ keratoconus if the steepest keratometry reading (Ks) was less than 45 dioptres (D) which, assuming a standard dioptric calibration refractive index value of 1.3375 for the entire cornea, equates to a radius of curvature of >7.50mm
- ‘Moderate’ keratoconus if the Ks reading was in-between 45 and 52 D (ie in-between 6.49mm and 7.50mm), or
- ‘Severe’ keratoconus if the Ks reading was greater than 52 D (ie <6.49mm).
While this approach is useful, there is clearly a reliance on the ECP’s ability to perform manual keratometry accurately, in both the flattest and steepest meridians. This can prove to be challenging, especially when large magnitudes of corneal distortion with irregular astigmatism have been induced, such that the principal meridians are no longer separated by 90 degrees.
Furthermore, Zadnik et al14 reported that, wherever necessary, the attending ECP increased the keratometer’s possible range of measurements, up to a maximum curvature reading of 68.30 D, by using an auxiliary lens whose power was either +1.25 D or +2.25 D.14 Again, performance of this specific technique would be heavily dependent upon the ECP’s skill levels.
Finally, other key parameters, which ought to be considered when classifying keratoconus, were unfortunately not included as part of this classification system. For example, residual corneal thickness measurements, the patient’s best-corrected visual acuity, measurements of the profile of the posterior corneal surface, and an assessment of corneal scarring.
Considering Aberrometry
In keratoconus patients, even relatively early alterations in the shape of the cornea can induce higher-order aberrations, which differ significantly from those measured in healthy normal eyes.34 Of the different higher-order aberration metrics, coma root-mean square (RMS) error (measured in microns, μm) are most commonly found to become significantly elevated in keratoconic eyes.21, 34 This is because the maximal region of stromal thinning is classically located inferiorly.23, 24 Consequently, light waves arriving at the keratoconic cornea, from a distant source, will become distorted by comparatively different amounts at the (‘flatter’) superior and (‘steeper’) inferior cornea.21
The cone’s apex will also further distort the incoming light waves by ‘rotating’ them, thereby inducing trefoil (or triangular astigmatic) aberrations. Additionally, the steepened cone’s apex will also induce spherical aberration.21
These notable differences in higher-order aberrations, compared to in healthy normal eyes, have supported the use of anterior corneal aberrometry measurements as an accurate tool to detect early keratoconus.35 Consequently, two sets of different authors have published studies that incorporated higher-order aberration measurements into two new keratoconus classification systems.36, 37
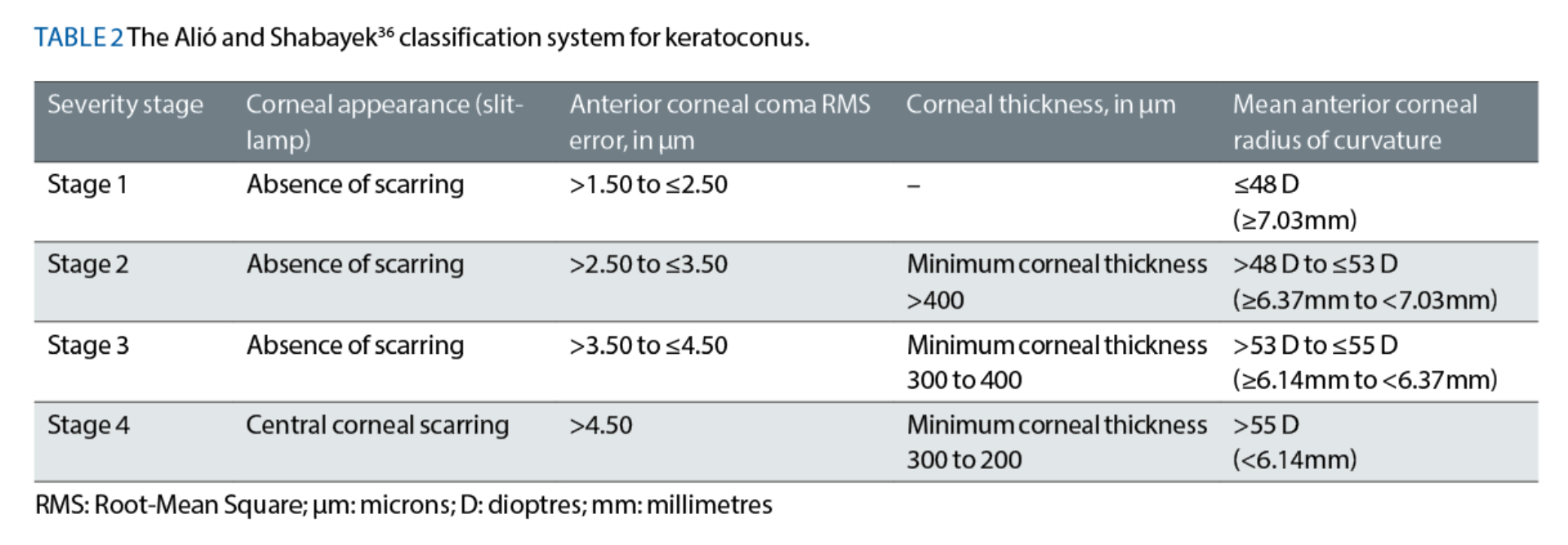
The Alió and Shabayek36 Classification System (June 2006)
Alió and Shabayek36 elected to modify and further develop the work of Krumeich et al33 by integrating Placido-ring-based, anterior corneal coma RMS error measurements into their classification system (see table 2). As it was based on the preceding A-K system,33 the Alió and Shabayek36 system also classifies keratoconus using four severity stages, from Stage 1 to Stage 4, where Stage 1 represents the earliest stage of the disease.
