Domains and learning outcomes (C110415)
• One distance learning CPD point for optometrists and dispensing opticians.
• Clinical practice
Upon completion of this CPD, ECPs will be able to list the different factors that affect visual acuity measurement in presbyopia, including aspects such as lighting (s7).
Upon completion of this CPD, ECPs will be able to identify earlyindicators of presbyopia, eg adaptations patients may make when reading (s7).
Upon completion of this CPD, ECPs will be able to describe recent global research into patient awareness of presbyopia (s5).
In clinical practice, measuring the range of clear focus in presbyopic patients is of foremost importance. This measurement is relevant for understanding the necessary optical correction to optimise patients’ near vision. The purpose of this article is to reinforce the evidence by investigating several approaches to the detection, monitoring, assessment and diagnosis of presbyopia progression.
The correct diagnosis of presbyopia and assessment of visual function in those with presbyopia involves the application of both subjective and objective procedures. Objective techniques, such as autorefraction, corneal topography and lens imaging, are more reliable than subjective techniques, like patient-reported outcome questionnaires and defocus curves.
Subjective Assessments
Almost all individuals over 40-45 years old become presbyopic, with symptoms often noticed before seeking professional care. People generally adapt by using larger fonts, more lighting and longer working distances before resorting to corrective measures.1
Patient-reported outcome measures (PROMs) are a good way to evaluate how presbyopia affects an individual’s quality of life and to systematically quantify symptomatology, shifting the focus to patient-driven care.2 PROMS also help evaluate treatment effectiveness and compare interventions, common examples include:
- Near Activity Visual Questionnaire (NAVQ)
- Presbyopia Impact and Coping Questionnaire (PICQ)
- Near Vision Presbyopia Task-Based Questionnaire (NVPTQ)
- National Eye Institute Refractive Error Quality of Life Instrument (NEI-RQL)
- The Refractive Status and Vision Profile (RSVP)
- Quality of Life Impact of Refractive Correction (QIRC)
- Contact Lens Impact on Quality of Life (CLIQ)
- Quality of Vision (QoV)
- Near Vision-Related Quality of Life (NVQL)
- Freedom from Glasses Value Scale (FGVS)
- Patient-Reported Spectacle Independence Questionnaire (PRSIQ)
Psychophysical Techniques
Acuity assessments
Static acuity where both the target and observer are stationary is the most common method used in practice. The preference is to measure LogMAR at various distances – far, intermediate and near for those with presbyopia or associated risk factors.
Defocus curves, which evaluate visual acuity across different levels of dioptric defocus, offer a detailed understanding of visual performance at multiple distances and are particularly valuable for comparing presbyopia correction methods.
Typically, measurements are made in increments of 0.50 D, covering a defocus range from +2.00 D to -5.00 D.3-5 For finer detail in visual acuity for distant viewing vergences, some experts advise using 0.25 D intervals within +0.50 D and -0.50 D.6
Although the process is time-consuming, defocus curves provide more detailed information than single-point measurements, and analysis methods like the area under the curve often correlate more strongly with functional outcomes than direct step-by-step comparisons.3,7
Dynamic visual acuity is the ability to perceive details of moving objects, whether the object, observer or both are in motion. It evaluates the eye capacity for proactive information retrieval, simulating tasks like driving and flying in the actual environment. The observer’s acuity decreases as the target speed increases. This ability decreases with age and it is also affected by contrast, target size and colour.8
Measurement parameters
Modifications to these parameters may have a major effect on visual acuity.
Target: Optotype presentation affects visual acuity; isolated single-line examinations frequently produce higher scores because of less crowding,10 which may lead to overestimating acuity. Different target types, like letters or symbols, are chosen based on recognition ability. To avoid memorisation, randomisation or varied charts are recommended.
Light levels significantly impact visual acuity, with low illumination worsening acuity, especially in the presence of refractive errors.11, 12
Time of the day may impact visual acuity, decreasing later during the day because of physiological and environmental factors. An illuminance meter is suggested to ensure consistency in lighting during the day.
Reading performance is influenced by the level of presbyopia, font size, type of text, duration of reading and word and line spacing.13-17
Contrast sensitivity impacts visual quality and decreases with age, especially in low-light environments. It is crucial to evaluate how well pseudo-accommodative optical devices work to correct presbyopia, as they reduce contract sensitivity by increasing spherical aberrations, which can jeopardize the optical system modulation transfer function.18
It is typically measured with a Pelli-Robson chart. Mobile apps provide faster testing, but it is uncertain if they are reliable for elderly patients. Computerised techniques offer higher accuracy.
Amplitude of accommodation (AoA) is important to measure to determine whether a near addition is required. Methods to determine the AoA include push-up or push-down method, minus lens method, retinoscopy and calculating the near addition from AoA (Dioptric working distance – ½ AoA).
Eye dominance is crucial in presbyopia correction, especially with multifocal lenses and monovision. Typically, the dominant eye is corrected for distance, but there is debate over whether sensory or sighting dominance is more relevant for success.
