Domains and learning outcomes (C110276)
• One distance learning CPD point for optometrists and dispensing opticians.
• Clinical practice
Upon completion of this CPD, ECPs will be able to recognise the different visual symptoms patients suffering from post-viral conditions (such as ME/CFS or post-Covid syndrome) may report, based on current research (s.5)
Upon completion of this CPD, ECPs will be able to describe ocular signs found in patients suffering from post-viral conditions (such as
ME/CFS or post-Covid syndrome), based on current research (s.5)
Despite the ongoing debate surrounding numerous case definitions for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and diagnosing against those differing definitions, estimates show there are approximately around 17 to 24 million sufferers of ME/CFS worldwide1 and there are over 250,000 people in the UK affected by it.2
ME/CFS is a heterogenous and consequently a multifaceted chronic disease (which lasts a duration of > six months). With no single definitive diagnostic test, ME/CFS is often misdiagnosed3 and while the condition itself can drastically affect the lives and livelihoods of sufferers causing cessation of employment in 50% of cases,4 the misdiagnosis and delays in diagnosis prolong any suffering.
The more commonly reported life changing sequelae of symptoms reported include unexplained fatigue, post-exertional malaise (PEM), unrefreshed sleep, cognitive dysfunction such as foggy thoughts and a whole range of symptoms affecting the cardiovascular, immune and central nervous system.5, 6
Many visual markers have also been reported in those with ME/CFS.7, 8 ME/CFS is thought to be autoimmune in origin9 and the result of a consequence of a host of viral infections; namely human cytomegalovirus (HCMV), human herpesvirus7 (HHV-7), Epstein Barr virus (EBV), human herpesvirus – six types A and B).10, 11
ME/CFS will be referred to as a post-viral condition in this article.
Pre-pandemic, there were fewer studies on visual symptoms in post-viral diseases, however Covid-19/ Post-Covid-19 syndrome (PCS) have given rise to a resurgence in these such studies. Both Covid-19 and ME/CFS have impacted populations worldwide and across all ethnicities, sexes and ages. More than 775 million cases of Covid have been confirmed.12 The World Health Organization (WHO) defines post-Covid-19 condition (or long Covid) as ‘the continuation or development of new symptoms three months after the initial SARS-CoV-2 infection, with these symptoms lasting for at least two months with no other explanation’.
The WHO acknowledge there are over 200 symptoms, which also include symptoms like fatigue and cognitive dysfunction. The NHS has cited extreme tiredness (fatigue), feeling short of breath, muscle aches, difficulty concentrating and joint pain, which are also common symptoms found in ME/CFS.13 The approved classifications of both post-viral conditions bear a striking similarity and perhaps ME/CFS and other post-viral conditions could benefit from the increasing data born from the pandemic, which in turn could lead to definitive diagnostic tests and fewer cases of misdiagnosis/non-diagnosis in ME/CFS and the like.
Visual Symptom Reports in ME/CFS
ME/CFS studies report of a loss of vision, visual fatigue, vision disturbances, pain in the eyes14 and blurred vision.15, 16 Further, commonly reported visual symptoms include photosensitivity, difficulty focusing on images, blurring of images, halos around images, poor depth perception, pain in the eyes, impaired visual attention, increased susceptibility to pattern glare and vision-related headaches.7, 17-20
There is also evidence for a significantly higher distribution of exophoria, lower functional vergence (near and far), a further point of convergence, and lower tear secretion and break-up time in ME patients, compared to healthy controls.21 Reduced accommodation,20, 22 impaired anti-saccadic and smooth pursuit eye movements deficits in visual attention (determined using visual cueing), visual search and selective visual attention tasks,23 increased susceptibility to pattern-glare/related visual stress8 and reduced contrast sensitivity.24
Visual symptom reports in Covid-19 and PCS
Reported presenting visual symptoms in Covid-19 are similar to that of ME/CFS (see table 1) but now that we are nearly over five years on from the start of the pandemic, long term and stable findings will be more relevant to the optometrist in practice.