Alió and Shabayek36 recommended two important changes to the older A-K classification system. First, they removed the requirement to consider measurements of manifest refraction, which assists ECPs in avoiding a significant ‘subjective’ element of the A-K system. Second, Alió and Shabayek36 altered the corneal thickness boundaries used within their new system, such that ‘Stage 3’ keratoconus would now only include corneas with a minimum thickness value of between 300 and 400μm.
Rather unusually, Alió and Shabayek36 implemented this boundary alteration without clearly describing how they had measured the corneal thicknesses of their 40 keratoconic eyes (from 25 patients) in the methods section of their paper. Nonetheless, the ‘Stage 3’ classification in the older A-K system33 included a significantly wider range of corneal thickness values, of between 200 to 400μm;33 hence, it can be argued that Alió and Shabayek’s36 amendment would assist ECPs in more accurately stratifying keratoconic patients into the appropriate classification.
Despite these positive changes, readers will recognise that use of the Alió-Shabayek36 classification system requires the ECP to have access to a topographer that provides anterior corneal higher-order aberration measurements. Also, this system does not take the contour profile of the posterior corneal surface, nor the patient’s best-corrected visual acuity into consideration.
The Keratoconus Severity Score (KSS)37 Classification System (August 2006)
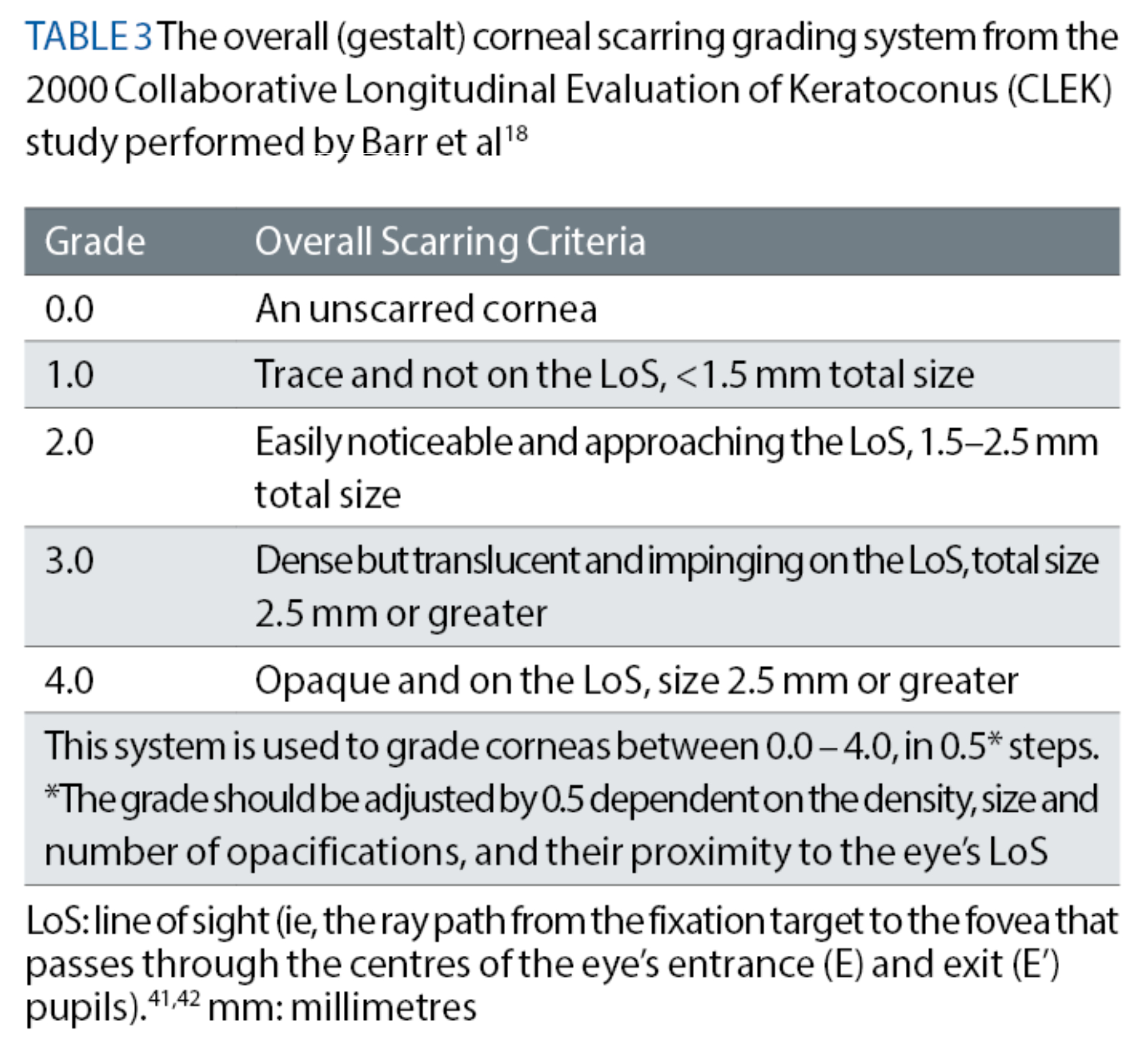
McMahon et al37 took a different approach while developing their KSS classification system. Their assessment process included a detailed slit-lamp examination of the cornea to detect any clinical signs of keratoconus. Specifically, the authors recommended concentrating on the presence or absence of Fleischer’s ring, Vogt’s striae and any corneal scarring associated with keratoconus. Whenever such corneal opacification is detected, a bespoke method for grading the scarring, as outlined in an earlier CLEK study conducted by Barr et al,18 must be used. This particular grading method utilises a scale of 0 to 4, where 0 refers to an unscarred cornea. This method also considers how close the opacity is, as a whole, to the eye’s line of sight, as well as how large and dense the opacity is, to give an estimate of the cornea’s overall (gestalt) scarring (see table 3).18
McMahon et al37 also recommended a careful evaluation of the anterior corneal surface’s topographical pattern, and drawing comparisons to the characteristic topographical patterns found in keratoconus. To assist readers, figure 1 (top picture) displays seven sets of anterior corneal topographical patterns, which range from symmetrical patterns (row 1), to asymmetrical patterns (row 2) and asymmetrical patterns with ‘skewed radial axes’ (row 3).