Despite common practices, no clear scientific consensus exists, and the subjective nature of dominance assessment adds complexity.
Objective Techniques
Keratometry and corneal topography equipment can be utilised in practice to accurately measure corneal optical power in normal eyes19 but may lack accuracy following eye surgery, due to corneal curvature alterations.20
Corneal topography is essential because it precisely determines the power of the intraocular lens (IOL) to be used following cataract surgery. It is also important in cases where the patient has previously undergone kerato-refractive surgery.21 Corneal power, anterior chamber depth and axial length are the three most important measurements for determining the correct IOL to implant.
Reflection techniques include keratometry and Placido disc corneal topographers.
Optical section techniques include tomography using slit-scanning technology, such as Scheimpflug imaging and optical coherence tomography (OCT).
Slit scanning devices, like the Orbscan 3, combine slit-scanning technology with a Placido disc system to obtain topographic measurements of both anterior and posterior corneal surfaces. An added feature is full corneal pachymetry.22-24
Scheimpflug imaging devices are based on the principle of rotating a lens about its horizontal or vertical axis to adjust the plane of focus.
Anterior segment OCT is a non-contact optical imaging technique capable of high-resolution cross-sectional imaging of biological tissue using infrared light.25 There are three types of OCTs available: time-domain OCT, spectral-domain OCT, swept-source OCT autorefraction/aberrometry.
Autorefraction is an objective measurement of the power of the eye. If possible, an open field autorefractor is preferable when trying to assess the accommodative element.26 Aberrometry is an advanced form of autorefraction that examines light refraction from many sites on the eye. The Hartmann-Shack is the most common one, which uses a lenslet array to evaluate visual disturbances of the wavefront existing in the eye (figure 1).
Figure 1: Hartmann-Shack aberrometry principle, which uses a lenslet array to evaluate the visual disturbances of the wavefront exiting the eye from a laser point source input. The deviation of the light spot from each lenslet from that of a perfect wavefront is measured with a charge-coupled device (CCD) digital camera

Depth of Field
Depth of field (DoF) is the perceptual tolerance of the human eye to retinal defocus. DoF can improve presbyopia symptoms as it can partially compensate for accommodation (pseudo-accommodation). An objective definition of DoF is ‘the focusing range for which no accommodation is detected’27(figure 2).
It is important to measure the DoF when conducting the presbyopia examination as it can influence the needs of the presbyope and thus influence the correction required.28 DoF can be measured both subjectively (eg by detecting target blur) and objectively (eg by analysing retinal image quality). Factors influencing DoF include: pupil size, age, luminance and neural adaptation. Measuring DoF is vital for optimising presbyopia correction strategies, especially with multifocal lenses and surgical interventions.
Figure 2: Depth of field (DoF)
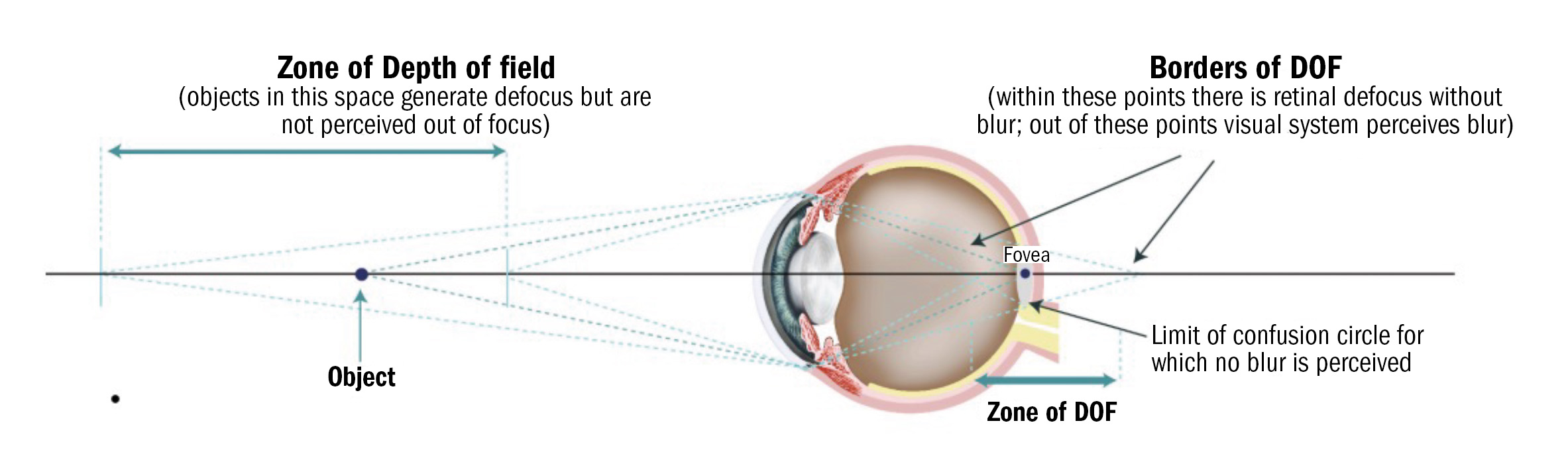
Pupil size
In presbyopes, pupil size, position and dynamics can assist in deciding which correction strategy to use, especially multifocal optical designs for contact lenses, IOLs, corneal inlays and corneal refractive surgery. Pupil size controls the amount of light entering the eye.