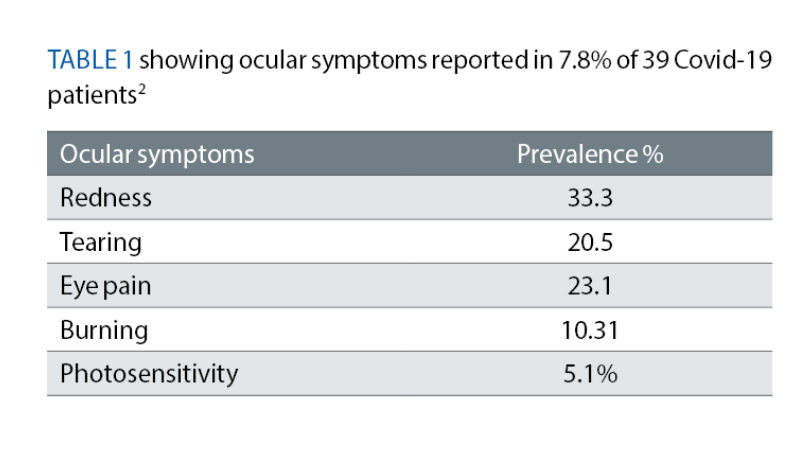
In one PCS study published in 2024, among a few of the visual symptoms recorded was an increased difficulty with near work and difficulty moving the eyes.25 Another study looked at 56 articles on PCS patients who suffered with Covid-19 between 2019 and 2022 and found that relative afferent pupillary defect, colour vision, papilloedema, arcuate, central, altitudinal field defects, larger blind spot and overall decreased visual field sensitivity26 were the most common findings.
Visual field (VF) anomalies were also documented along with neuritis (ON), cranial nerve palsies 27, 28 in two other studies.
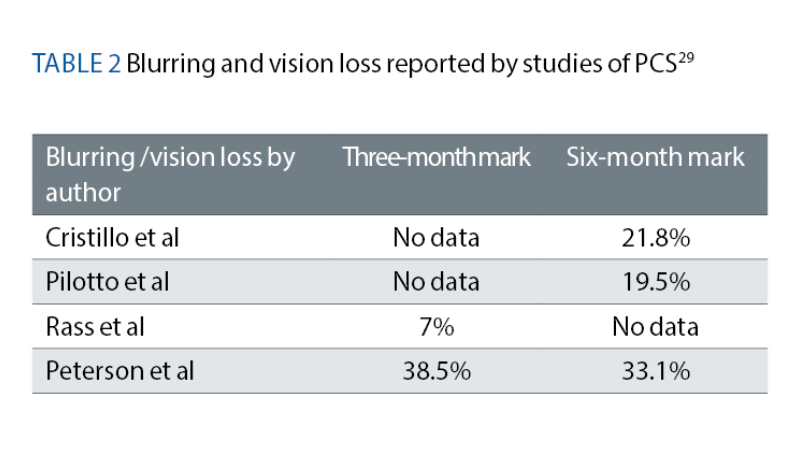
A meta-analysis was conducted by Trott et al, which collated symptoms of blurring/vision loss from other studies reported by PCS patients at both the three-month and six-month mark29 (see table 2).
1: Colour Vision
Difficulties in colour discrimination have been reported frequently in Covid-19 patients and PCS.26, 30, 31 Colour vision symptoms are also often reported in ME/CFS.7
When 63 Covid-19 patients were tested with an online version of the FM 100 hue test (FM-H100), provided by X-Rite, difficulties in blue-yellow colour discrimination were found to be more prevalent than compared to the control group (P< 0.001). This particular study believed the cause to be due to poor perfusion in the deep capillary plexuses and superficial plexus in Covid-19 patients.32
In a separate study where a cohort of 77 PCS patients were tested for colour vision defects, mild blue and/or blue-yellow defects were found again using the FM-H10033 and this group postulated that there was poor retinal perfusion.
Optometrists will be aware of Köllner’s rule which suggests that blue-yellow (B-Y) acquired colour vision (or s-cone mediated) deficiencies have a retinal origin and red-green (R-G), or M-L-cone, deficiencies occur due to optical nerve damage. Many optic neuropathies have been reported in PCS.28
Though studies mentioned thus far suggest an acquired B-Y deficiency, reports of optic neuritis reports are vast in Covid-19 sufferers, 28, 35 R-G should therefore not be discounted in the test room as optic neuritis has been reported in 12.7% around 33 days from diagnosis34 and therefore, it could be worthwhile for the optometrist to investigate both R-G and B-Y colour deficiencies.
Furthermore, the relationship between demyelination of the peripheral and CNS and post viral infection has been explored pre-pandemic.38 Optometrists should therefore consider any history of a unilateral (rarely bilateral) deterioration in vision and pain on moving the eyes as it is often the hallmark of MS but also a characteristic found in post-Covid patients.