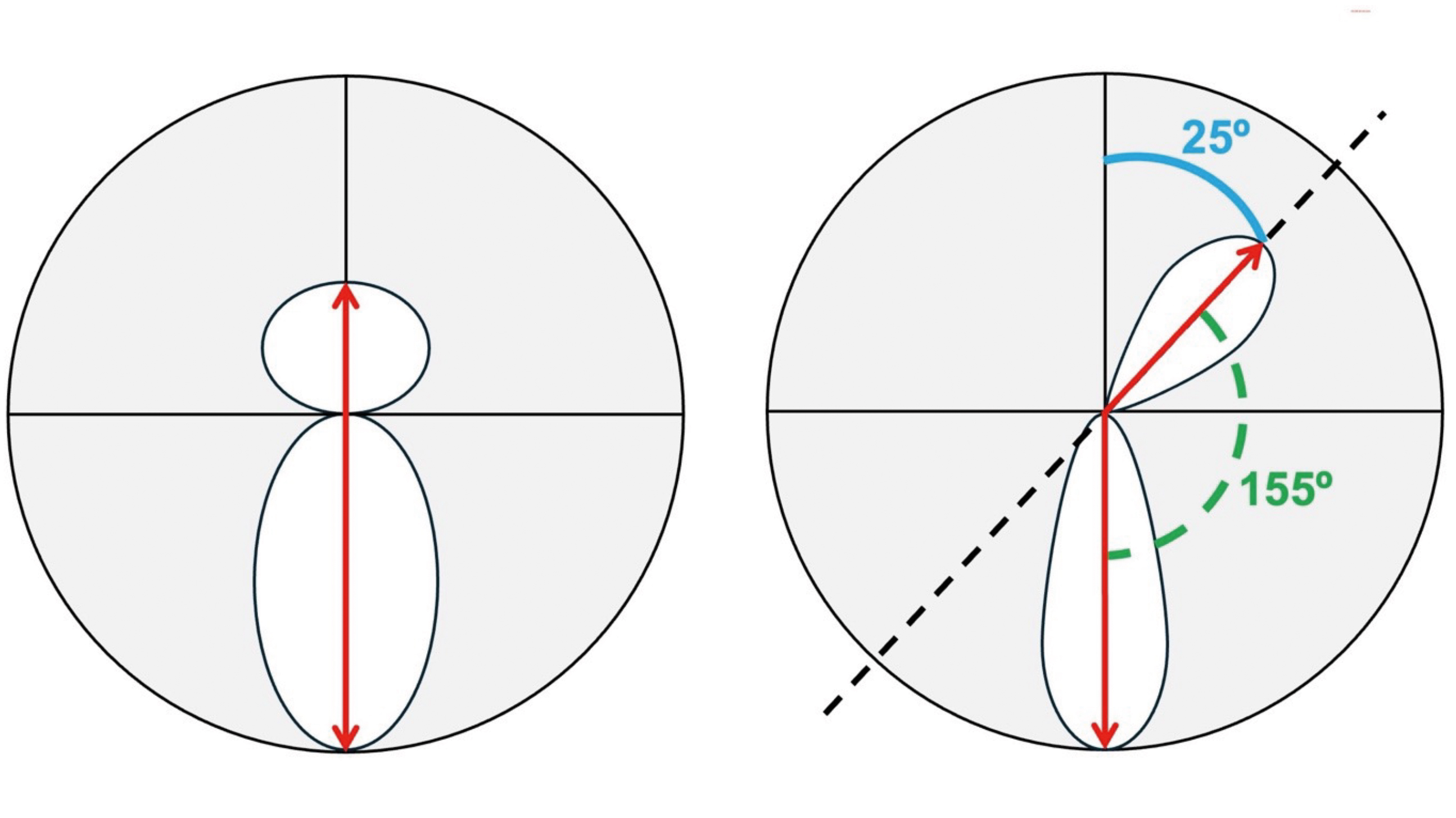
Figure 2 (above, bottom picture) illustrates how ECPs can differentiate between an ‘asymmetric bowtie with inferior steepening’ pattern from an ‘asymmetric bowtie with skewed radial axes’ pattern.