The DoF decreases by approximately 0.12 D per millimetre increase in pupil diameter over a range of 2.5mm to 8.0mm.29 Pupil size is influenced by the illumination level and decreases with age. Measuring pupil size can be challenging, but recently developed infrared pupillometry instruments offer reliable results.
Proper alignment of optical devices with the pupil is vital, especially in multifocal contact lenses and IOLs, as misalignment can adversely impact visual quality.
Neural factors
Neuroadaptation may help counteract visual issues like contrast sensitivity loss caused by spherical aberrations in multifocal lenses, IOLs and corneal inlays.30 This adaptation, possibly a blur-adaptive phenomenon, can influence image focus perception and alleviate presbyopia symptoms by interacting with correction strategies like increased DoF.
Studies using neuroimaging and electrophysiology suggest that various brain regions, from the retina to the visual cortex, are involved,31-34 but a complete understanding of these mechanisms is still lacking.
Lens shape and position
The shape and positioning of the crystalline lens are crucial for determining lens power during accommodation, though this assessment involves assumptions about the lens refractive index, which varies with age.35 Various imaging methods, including Scheimpflug imaging, ultrasound biomicroscopy, OCT and magnetic resonance imaging (MRI), are employed to evaluate lens shape and position.
Each technique has distinct benefits in terms of precision and application. Scheimpflug imaging generates 3D images of the anterior eye, though distortions can occur due to the tilt of the camera and refractive surfaces. Ultrasound biomicroscopy uses high-frequency sound waves to provide detailed images of the lens and surrounding structures, even in the presence of opaque tissues. OCT delivers high-resolution images by analysing light reflections, frequently used to examine the crystalline lens during accommodation and to pinpoint the location of implanted IOLs.
MRI leverages nuclear magnetic resonance to create detailed 3D images of the eye, which is particularly valuable for observing changes in the ciliary muscle during accommodation. Although each technique has its own strengths and limitations, together, they offer a comprehensive understanding of lens mechanics and positioning, essential for enhancing accommodative function in both natural and artificial lenses.
Diagnosis
Presbyopia is diagnosed when the eye’s ability to accommodate is insufficient to meet an individuals’ near vision requirements. For further detail, please refer to the BCLA CLEAR Presbyopia: Definitions Report.36 The amplitude of accommodation is high until the age of 20; it declines dramatically by the age of 30 and stabilises by the age of 50 at the level of one dioptre (figure 3).
Figure 3: Maximum accommodation amplitudes for subjects pooled from four studies41-44
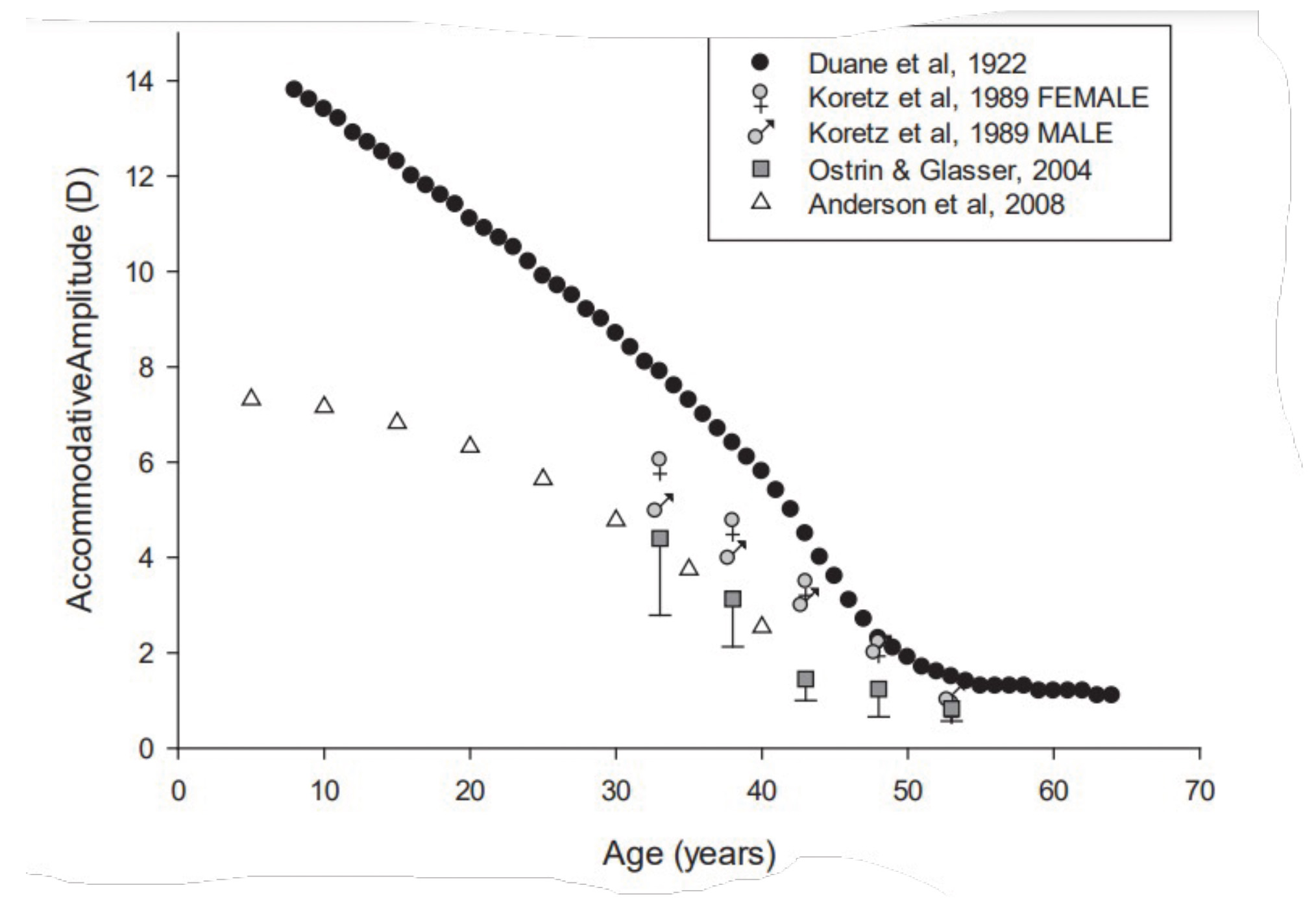
The scatter of data points illustrates that exact accommodation loss cannot be predicted based only on biological age.
As the presbyope becomes aware of the gradual loss of near vision, they try to compensate for or adapt to this new experience. The early presbyope begins to hold the near objects further away to see them clearly. This may be more difficult in low-light conditions. There might be feelings of eye fatigue or headaches.