2: Accommodative Insufficiency and poor convergence
Caffery et al found that visual problems in a cohort of 25 ME/CFS patients were often reported and while tear film abnormalities were most common (76%) the next most frequently reported symptom was poor accommodation, which was found in 18 of those patients, ie 72%.22 Godts et al found that fusional amplitudes were significantly smaller (P < 0.001), convergence was reduced (P < 0.001) and accommodative was also reduced (P < 0.001) in a cohort of 41 participants with ME/CFS compared to the control group.37
Accommodative insufficiency is characterised by blurred vision at near that is measured at lower levels than the patients’ age. There are many early reports of vision problems and specifically near vision soon after diagnosis of Covid-19.38, 25
Johansson et al tested 38 PCS sufferers against a battery of binocular function and accommodative tests following questionnaires that found symptoms of ‘re-reading the same line’ in 80% of participants. Fusional reserves were tested using the prism bar at 40cm, NPC tested using the RAF rule, near point of accommodation using the RAF, prism cover test and vergence reserves using 3 Base In and 12 Base Out prisms.25
Johansson et al found around 5.2% of the cohort of 38 PCS sufferers were found to have a reduced amplitude of accommodation, 65.8 % a reduced convergence facility, 23.7 % reduced NPC, 23.7% and reduced vergence at 40cm.25
3: Stereopsis
Other studies have observed headaches as one of the most reported symptoms after an extended period of six months in PCS.39-41 One potential factor behind headaches may relate to reduced stereopsis.
We have numerous handheld stereoscopic tests available to us in the test room, however virtual reality (VR) assessment can be a more modern way of checking for stereopsis. The Random Forest classifier takes about a minute to complete and is about 71% accurate.42
One study group that used the Random Forest VR found there was worse stereopsis in 20 participants with PCS compared to controls. The self-critical limitation of this study, however, is that participants were asked to remove spectacles and so this could have influenced results.42 Other limitations included the proximity between the head display and the eyes.42
Another group investigated the potential relationship between PCS and stereopsis and found that stereopsis was affected more in the severe PCS (P=0.008) compared to the mild PCS (P= 0.029) control group.43
4: Pupil Reactions
Bansal noted that in patients with ME/CFS, prolonged illumination of the pupils revealed uncharacteristic responses. A rhythmic dilation and contraction was seen in 75% of ME/CFS participants. Bansal also noted a pupil anomaly normally seen in patients with autoimmune autonomic neuropathy that is caused by IgG antibodies to the ganglionic acetylcholine receptor but is absent in ME/CFS sufferers, ie after light is shone into the eye, there was an unusual dilation of the pupils.6
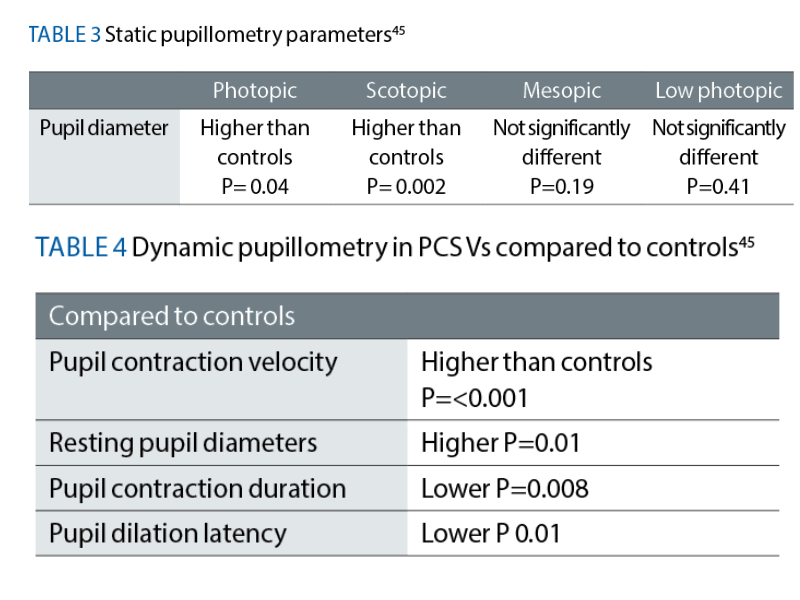
In those with PCS, Peterson et al summarises that Gonzalez-Hermosillo et al reported light sensitivity in 18.5% and 20% of participants after three and six months respectively.44 In a substantial pupil study, Kaharan et al found pupil diameters in recovering Covid patients to be significantly larger in both scotopic and mesopic conditions compared to healthy controls45 (see table 3).