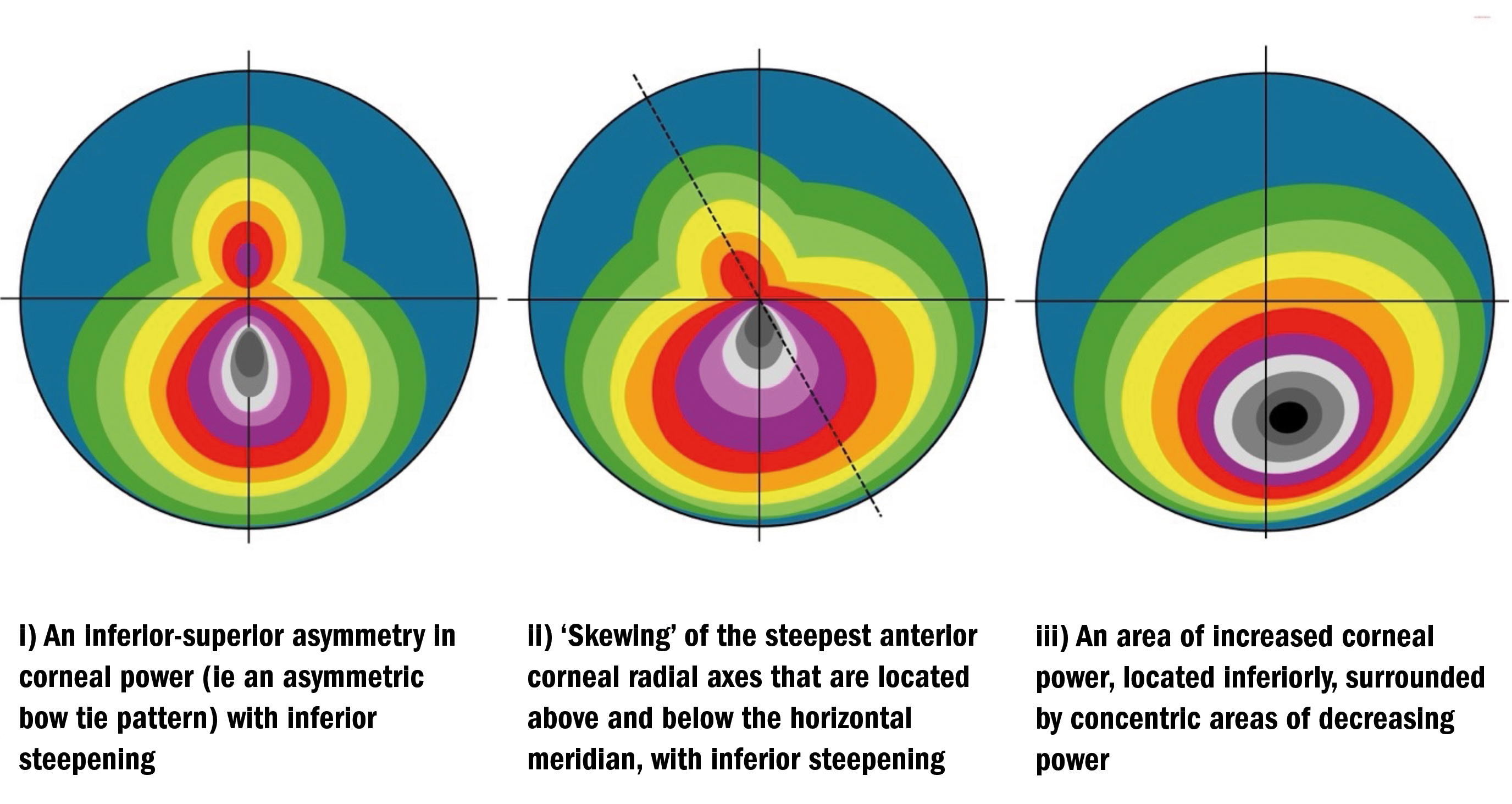
Figure 3 (above) presents three of the most common topographical patterns typically associated with keratoconus including:
- An inferior-superior asymmetry in corneal power (ie, an ‘asymmetric bow tie’ pattern) most typically with inferior steepening2
- ‘Skewing’ of the steepest anterior corneal radial axes that are located above and below the horizontal meridian (through induced irregular astigmatism), most typically with inferior steepening2
- An area of increased corneal power, most typically located inferiorly, surrounded by concentric areas of decreasing power2
The reference to ‘radial axes’ in point ii), above, relates to the fact that topographical data points are produced in the form of ‘polar coordinates’ using >250 radial lines that have been scanned across >20 concentric rings mapped out across the cornea, generating, as a minimum, approximately 7,000 data points.
McMahon et al37 also mandated that the average corneal power (ACP), measured in D and derived from (simulated) topographical keratometric readings, should be considered as part of the classification process.
Finally, the authors advised that topography-based anterior corneal higher-order RMS (HORMS) error, measured in μm, should be considered too. McMahon et al37 most likely chose this specific criterion for their key metric because several other preceding studies had successfully demonstrated that anterior corneal HORMS error corresponded well, with the corneal distortions typically found in all stages of keratoconus.35, 39, 40
Depending on the KSS stage, which ranges from zero – representing a ‘normal’ cornea, to five – representing ‘severe’ disease, some of the intra-stage clinical criteria are deemed to be ‘required’, whereas others are considered to be ‘additional’. Table 4 sets out how such criteria vary between different severity classifications. McMahon et al37 have strictly mandated that, within each severity stage, the ‘required’ clinical criteria must all be met first. Only after this should the clinical features from any of the indicated ‘additional’ criteria be assessed, with the ‘worst’ criterion carrying ‘the greater weight’.