As the presbyopia progresses, the compensation increases, such as the font size and brighter light. In the cases of advanced presbyopia, to be able to recognise objects or print close, a reading aid is required. When examining presbyopic myopes, the eye care professional (ECP) must know whether the patient wears contact lenses.
If this is the case, then reading spectacles or multifocal contact lenses will be required as the patient cannot remove their contact lenses to read like they do when wearing spectacles. Myopic contact lens wearers experience symptoms earlier than spectacle wearers.
In the case of hyperopes, the opposite occurs.37 Myopic patients without their spectacles adjust their near clear point in relation near clear point in relation to the equivalent addition relative to their distance prescription.
For example, if their distance prescription is -3.00 D, when they remove their spectacle effectively, they have a +3.00 D near add; thus, their working distance is 33cm. The working distance places a high demand on accommodation and vergence, causing symptoms of headaches, blurred vision and eye strain.38
Practitioners must consider the closer distances adopted while viewing material on smartphones when they examine patients and prescribe refractive corrections for near, as well as when treating patients suffering from asthenopia associated with near work.39 It has been shown that when looking at a smartphone, the instrument is held much closer than when viewing written text.39,40
Patient communication and awareness
Early discussions about presbyopia, ideally around age 40, are recommended to prepare patients for the condition. Customising the prescription based on the patient’s specific requirements will help improve their overall visual function and quality of life.
A prospective study conducted in Japan revealed awareness of presbyopia significantly increases with age, with 50% of individuals aged 45-49 being aware of the condition, 87.5% awareness in those aged 50-54, and full awareness among those aged 55-59.45 In contrast, none of the participants under 44 were aware of presbyopia.
Another study, however, reported that 38% of participants recalled having difficulty focusing before the age of 40.46 The Japanese study also found that as binocular near visual acuity decreased to 0.0 logMAR (20/20), awareness of presbyopia and difficulties with near tasks increased significantly, with over 80% of patients having trouble reading comfortably.45
This indicates that maintaining a near visual acuity better than 0.0 logMAR may be crucial for comfortable near vision and could serve as a useful threshold for diagnosing presbyopia. A UK based survey of 135 patients aged 40 years and over, revealed that 70% were aware of presbyopia, but only 15% were aware of the correct definition of presbyopia.47
It is important to educate people about presbyopia early, particularly before symptoms begin, and to emphasise the need for regular eye exams to ensure comfortable near vision as they age.
In many countries, many presbyopes are under-corrected due to the lack of correct diagnosis and management of the condition. The protocol for diagnosing and managing presbyopia focuses on a comprehensive approach to ensure accurate correction and patient satisfaction.