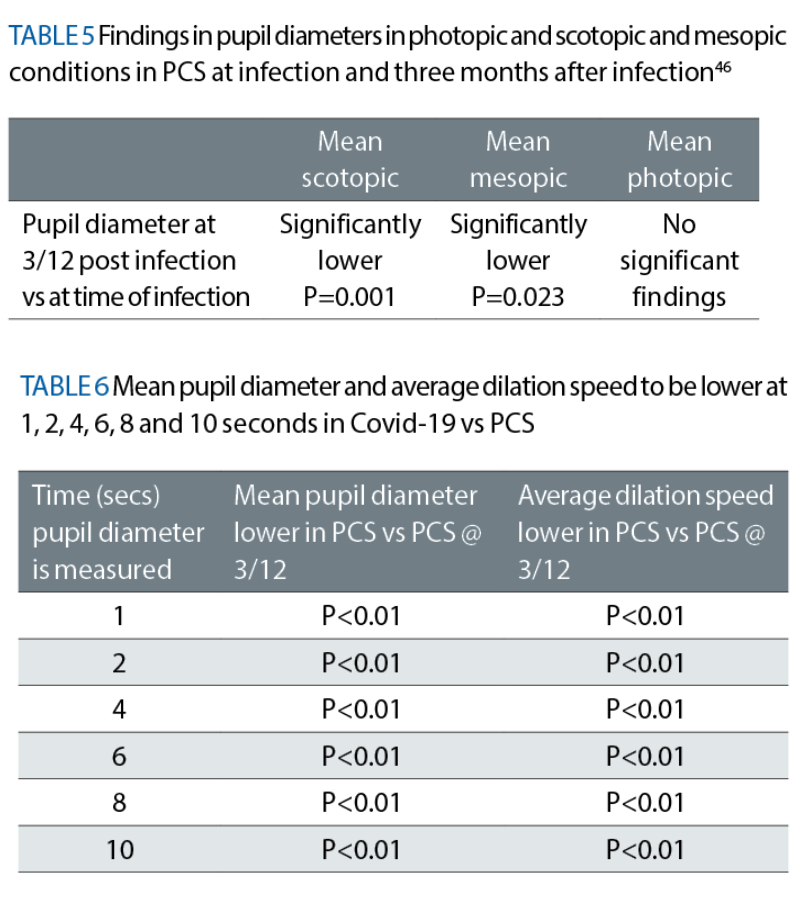
A separate study looked at how pupil diameters changed in infected versus PCS at three months. See table 5.
The same group of researchers found significant findings where the average dilation speed was lower and the mean pupil diameter smaller (for every second light was presented) in the Covid-19 infected group compared to three months post infection (P <0.01). See table 6.46
Bitegen et al concluded that dynamic pupillometry reveals significant alterations in contractile pupillary light responses in 35 PCS subjects compared to their controls, which is indicative of parasympathetic dysfunction after Covid-19.47 The median time after the diagnosis of acute Covid-19 was 4.0 (2.0-5.0) months.
There was an increase in the latency of pupil contraction (P = 0.001) and a reduction in the duration of pupil contraction (P = 0.039) in post-Covid-19 subjects compared to healthy controls. No significant differences were observed in the initial pupil diameter, amplitude and velocity of pupil contraction or latency, velocity and duration of pupil dilation.47
Gunduz et al conducted dynamic and static pupillometry measurements using quantitative infrared pupillography. They had 42 participants diagnosed with Covid-19 and 44 health controls. Results of this experiment found that pupil diameters measured in photopic, mesopic and scotopic conditions were significantly larger in Covid-19 patients in the first month compared to controls.48
Gunduz et al found there was no significant finding in pupil diameters at six months post diagnosis in static pupillometry. They also found that dynamic pupil diameters were larger in photopic, scotopic and mesopic conditions at the first month but not significantly so. They also found that pupil diameters were similar between the photopic, mesopic and scotopic groups at six months compared to the controls.