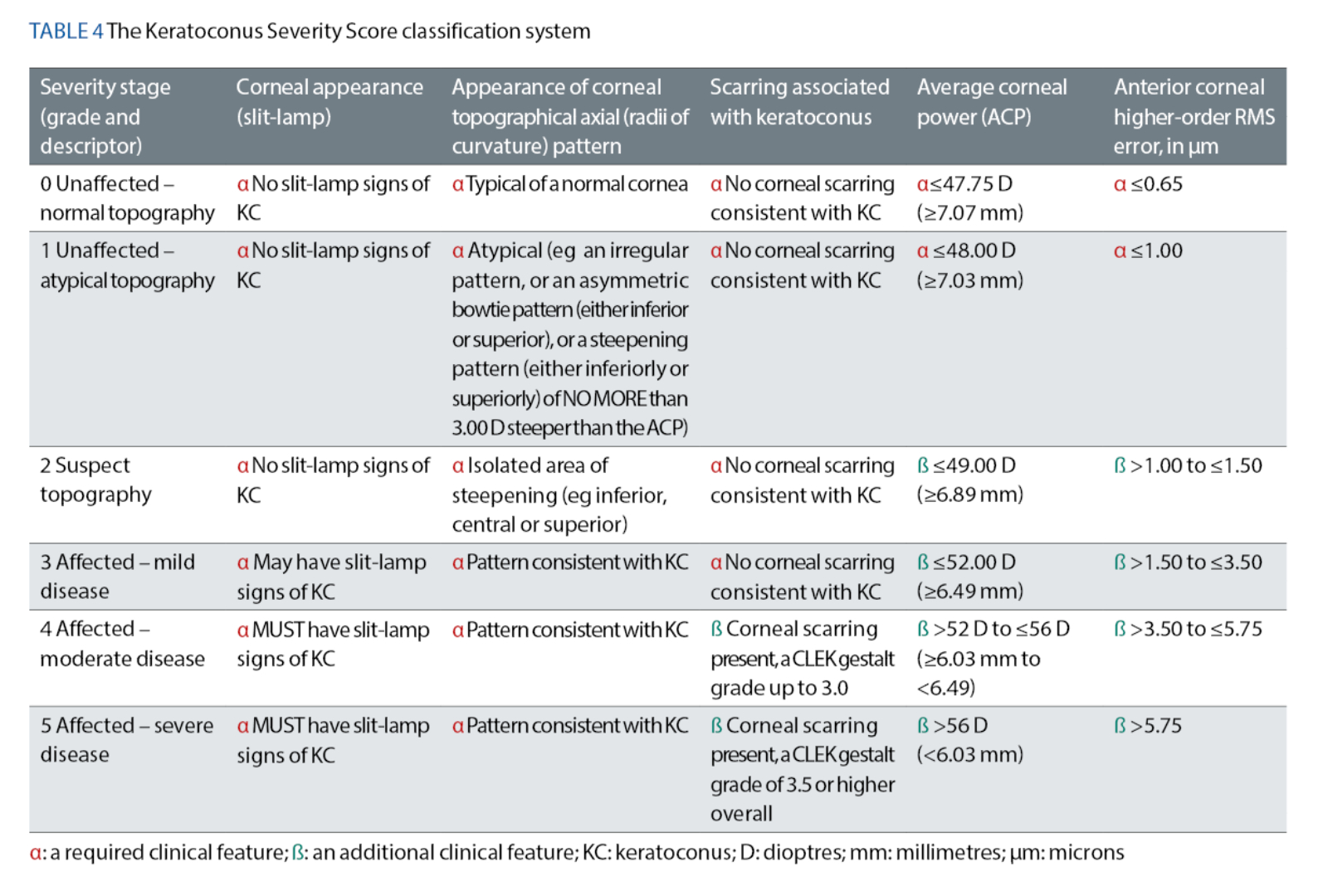
At first glance, ECPs may feel that using the KSS system will be complicated. However, by considering the anterior cornea’s topographical pattern and any potential corneal slit-lamp signs, it may be argued that the KSS system37 offers ECPs a more holistic approach to classifying keratoconus compared to the CLEK system.14 The KSS system also offers the added flexibility of the ‘additional’ criteria, which means that ECPs do not have to always rely on every single clinical criterion being present.37
Nevertheless, the KSS system does not consider residual corneal thickness measurements, the patient’s best-corrected visual acuity or the contour profile of the posterior corneal surface. Furthermore, for appropriate stratification into the correct severity score, this system requires an ECP who is competent in interpreting anterior corneal topography maps.
Considering the Posterior Corneal Surface
Currently, in high-street clinical practice, the detection and classification of keratoconus may still be undertaken using measurements from Placido-ring-based corneal topographers. This is likely because these devices are relatively inexpensive to purchase compared to more costly Scheimpflug-based tomographers. Unfortunately, Placido-based corneal imaging can suffer from the following drawbacks:
- It relies heavily upon reflection; therefore, any tear film irregularities can impact upon the quality of the ring images (and any subsequent data/measurements)
- It offers limited corneal coverage, which could hinder the measurement of important data from the para-central and peripheral anterior corneal surface
- During measurements, the topographer device’s axis is typically aligned (by the user) along an axis which is neither the visual axis nor the eye’s line of sight – ie the device’s axis is typically aligned perpendicular to the cornea, as if directed towards the cornea’s centre of curvature, which could induce small unwanted measurement errors41, 42
- It is limited to measuring the anterior corneal surface only, ignoring any posterior corneal surface irregularities that might be present
Modern Scheimpflug-based tomographers, such as the Oculus Pentacam (Oculus Optikgeräte GmbH; Wetzlar, Germany), are capable of imaging both the anterior and posterior corneal surfaces simultaneously. Additionally, Scheimpflug-based tomographers can also achieve near limbus-to-limbus corneal analysis, thereby offering comparatively more surface contour information than Placido-ring-based topographers.
Using the Oculus Pentacam device, a plethora of studies have identified that the detection of discrete areas of elevation on the posterior corneal surface is a good predictor of early keratoconus.11, 12, 43-45 It is widely accepted that the pre-screening of corneal refractive surgery candidates has revealed that early keratoconus can not only be diagnosed prior to any loss in visual performance, but that its incidence was far higher than previously predicted.29 Currently, rather than having to treat significant irreversible vision loss, clinicians can helpfully refer suitable keratoconic patients for corneal collagen cross-linking (CCXL).
CCXL treatment provides an opportunity to proactively slow down or even arrest the progression of keratoconus, and if provided early enough, it might even prevent any consequent visual loss.46 Consequently, Belin and co-workers47 have argued that the requirement to carefully screen refractive surgery candidates and the advent of CCXL had highlighted the need for a new keratoconus classification system that included an evaluation of the posterior corneal surface.