The key steps are as follows:
- Comprehensive history: Gather detailed information about the patient’s current eyewear (glasses/contact lenses), near vision tasks, any symptoms like eye strain or headaches, and any coping strategies they use. Consider using presbyopia-specific patient-reported outcome measures if appropriate.
- Update visual prescription: Assess and update the patient’s prescription for distance, intermediate, and near vision.
- Evaluate visual parameters: Measure pupil size, residual accommodation, and binocular vision to identify any visual anomalies that might be contributing to the patient’s symptoms.
- Determine required near vision addition: Fine-tune the reading addition based on the patient’s preferred working distance and other factors such as age, gender, ethnicity and near visual acuity. Assess eye dominance while considering multifocal lenses or monovision.
- Confirm visual range: Verify that the prescribed addition allows the patient to perform their tasks comfortably across the required range of distances. Discuss the benefits of appropriate lighting or the use of additional reading glasses. If no addition is needed, educate the patient on potential presbyopic changes and advise them to seek help if their visual demands change.
- Demonstrate and explain correction: Show the patient how presbyopia correction affects their vision, particularly for distant tasks, and explain when the correction should be used.
- Effective communication: Discuss the adaptation process to the new correction, potential future adjustments and alternative correction options, such as contact lenses. Emphasise the importance of understanding presbyopia and tailoring treatment strategies to the patient’s individual visual needs and preferences.
Evaluating between presbyopia management strategies
There are numerous strategies available to correct presbyopia. The key issues to be addressed are the relative safety, efficacy and the patient suitability of the intervention used. The ECP must understand the presbyope’s lifestyle, their visual requirements, and their expectations before deciding which intervention to recommend.
Many management approaches are available or being developed, such as contact lenses, corneal techniques, scleral techniques, pharmaceuticals and IOLs. Refer to BCLA CLEAR Presbyopia: Management Reports.48-51
The most important considerations when deciding which intervention to choose are the overall efficacy and safety of the intervention (table 1). The individual can determine value for money and make an informed decision based on considerations such as access, convenience, effectivity and cost of each option available.
Table 1: Key considerations when comparing the effectiveness and safety of current presbyopia correction strategies (refer to BCLA CLEAR Presbyopia: Management reports)48-51
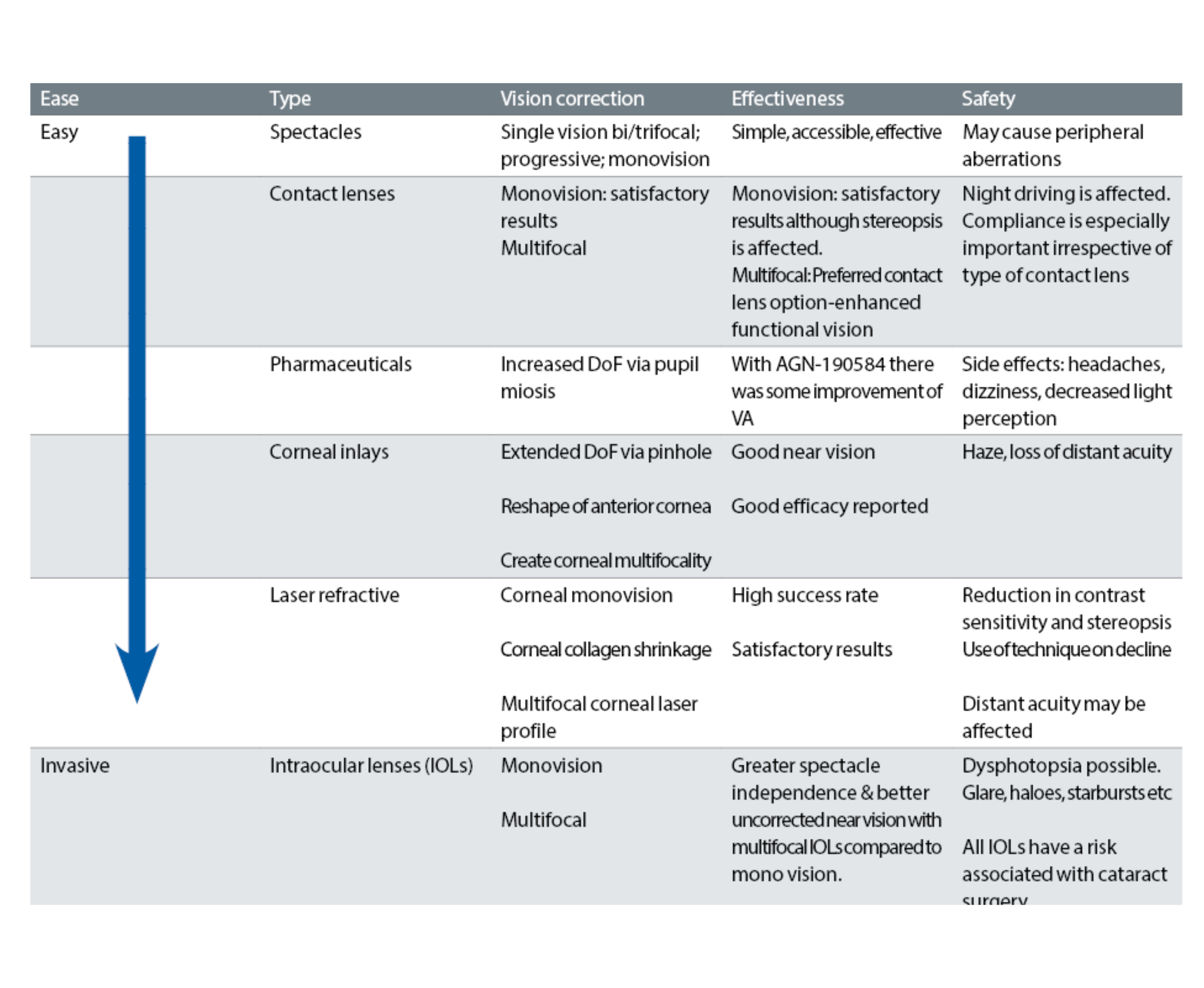
There is a lack of published information to help the ECP advise the presbyope on the best option to select. Usually, the first choices are multifocal spectacles and multifocal contact lenses. Both choices enable reversibility and flexibility.