In another study, Ozturk et al, dynamic and static pupillometry measurements were performed with the Sirius (CSO, Italy) corneal topography device on 32 PCS participants and 32 controls. This team found larger pupil diameters in both static and dynamic pupillary measures however did they not find any difference in the average speed of pupillary dilatation.49
OCT, OCTA and ED-OCT
An exciting branch of research is that of OCT, optical coherence tomography angiography (OCTA) and enhanced-depth imaging optical coherence tomography (ED-OCT) and retinal findings in PCS and ME/CFS using those methods. One such study has already investigated the relationship between ME/CFS (self-reported) and the retinal microcirculation in PCS.50
Differences were found in vessel density in the intermediate capillary plexus between the PCS and control group. The same team also found significant differences in vessel density in the superior vascular plexus between those with and without ME/CFS.
Fifty-one PCS patients were tested against 37 controls by Naderi et al. They underwent macula OCT and peripapillary OCT and OCTA. Naderi et al found that those with PCS had thicker RNFL in the peripapillary area compared to healthy controls. The superficial and deeper macular vessel densities parafoveally and superficial and deeper macular vessel densities perifoveally were higher compared to the healthy eyes. Interestingly, they also found the inner layers to be thicker in those with PCS.51
Karkhur et al conducted SD- OCT, OCTA and EDI- OCT in a total of 180 PCS (three months after recovery) and control.52
The results were as follows:
- Decreased mean FAZ area in superficial capillary plexus (P= 0.03)
- Decreased mean FAZ area in deep capillary plexus (P<0.01)
- Reduced average inner plexiform layer – ganglion cell layer thickness (P=0.04)
- Increased subfoveal choroidal thickness (P<0.001)
Conclusion
The tests above highlight the shared visual symptoms between ME/CFS and PCS and, in many cases, this is no surprise considering ME/CFS is considered by many to be pathogenically post viral. While those with ME/CFS and PCS may lack the confidence, knowledge or language to explain their condition, the role of an understanding optometrist could vastly affect the outcome of a visit particularly if those patients are not yet diagnosed.
For example, as more research emerges perhaps there may be a move towards the earlier prescribing of a low near additions. Thorough binocular vision assessments may help identify and subsequently address ocular motor balance problems.
Pupil reactions might not be typical in which case RAPD will need to be ruled out and atypical reactions noted and potentially referred. Time permitting, some OCTs may have an automated pupil programme and, of course, the thicknesses of the retinal layers could be assessed here.
Finally, optometrists should not underestimate the help we can offer ME/CFS and PCS sufferers. ME/CFS sufferers have traditionally suffered from stigma from health professionals, however, even without diagnosis there are many ways in which we may be able to assist.
It may be a few years before a single diagnostic test is created but the current research is looking hopeful.
Nadia Ahmed is an experienced optometrist and has worked across primary and secondary care optometry. She completed a master’s in research; her experimental work and dissertation focused on the impact ME/CFS has upon the eye.
References
- 1Lim, EJ, Son, CG. Review of case definitions for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J Transl Med 18, 289 (2020). https://doi.org/10.1186/s12967-020-02455-0.
- 2Nacul, LC, Lacerda, EM, Pheby, D et al. Prevalence of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) in three regions of England: a repeated cross-sectional study in primary care. BMC Med 9, 91 (2011).
- 3Jason, LA, Ravichandran, S, Katz, BZ, Natelson, BH, & Bonilla, HF. (2022). Establishing a consensus on ME/CFS exclusionary illnesses. Fatigue: Biomedicine, Health & Behavior, 11(1), 1–13.
- 4Collin SM, Crawley E, May MT, Sterne JA, Hollingworth W; UK CFS/ME National Outcomes Database. The impact of CFS/ME on employment and productivity in the UK: a cross-sectional study based on the CFS/ME national outcomes database. Collaborators: O’Dowd H, Butt K, Dunn D, Pemberton S, White P, Murphy M, Mullick Y, Bansal A. BMC Health Serv Res. 2011; 11:217. doi:10.1186/1472-6963-11-217.
- 5Michael Maes, Frank N.M. Twisk, Cort Johnson, Myalgic Encephalomyelitis (ME), Chronic Fatigue Syndrome (CFS), and Chronic Fatigue (CF) are distinguished accurately: Results of supervised learning techniques applied on clinical and inflammatory data, Psychiatry Research,Volume 200, Issues 2–3,2012,Pages 754-760,ISSN 0165-1781.