The ABCD Keratoconus Classification System47
All of the aforementioned classification systems have, thus far, failed to consider the contour of the posterior corneal surface and the patient’s visual acuity. Cognisant of these inherent weaknesses, Belin and co-workers47 designed their ABCD keratoconus classification system to specifically overcome these deficiencies (see table 5). The ABCD system is based on four clinical parameters, which are:
- A: Anterior radius of curvature in the 3.0mm zone centred on the thinnest point of the cornea
- B: Posterior radius of curvature in the 3.0mm zone centred on the thinnest point of the cornea
- C: Thinnest corneal pachymetry measurement, in μm
- D: Best-corrected distance visual acuity (measured using the patient’s rigid gas-permeable contact lenses or spectacles)
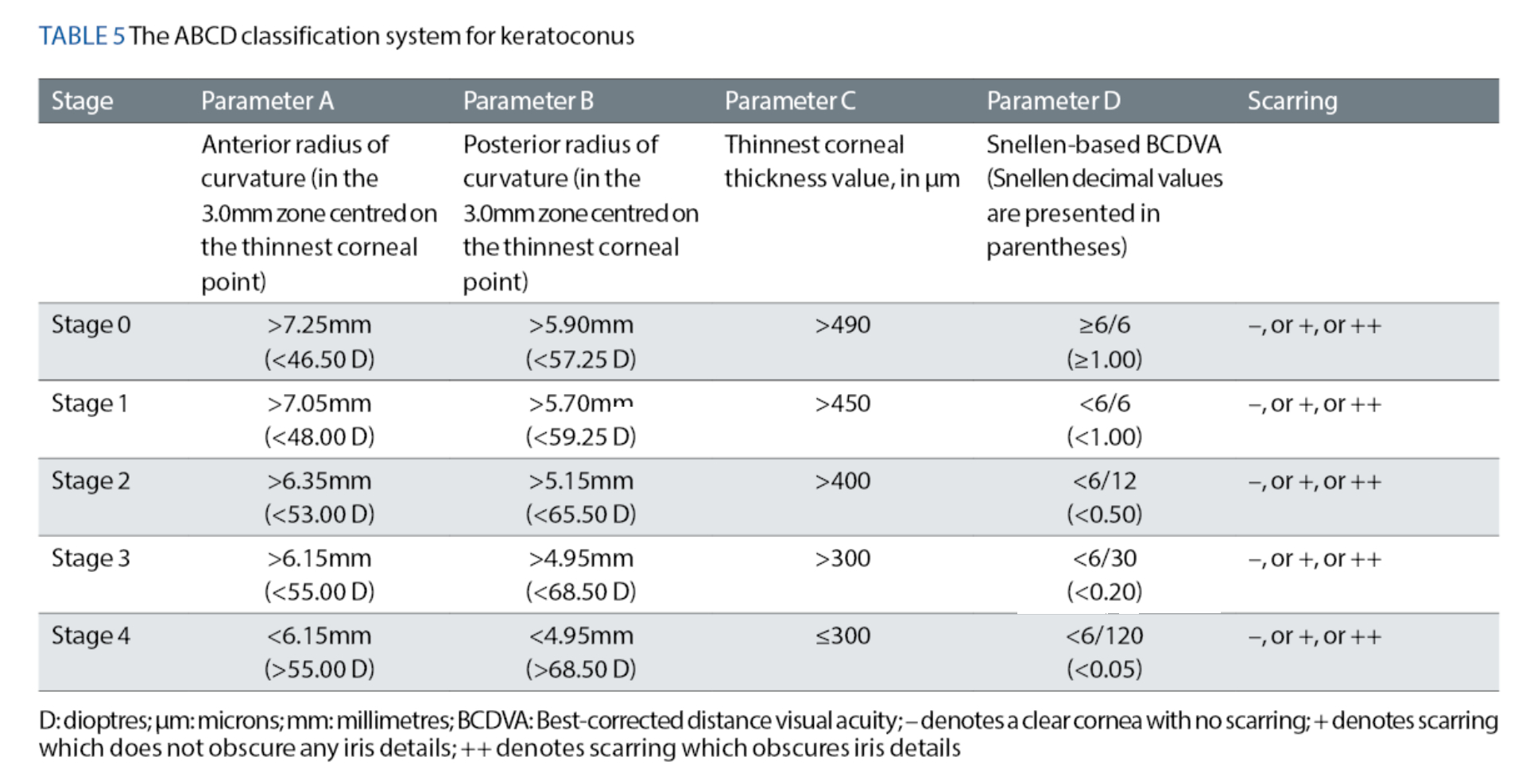
The authors proposed the ‘ABCD’ acronym because their system involves consideration of the Anterior surface curvature, the Back surface curvature, Corneal thickness and Distance visual acuity.47
Each parameter is graded on a scale of 0 to 4, where ‘Stage 0’ represents a ‘normal’, clear cornea, and ‘Stage 4’ represents a cornea with ‘advanced’ keratoconus. In addition, this system includes a separate grading for any corneal scarring, which is based on the visibility of the patient’s iris while using a slit-lamp. The grades are as follows:
- – denotes a clear cornea with no scarring,
- + denotes scarring which does not obscure any iris details, and
- ++ denotes scarring that obscures iris details.
In comparison to the KSS system,37 the ABCD system47 does not consider the axial topographical pattern of the anterior corneal surface, nor any anterior corneal surface higher-order aberration measurements. However, Belin and colleagues47 argue that these shortfalls are easily overcome by considering measurements of the posterior radius of curvature and the patient’s best-corrected distance visual acuity, respectively.
Furthermore, it appears that ECPs will likely favour the ABCD system’s47 method for grading corneal scarring over that of the KSS system,37 which is more complex and time-consuming, as it requires individual opacities to be counted and measured.
Ectasia Progression: Is there an accepted definition?
Having reviewed the most common keratoconus classification systems, the final matter is to consider how the progression of this ectatic condition might be defined. Prior to the Global Consensus on Keratoconus and Ectatic Diseases study,31 there was no consistent definition for ectasia progression within the peer-reviewed literature. Consequently, the study’s panel determined that ectasia progression required a consistent change in at least two of the following clinical parameters:
- Steepening of the anterior corneal surface
- Steepening of the posterior corneal surface
- Corneal thinning, and/or an increase in the rate at which the corneal thickness is reducing, from the corneal periphery to the cornea’s thinnest point
The panel further mandated that these clinical changes must be consistent over time and greater in magnitude than the normal degree of variability (ie noise) typically found for each parameter’s measurement device.31
Unfortunately, the panel did not specify which measurement metric should be used for each individual parameter, or even the type of device that should be used, thus leaving the guidance open to different interpretations by ECPs. For example, steepening of either corneal surface could be confirmed using asphericity metrics (eg P, Q or E values) or keratometric measurements.