When considering the safety issue, it is important to explain the gaze location when wearing multifocal spectacles. If the wearer does not look through the correct portion of the spectacle lens, they may fall due to unclear vision. For contact lens wearers, educating on strategies to minimise risk of complications and to promote compliant wear should be a priority.
Some people are concerned about their cosmetic appearance when wearing spectacles, while others feel that spectacles are a fashion accessory and prefer to wear them.
However, it seems that presbyopic females prefer multifocal contact lenses over spectacles, especially if they previously wore single-vision contact lenses.53
Factors that can influence a patient’s choice include:
- Accessibility
- Affordability
- Presbyope’s expectations
- Cosmetic factors
- Flexibility between interventions
Summary
Key recommendations for managing patients with presbyopia:
- Measure the range of clear focus of the patient to recommend which presbyopic technique is advisable.
- The wrong near vision correction will cause frustration. Sub-optimal correction will influence productivity and quality of life. An over-correction will limit the range of focus and make adaptation more difficult. The advantage of optical options are they can be changed along the way as the patient’s needs evolve.
- Both subjective and objective techniques must be used when diagnosing and measuring the degree of presbyopia.
- The journey of presbyopia offers diverse options. Therefore, the presbyope must be prepared in advance to try various possibilities54 and may need an adaption period.
- There is a lack of patient education on presbyopia and the available options, highlighting the importance of effective communication.55
- Dr Daddi Fadel is an optometrist working as a clinical scientist and adjunct assistant professor at the Centre for Ocular Research and Education University of Waterloo in Canada. She is a globally renowned speaker, author and is the editor-in-chief of the Journal of Contact Lens Research & Science. She has committee/advisory/chair roles and fellowships from numerous organisations across the globe.
- Dr David Berkow is an optometrist working in his private practice in Haifa, Israel. He completed his Doctor of Optometry at Aston University. He is a fellow of numerous optometric associations and a global ambassador of the BCLA. He lectures extensively both locally and abroad at optometric conferences and engages in promoting optometric education in Israel.
- Acknowledgement and recognition to the authors of the original paper- James S Wolffsohn, David Berkow, Ka Yin Chan, Suraj K. Chaurasiya, Daddi
 Fadel, Mera Haddad, Tarib Imane, Lyndon Jones, Amy Sheppard, Marta Vianya-Estopa, Karen Walsh, Jill Woods, Fabrizio Zeri and Philip Morgan.
Fadel, Mera Haddad, Tarib Imane, Lyndon Jones, Amy Sheppard, Marta Vianya-Estopa, Karen Walsh, Jill Woods, Fabrizio Zeri and Philip Morgan. - The paper is open access and available at BCLA CLEAR Presbyopia: Evaluation and diagnosis - Contact Lens and Anterior Eye (contactlensjournal.com) or via the QR code above.
- BCLA CLEAR Presbyopia was facilitated by the BCLA, with financial support by way of educational grants for collaboration, publication and dissemination provided by Alcon, Bausch + Lomb, CooperVision, EssilorLuxottica, and Johnson & Johnson Vision.
- The editors for this article series are Neil Retallic and Dr Debarun Dutta.
References
- Patel, I, et al, Impact of presbyopia on quality of life in a rural African setting. Ophthalmology, 2006. 113(5): p. 728-34.
- Black, N, Patient reported outcome measures could help transform healthcare. Bmj-British Medical Journal, 2013. 346.
- Wolffsohn, JS and LN Davies, Presbyopia: Effectiveness of correction strategies. Prog Retin Eye Res, 2019. 68: p. 124-143.