- 6Bansal, AS. Investigating unexplained fatigue in general practice with a particular focus on CFS/ME. BMC Fam Pract 17, 81 (2016).
- 7Hutchinson CV, Maltby J, Badham SP, et al Vision-related symptoms as a clinical feature of chronic fatigue syndrome/myalgic encephalomyelitis? Evidence from the DePaul Symptom Questionnaire British Journal of Ophthalmology 2014;98:144-145.
- 8Wilson, Paterson McGowan, Front.Visual Aspects of Reading Performance in Myalgic Encephalomyelitis (ME)Psychol., 17 August 2018Sec. Cognition. Volume 9 - 2018 |
- 9Morris, G, Berk, M, Galecki, P et al. The Emerging Role of Autoimmunity in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/cfs). Mol Neurobiol 49, 741–756 (2014).
- 10Franziska Sotzny, Julià Blanco, Enrica Capelli, Jesús Castro-Marrero, Sophie Steiner, Modra Murovska, Carmen Scheibenbogen,Myalgic Encephalomyelitis/Chronic Fatigue Syndrome – Evidence for an autoimmune disease, Autoimmunity Reviews,Volume 17, Issue 6,2018,Pages 601-609,ISSN 1568-9972.
- 11Rasa, S, Nora-Krukle, Z, Henning, N et al. Chronic viral infections in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J Transl Med 16, 268 (2018). https://doi.org/10.1186/s12967-018-1644-y
- 12WHO https://data.who.int/dashboards/covid19/cases
- 13NHS https://www.nhs.uk/conditions/chronic-fatigue-syndrome-cfs/#:~:
- 14Dry eye symptoms and signs in United States Gulf War era veterans with myalgic encephalomyelitis/chronic fatigue syndrome Victor Sanchez BS, Colin K. Kim BS, Elyana VT. Locatelli BS, Adam K. Cohen, Kimberly Cabrera MS, Kristina Aenlle PhD, Nancy G. Klimas MD, Robert O’Brien PhD, Anat Galor MD, MSPH
- 15Martínez-Lapiscina, EH; Fraga-Pumar, E; Gabilondo, I; Martínez-Heras, E; Torres-Torres, R; Ortiz-Pérez, S; Llufriu, S; Tercero, A; Andorra, M; Roca, MF; et al. The multiple sclerosis visual pathway cohort: Understanding neurodegeneration in MS. BMC Res. Notes 2014, 7, 910.
- 16Chen, L; Gordon, LK. Ocular manifestations of multiple sclerosis. Curr. Opin. Ophthalmol. 2005, 16, 315–320. [Google Scholar]
- 17Potaznick, W.; Kozol, N. Ocular manifestations of Chronic Fatigue and Immune Dysfunction Syndrome. Optom. Vis. Sci. 1992, 69, 811–814.
- 18Leslie, S. Chronic Fatigue Syndrome: Optometric clinical presentation and management. J. Behav. Optom. 1997, 8, 155–161.
- 19Vedelago, LJ. Visual dysfunction in Chronic Fatigue Syndrome: Behavioural optometric assessment and management. J. Behav. Optom. 1997, 8, 149–154.
- 20Loew, S; Marsh, NV; Watson, K. Symptoms of Meares-Irlen/Visual Stress Syndrome in subjects diagnosed with Chronic Fatigue Syndrome. Int. J. Clin. Health Psychol. 2014, 14, 87–92.
- 21Mastropasqua, L; Ciancaglini, M; Carpineto, P; Iezzi, A; Racciatti, D; Falconio, G; Zuppardi, E; Pizzigallo, E. Ocular manifestations in Chronic Fatigue Syndrome. Ann. Ophthalmol. 2000, 32, 219–224.
- 22Caffery, BE; Josephson, JE; Samek, MJ. The ocular signs and symptoms of chronic fatigue syndrome. J. Am. Optom. Assoc. 1994, 65, 187–191.
- 23Badham, SP, Hutchinson, CV. Characterising eye movement dysfunction in myalgic encephalomyelitis/chronic fatigue syndrome. Graefes Arch Clin Exp Ophthalmol 251, 2769–2776 (2013).