Moreover, the panel did not clearly define numerical cut-off values for judging the levels of deterioration required for each parameter. Nor did they specify a minimum time interval over which any relevant deteriorations must be measured.
ECPs need to be aware that without a universally accepted definition for ectasia progression, it is likely that inconsistencies in CCXL treatment strategies, between different clinical trials, will continue.48 Furthermore, if the progression of a patient’s keratoconus is missed, this could lead to a delay in referring for CCXL treatment, a poorer final visual outcome and an increased risk of requiring a corneal transplant. Each of these factors is likely to have a significant detrimental effect on the patient’s quality of life.
Looking to the Future
Artificial intelligence (AI) is rapidly evolving within the field of corneal research. Currently, several studies have reported success in the ‘automated’ detection of early keratoconus, thanks to the assistance of computerised algorithms and a continuous progression in the level of sophistication of corneal imaging devices.49
Hence, AI can be used to evaluate substantial numbers of existing pachymetry and topography datasets measured from keratoconic patients, thereby facilitating ‘machine learning’ of the characteristic features of keratoconus, to essentially learn how to detect the disease in future patients.50
It is therefore increasingly likely that, in the future, AI-based algorithms will also eventually be able to classify keratoconic corneas into their various different disease stages, and then monitor any ectasia progression over time. Such valuable information would support ECPs in determining the optimal timeframe within which to recommend CCXL treatment. However, in the meantime, ECPs may use the information presented within this article to classify the keratoconic patients that they are currently managing in clinical practice.
- Dr Amit N Jinabhai is a qualified optometrist with a proven track record of excellence in optometric education, optometric research, regulatory decision-making and clinical auditing. He is the founder of Jinabhai Regulatory Consultancy and works as a freelance consultant.
- Dr Amit N Jinabhai has no declarations of interest to make.
References
- Maharana PK, Dubey A, Jhanji V, Sharma N, Das S, Vajpayee RB. Management of advanced corneal ectasias. Br J Ophthalmol. 2016;100(1):34-40.
- Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42(4):297-319.
- Santodomingo-Rubido J, Carracedo G, Suzaki A, Villa-Collar C, Vincent SJ, Wolffsohn JS. Keratoconus: An updated review. Cont Lens Anterior Eye. 2022;45(3):101559.
- Romero-Jiménez M, Santodomingo-Rubido J, Wolffsohn JS. Keratoconus: a review. Cont Lens Anterior Eye. 2010;33(4):157-66.
- Falgayrettes N, Patoor E, Cleymand F, Zevering Y, Perone JM. Biomechanics of keratoconus: Two numerical studies. PLoS One. 2023;18(2):e0278455.
- Hayes S, Boote C, Tuft SJ, Quantock AJ, Meek KM. A study of corneal thickness, shape and collagen organisation in keratoconus using videokeratography and X-ray scattering techniques. Exp Eye Res. 2007;84(3):423-34.
- Zhou D, Abass A, Lopes B, Eliasy A, Hayes S, Boote C, et al. Fibril density reduction in keratoconic corneas. J R Soc Interface. 2021;18(175):20200900.
- Meek KM, Tuft SJ, Huang Y, Gill PS, Hayes S, Newton RH, et al. Changes in collagen orientation and distribution in keratoconus corneas. Invest Ophthalmol Vis Sci. 2005;46(6):1948-56.
- Padmanabhan P, Lopes BT, Eliasy A, Abass A, Elsheikh A. In Vivo Biomechanical Changes Associated With Keratoconus Progression. Curr Eye Res. 2022;47(7):982-6.
- Tomidokoro A, Oshika T, Amano S, Higaki S, Maeda N, Miyata K. Changes in anterior and posterior corneal curvatures in keratoconus. Ophthalmology. 2000;107(7):1328-32.
- Kamiya K, Ishii R, Shimizu K, Igarashi A. Evaluation of corneal elevation, pachymetry and keratometry in keratoconic eyes with respect to the stage of Amsler-Krumeich classification. Br J Ophthalmol. 2014;98(4):459-63.
- Pinero DP, Alio JL, Aleson A, Escaf Vergara M, Miranda M. Corneal volume, pachymetry, and correlation of anterior and posterior corneal shape in subclinical and different stages of clinical keratoconus. J Cataract Refract Surg. 2010;36(5):814-25.
- Zadnik K, Steger-May K, Fink BA, Joslin CE, Nichols JJ, Rosenstiel CE, et al. Between-eye asymmetry in keratoconus. Cornea. 2002;21(7):671-9.
- Zadnik K, Barr JT, Gordon MO, Edrington TB. Biomicroscopic signs and disease severity in keratoconus. Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study Group. Cornea. 1996;15(2):139-46.
- Davis LJ, Schechtman KB, Wilson BS, Rosenstiel CE, Riley CH, Libassi DP, et al. Longitudinal changes in visual acuity in keratoconus. Invest Ophthalmol Vis Sci. 2006;47(2):489-500.
- Wagner H, Barr JT, Zadnik K. Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study: methods and findings to date. Cont Lens Anterior Eye. 2007;30(4):223-32.
- Rathi VM, Mandathara PS, Dumpati S. Contact lens in keratoconus. Indian J Ophthalmol. 2013;61(8):410-5.
- Barr JT, Zadnik K, Wilson BS, Edrington TB, Everett DF, Fink BA, et al. Factors associated with corneal scarring in the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study. Cornea. 2000;19(4):501-7.
- Krachmer JH, Feder RS, Belin MW. Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol. 1984;28(4):293-322.
- Kim J, Whang WJ, Kim HS. Analysis of total corneal astigmatism with a rotating Scheimpflug camera in keratoconus. BMC Ophthalmol. 2020;20(1):475.