- Wolffsohn, JS, et al, Exploring the optimum step size for defocus curves. J Cataract Refract Surg, 2013. 39(6): p. 873-80.
- Nuijts, RMMA, et al, Bilateral implantation of+2.5 D multifocal intraocular lens and contralateral implantation of+2.5 D and+3.0 D multifocal intraocular lenses: Clinical outcomes. Journal of Cataract and Refractive Surgery, 2016. 42(2): p. 194-202.
- Kohnen, T, J Lemp-Hull, and R Suryakumar, Defocus curves: focusing on factors influencing assessment. Journal of Cataract and Refractive Surgery, 2022. 48(8): p. 961-968.
- Law, EM, et al, Optimising curve fitting techniques to look for standardisation of the analysis of defocus curves derived from multifocal intraocular lenses. Ophthalmic Physiol Opt, 2022. 42(4): p. 887-896.
- Erdinest, N and N London, Dynamic visual acuity and methods of measurement. Journal of Optometry, 2022. 15(3): p. 247-248.
- Wu, TY, YX Wang, and XM Li, Applications of dynamic visual acuity test in clinical ophthalmology. Int J Ophthalmol, 2021. 14(11): p. 1771-1778.
- Morad, Y, E Werker, and P Nemet, Visual acuity tests using chart, line, and single optotype in healthy and amblyopic children. J AAPOS, 1999. 3(2): p. 94-7.
- Tidbury, LP, G Czanner, and D Newsham, Fiat Lux: the effect of illuminance on acuity testing. Graefes Arch Clin Exp Ophthalmol, 2016. 254(6): p. 1091-7.
- Baker, PA, et al, Visual Acuity During Direct Laryngoscopy at Different Illuminance Levels. Anesthesia and Analgesia, 2013. 116(2): p. 343-350.
- Mansfield, JS, GE Legge, and M.C. Bane, Psychophysics of reading. XV: Font effects in normal and low vision. Invest Ophthalmol Vis Sci, 1996. 37(8): p. 1492-501.
- Zoccolotti, P, et al, Reading development in an orthographically regular language: effects of length, frequency, lexicality and global processing ability. Reading and Writing, 2009. 22(9): p. 1053-1079.
- Slattery, TJ and K Rayner, Effects of intraword and interword spacing on eye movements during reading: Exploring the optimal use of space in a line of text. Attention, Perception, & Psychophysics, 2013. 75(6): p. 1275-1292.
- Wang, HF, To space or not space? Interword spacing effects on Chinese children’s reading materials. Ergonomics, 2015. 58(12): p. 1947-1959.
- Daxer, B, et al, Towards a standardisation of reading charts: Font effects on reading performance-Times New Roman with serifs versus the sans serif font Helvetica. Ophthalmic Physiol Opt, 2022. 42(6): p. 1180-1186.
- Bakaraju, RC, et al, Inherent ocular spherical aberration and multifocal contact lens optical performance. Optom Vis Sci, 2010. 87(12): p. 1009-22.
- Alió, JL, A Grzybowski, and D Romaniuk, Refractive lens exchange in modern practice: when and when not to do it? Eye and Vision, 2014. 1(1): p. 10.
- Hamed, AM, et al, A comparative analysis of five methods of determining corneal refractive power in eyes that have undergone myopic laser in situ keratomileusis. Ophthalmology, 2002. 109(4): p. 651-8.
- Anders, P, et al, Intraocular lens power calculation in eyes with previous corneal refractive surgery. Ther Adv Ophthalmol, 2022. 14: p. 25158414221118524.
- Gharieb, HM, IS Othman, and RS Elkitkat, Orbscan 3 Versus Pentacam HR: Evaluating the Possible Interchangeable Use of Various Parameters. Cornea, 2020. 39(5): p. 649-653.
- Cairns, G and CN McGhee, Orbscan computerized topography: attributes, applications, and limitations. J Cataract Refract Surg, 2005. 31(1): p. 205-20.
- Oliveira, CM, C Ribeiro, and S Franco, Corneal imaging with slit-scanning and Scheimpflug imaging techniques. Clin Exp Optom, 2011. 94(1): p. 33-42.
- Simpson, T and D Fonn, Optical Coherence Tomography of the Anterior Segment. The Ocular Surface, 2008. 6(3): p. 117-127.
- Mallen, EA, et al, Clinical evaluation of the Shin-Nippon SRW-5000 autorefractor in adults. Ophthalmic Physiol Opt, 2001. 21(2): p. 101-7.
- Vasudevan, B, KJ Ciuffreda, and B Wang, An objective technique to measure the depth-of-focus in free space. Graefe’s Archive for Clinical and Experimental Ophthalmology, 2006. 244(8): p. 930-937.
- Wang, B and KJ Ciuffreda, Depth-of-focus of the human eye: theory and clinical implications. Surv Ophthalmol, 2006. 51(1): p. 75-85.