- 24Restricted Spatial Windows of Visibility in Myalgic Encephalomyelitis (ME)by Nadia S. Ahmed, Irene Gottlob, Frank A. Proudlock and Claire V. Hutchinson Vision 2018, 2(1), 2;
- 25Johansson, J, Möller, M, Markovic, G, & Borg, K. (2023). Vision impairment is common in non-hospitalised patients with post-Covid-19 syndrome. Clinical and Experimental Optometry, 107(3), 324–331.
- 26MA Vélez Cevallos, AM Vásquez, Alterations in the optic nerve and retina in patients with Covid-19. A theoretical review, Archivos de la Sociedad Española de Oftalmología (English Edition), Volume 98, Issue 8, 2023,Pages 454-469, ISSN 2173-5794
- 27Shaikh, N, Al Mahdi, H, Pai, A, Pathare, A, Abujaber, AA, Dsliva, A, Khatib, MY. (2022). Ocular manifestations of Covid-19: facts and figures from a tertiary care center. Annals of Medicine, 54(1), 310–313.
- 28Abdul-Salam State SE, Sfredel V, Mocanu CL, Albu CV, Bălășoiu AT. Optic neuropathies post-Covid 19 - review. Rom J Ophthalmol. 2022 Oct-Dec;66(4):289-298.
- 29Trott, Driscoll, Pardhan, The prevalence of sensory changes in post-Covid syndrome: A systematic review and meta-analysisFront. Med., 25 August 202Sec. Infectious Diseases: Pathogenesis and Therapy. Volume 9 - 2022 |
- 30Gascon, P, Briantais, A, Bertrand, E, Ramtohul, P, Comet, A, Beylerian, M, Denis, D. (2020). Covid-19-Associated Retinopathy: A Case Report. Ocular Immunology and Inflammation, 28(8), 1293–1297.
- 31Nagy, ZZ. “Ophthalmic signs and complications of the Covid-19 infection.” Developments in Health Sciences 3.4 (2021): 79-82
- 32Sharma, Aditi1; Singh, Aditya1; Bansal, Yashik2; Mohan, Aditi1; Gnanaraj, Ramya3; Khulbe, Pranita1; Pangtey, Kavita1; Tripathi, Kaushiki1; Khan, Mohammad Ali4; Sharma, Akshita1; Jain, Manish1,5. Dyschromatopsia and contrast sensitivity changes in Covid-19 patients. Indian Journal of Ophthalmology 72(5):p 664-671, May 2024.
- 33Griber, Yulia Aleksandrovna, and Galina Vladimirovna Paramei. “Colour discrimination in post-Covid-19 observers assessed by the Farnsworth-Munsell 100-Hue test.” Russian Psychological Journal 21.1 (2024): 6-32.
- 34Jaafar Omer Ahmed, Shwan Abdubakr Ahmad, Marwan Nasih Hassan, Fahmi H. Kakamad, Rawezh Q. Salih, Berwn A. Abdulla, Fattah Hama Rahim Fattah, Shvan H. Mohammed, Razhan K. Ali, Abdulwahid M. Salih, Post Covid-19 neurological complications; a meta-analysis, Annals of Medicine and Surgery,Volume 76,2022,103440,ISSN 2049-0801.
- 35Mohammed A. Azab, Sharef Fawzy Hasaneen, Hassan Hanifa, Ahmed Y. Azzam,Optic neuritis post-Covid-19 infection. A case report with meta-analysis,Interdisciplinary Neurosurgery,Volume 26,2021,101320,
- 36Ismail Ibrahim Ismail, Jasem Al-Hashel, Raed Alroughani, Samar Farouk Ahmed,A case report of multiple sclerosis after Covid-19 infection: causality or coincidence?,Neuroimmunology Reports,Volume 1,2021,100008,ISSN
- 37Godts, D.; Moorkens, G.; Mathysen, D.G. Binocular Vision in Chronic Fatigue Syndrome. Am. Orthopt. J. 2016, 66, 92–97.
- Umapathi, Thirugnanam MBBS, MRCP (UK); Li, Kelvin Zhenghao MBBS, FRCOphth; Chin, Chee Fang MBChB; Vijakumar, Kalpana MBBS, MRCP (UK); Tan, Glorijoy Shi En MBBS, MRCP (UK); Ung, Peck Houy MD; Yeo, Tun Kuan MBBS, FRCOphth; Agrawal, Rupesh MD, FRCS. Acute Isolated Near Vision Difficulty in Patients With Covid-19 Infection. Journal of Neuro-Ophthalmology 41(3):p e279-e282, September 2021.