- Jinabhai A, Radhakrishnan H, O’Connell C. Higher-Order Aberrations in Keratoconus: A Review. Optometry in Practice. 2009;10:141-60.
- Jinabhai A, O’Donnell C, Radhakrishnan H, Nourrit V. Forward light scatter and contrast sensitivity in keratoconic patients. Cont Lens Anterior Eye. 2012;35(1):22-7.
- Wilson SE, Lin DT, Klyce SD. Corneal topography of keratoconus. Cornea. 1991;10(1):2-8.
- Auffarth GU, Wang L, Völcker HE. Keratoconus evaluation using the Orbscan Topography System. J Cataract Refract Surg. 2000;26(2):222-8.
- Weed KH, McGhee CN, MacEwen CJ. Atypical unilateral superior keratoconus in young males. Cont Lens Anterior Eye. 2005;28(4):177-9.
- Ertan A, Kamburoglu G, Colin J. Location of steepest corneal area of cone in keratoconus stratified by age using Pentacam. J Refract Surg. 2009;25(11):1012-6.
- Sedaghat MR, Momeni-Moghaddam H, Azimi Khorasani A, Belin MW, Monfared N, Wolffsohn JS, et al. Comparison of Keratoconus Cone Location of Different Topo/tomographical Parameters. Curr Eye Res. 2021;46(11):1666-72.
- Demirbas NH, Pflugfelder SC. Topographic pattern and apex location of keratoconus on elevation topography maps. Cornea. 1998;17(5):476-84.
- Moshirfar M, Tukan AN, Bundogji N, Liu HY, McCabe SE, Ronquillo YC, et al. Ectasia After Corneal Refractive Surgery: A Systematic Review. Ophthalmol Ther. 2021;10(4):753-76.
- Kennedy RH, Bourne WM, Dyer JA. A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthalmol. 1986;101(3):267-73.
- Gomes JA, Tan D, Rapuano CJ, Belin MW, Ambrósio R, Jr, Guell JL, et al. Global consensus on keratoconus and ectatic diseases. Cornea. 2015;34(4):359-69.
- Amsler M. [Classic keratoconus and crude keratoconus; Unitary arguments]. Ophthalmologica. 1946;111(2-3):96-101.
- Krumeich JH, Daniel J, Knülle A. Live-epikeratophakia for keratoconus. J Cataract Refract Surg. 1998;24(4):456-63.
- Ortiz-Toquero S, Fernandez I, Martin R. Classification of Keratoconus Based on Anterior Corneal High-order Aberrations: A Cross-validation Study. Optom Vis Sci. 2020;97(3):169-77.
- Gobbe M, Guillon M. Corneal wavefront aberration measurements to detect keratoconus patients. Cont Lens Anterior Eye. 2005;28(2):57-66.
- Alió JL, Shabayek MH. Corneal higher order aberrations: a method to grade keratoconus. J Refract Surg. 2006;22(6):539-45.
- McMahon TT, Szczotka-Flynn L, Barr JT, Anderson RJ, Slaughter ME, Lass JH, et al. A new method for grading the severity of keratoconus: the Keratoconus Severity Score (KSS). Cornea. 2006;25(7):794-800.
- Klyce SD. Computer-assisted corneal topography. High-resolution graphic presentation and analysis of keratoscopy. Invest Ophthalmol Vis Sci. 1984;25(12):1426-35.
- Barbero S, Marcos S, Merayo-Lloves J, Moreno-Barriuso E. Validation of the estimation of corneal aberrations from videokeratography in keratoconus. J Refract Surg. 2002;18(3):263-70.
- Applegate RA, Hilmantel G, Howland HC, Tu EY, Starck T, Zayac EJ. Corneal first surface optical aberrations and visual performance. J Refract Surg. 2000;16(5):507-14.
- Mandell RB. Everett Kinsey Lecture. The enigma of the corneal contour. CLAO J. 1992;18(4):267-73.
- Mandell RB, Chiang CS, Klein SA. Location of the major corneal reference points. Optom Vis Sci. 1995;72(11):776-84.
- de Sanctis U, Loiacono C, Richiardi L, Turco D, Mutani B, Grignolo FM. Sensitivity and specificity of posterior corneal elevation measured by Pentacam in discriminating keratoconus/subclinical keratoconus. Ophthalmology. 2008;115(9):1534-9.
- Miháltz K, Kovács I, Takács A, Nagy ZZ. Evaluation of keratometric, pachymetric, and elevation parameters of keratoconic corneas with pentacam. Cornea. 2009;28(9):976-80.
- Muftuoglu O, Ayar O, Ozulken K, Ozyol E, Akıncı A. Posterior corneal elevation and back difference corneal elevation in diagnosing forme fruste keratoconus in the fellow eyes of unilateral keratoconus patients. J Cataract Refract Surg. 2013;39(9):1348-57.
- Galvis V, Tello A, Ortiz AI, Escaf LC. Patient selection for corneal collagen cross-linking: an updated review. Clin Ophthalmol. 2017;11:657-68.
- Belin MW, Kundu G, Shetty N, Gupta K, Mullick R, Thakur P. ABCD: A new classification for keratoconus. Indian J Ophthalmol. 2020;68(12):2831-4.
- Kobashi H, Rong SS. Corneal Collagen Cross-Linking for Keratoconus: Systematic Review. Biomed Res Int. 2017;2017:8145651.
- Niazi S, Jiménez-García M, Findl O, Gatzioufas Z, Doroodgar F, Shahriari MH, et al. Keratoconus Diagnosis: From Fundamentals to Artificial Intelligence: A Systematic Narrative Review. Diagnostics (Basel). 2023;13(16).
- Xu Z, Feng R, Jin X, Hu H, Ni S, Xu W, et al. Evaluation of artificial intelligence models for the detection of asymmetric keratoconus eyes using Scheimpflug tomography. Clin Exp Ophthalmol. 2022;50(7):714-23.