- Ogle, KN and JT Schwartz, Depth of Focus of the Human Eye*. Journal of the Optical Society of America, 1959. 49(3): p. 273-280.
- Rosen, E, et al, Efficacy and safety of multifocal intraocular lenses following cataract and refractive lens exchange: Metaanalysis of peer-reviewed publications. J Cataract Refract Surg, 2016. 42(2): p. 310-28.
- Zeri, F, et al, Immediate cortical adaptation in visual and non-visual areas functions induced by monovision. J Physiol, 2018. 596(2): p. 253-266.
- Rosa, AM, et al, Functional Magnetic Resonance Imaging to Assess the Neurobehavioral Impact of Dysphotopsia with Multifocal Intraocular Lenses. Ophthalmology, 2017. 124(9): p. 1280-1289.
- Rosa, AM, et al, Functional magnetic resonance imaging to assess neuroadaptation to multifocal intraocular lenses. Journal of Cataract and Refractive Surgery, 2017. 43(10): p. 1287-1296.
- Fernandes, P, et al, Short-term delay in neural response with multifocal contact lens might start at the retinal level. Documenta Ophthalmologica, 2022. 145(1): p. 37-51.
- Kasthurirangan, S, et al, In vivo study of changes in refractive index distribution in the human crystalline lens with age and accommodation. Invest Ophthalmol Vis Sci, 2008. 49(6): p. 2531-40.
- Wolffsohn, JS, et al, BCLA CLEAR Presbyopia: Definitions. Cont Lens Anterior Eye, 2024. 47(4): p. 102155.
- Hunt, OA, JS Wolffsohn, and C Garcia-Resua, Ocular motor triad with single vision contact lenses compared to spectacle lenses. Cont Lens Anterior Eye, 2006. 29(5): p. 239-45.
- Das, A, et al, Computer vision syndrome, musculoskeletal, and stress-related problems among visual display terminal users in Nepal. PLoS One, 2022. 17(7): p. e0268356.
- Bababekova, Y, et al, Font size and viewing distance of handheld smart phones. Optom Vis Sci, 2011. 88(7): p. 795-7.
- Wolffsohn, JS, et al, TFOS Lifestyle: Impact of the digital environment on the ocular surface. Ocul Surf, 2023. 28: p. 213-252.
- Anderson, HA, et al, Minus-lens-stimulated accommodative amplitude decreases sigmoidally with age: a study of objectively measured accommodative amplitudes from age 3. Invest Ophthalmol Vis Sci, 2008. 49(7): p. 2919-26.
- Koretz, JF, et al, Accommodation and presbyopia in the human eye--aging of the anterior segment. Vision Res, 1989. 29(12): p. 1685-92.
- Ostrin, LA and A Glasser, Accommodation measurements in a prepresbyopic and presbyopic population. J Cataract Refract Surg, 2004. 30(7): p. 1435-44.
- Duane, A, Studies in Monocular and Binocular Accommodation, with Their Clinical Application. Trans Am Ophthalmol Soc, 1922. 20: p. 132-57.
- Tsuneyoshi, Y, et al, Determination of the Standard Visual Criterion for Diagnosing and Treating Presbyopia According to Subjective Patient Symptoms. J Clin Med, 2021. 10(17).
- Negishi, K, et al, Sleep and subjective happiness between the ages 40 and 59 in relation to presbyopia and dry eye. PLOS ONE, 2021. 16(4): p. e0250087.
- Hutchins, B and B Huntjens, Patients’ attitudes and beliefs to presbyopia and its correction. J Optom, 2021. 14(2): p. 127-132.
- Craig, JP, et al, BCLA CLEAR Presbyopia: Management with corneal techniques. Cont Lens Anterior Eye, 2024. 47(4): p. 102190.
- Schnider, C, et al, BCLA CLEAR presbyopia: Management with intraocular lenses. Cont Lens Anterior Eye, 2024. 47(4): p. 102253.
- Morgan, PB, et al, BCLA CLEAR Presbyopia: Management with contact lenses and spectacles. Cont Lens Anterior Eye, 2024. 47(4): p. 102158.
- Naroo, SA, et al, BCLA CLEAR presbyopia: Management with scleral techniques, lens softening, pharmaceutical and nutritional therapies. Cont Lens Anterior Eye, 2024. 47(4): p. 102191.
- Stapleton, F, et al, CLEAR-Contact lens complications. Contact Lens & Anterior Eye, 2021. 44(2): p. 330-367.
- Morgan, PB, N. Efron, and CA Woods, An international survey of contact lens prescribing for presbyopia. Clin Exp Optom, 2011. 94(1): p. 87-92.
- Sivardeen, A, C McAlinden, and JS Wolffsohn, Presbyopic correction use and its impact on quality of vision symptoms. Journal of Optometry, 2020. 13(1): p. 29-34.
- Hutchins, B and B Huntjens, Patients’ attitudes and beliefs to presbyopia and its correction. Journal of Optometry, 2021. 14(2): p. 127-132.