- Longitudinal evaluation of neurologic-post acute sequelae SARS-CoV-2 infection symptomsJacqueline E Shanley, Andrew F Valenciano, Garrett Timmons, Annalise E Miner, Visesha Kakarla, Torge Rempe, Jennifer H Yang, Amanda Gooding, Marc A Norman, Sarah J Banks, Michelle L Ritter, Ronald J Ellis, Lucy Horton, Jennifer S Graves. 15 June 2022
- Samantha A. Cintron, Stuart Hitchcock, Qiuhua Shen, Lalon Kasuske, Frances M. Yang, Janet Pierce,Symptom science and post-Covid-19 conditions,Journal of Medicine, Surgery, and Public Health,Volume 2,2024,100092,
- César Fernández-de-las-Peñas, Marcos Navarro-Santana, Víctor Gómez-Mayordomo, María L. Cuadrado, David García-Azorín, Lars Arendt-Nielsen, Gustavo Plaza-Manzano as an acute and post-Covid-19 symptom in Covid-19 survivors: A meta-analysis of the current literature: 30 July 2021
- Mehringer W, Stoeve M, Krauss D, Ring M, Steussloff F, Güttes M, Zott J, Hohberger B, Michelson G, Eskofier B. Virtual reality for assessing stereopsis performance and eye characteristics in Post-Covid. Sci Rep. 2023 Aug 13;13(1):13167.
- Valldeflors Vinuela-Navarro, Joan Goset, Mikel Aldaba, Clara Mestre, Cristina Rovira-Gay, Neus Cano, Mar Ariza, Bàrbara Delàs, Maite Garolera, and Meritxell Vilaseca, “Eye movements in patients with post-Covid condition,” Biomed. Opt. Express 14, 3936-3949 (2023)
- Peterson J, Welch V, Losos M, Tugwell PJOOHRI. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. (Vol. 2). Ottawa, ON: Ottawa Hospital Research Institute (2011). p. 1–12.
- Karahan, M, Demirtaş, AA, Hazar, L. et al. Autonomic dysfunction detection by an automatic pupillometer as a non-invasive test in patients recovered from Covid-19. Graefes Arch Clin Exp Ophthalmol 259, 2821–2826 (2021).
- Yurttaser Ocak, S, Ozturan, SG & Bas, E. Pupil responses in patients with Covid-19. Int Ophthalmol 42, 385–391 (2022).
- Bitirgen, G, Korkmaz, C, Zamani, A. et al. Abnormal quantitative pupillary light responses following Covid-19. Int Ophthalmol 42, 2847–2854 (2022).
- Ucan Gunduz G, Mavi Yildiz A, Yalcinbayir O, Baykara M, Sogutlu Sari E, Isleker S, et al. Pupillographic Analysis of Covid-19 Patients: Early and Late Results After Recovery. Beyoglu Eye J 2023; 8(3): 149-156.
- Öztürk, Y., Yıldız, MB, & Bolaç, R. (2021). Evaluation of Pupillometric Parameters in Patients with Covid-19. Ocular Immunology and Inflammation, 31(1), 39–43.
- Schlick S, Lucio M, Wallukat G, Bartsch A, Skornia A, Hoffmanns J, Szewczykowski C, Schröder T, Raith F, Rogge L, Heltmann F, Moritz M, Beitlich L, Schottenhamml J, Herrmann M, Harrer T, Ganslmayer M, Kruse FE, Lämmer R, Mardin C, Hohberger B. Post-Covid-19 Syndrome: Retinal Microcirculation as a Potential Marker for Chronic Fatigue. Int J Mol Sci. 2022 Nov 8;23(22):13683.
- Naderi Beni A, Dehghani A, Kianersi F, Ghanbari H, Habibidastenae Z, Memarzadeh SE, Naderi Beni Z. Retinal findings of Covid-19 patients using ocular coherence tomography angiography two to three months after infection: Ocular appearance recovered Covid-19 patient. Photodiagnosis Photodyn Ther. 2022 Jun;38:102726.
- Karkhur S, Chauhan K, Soni D, Sharma B, Yadav N, Banerjee L, Nyodu R, Verma S. Optical coherence tomography-based assessment of macular vessel density, retinal layer metrics and sub-foveal choroidal thickness in Covid-19 recovered patients. Indian J Ophthalmol. 2023 Feb;71(2):385-395.
