2Domains and learning outcomes (C110989)
• One distance learning CPD point for optometrists, dispensing opticians and contact lens optician.
• Clinical practice
Upon completion of this CPD, ECPs will be able to list the typical signs and symptoms of Demodex blepharitis (s5)
Upon completion of this CPD, ECPs will be able to describe the aetiological processes, which may underlie demodex blepharitis and associated inflammation (s5)
Upon completion of this CPD, ECPs will be able to describe the potential treatments used for demodex blepharitis as related to
their scope of practice including, where necessary, referral (s6, s7)
• Specialty CPD – contact lens optician
Upon completion of this CPD, contact lens opticians will be able to differentially diagnose demodex blepharitis from other similar anterior eye conditions during slit lamp examination and through history taking (s5)
The ocular surface comprises the cornea and conjunctiva and is in constant, direct contact with the environment.1 The eyelids, eyelashes and tear-producing glands make up the nearby structures. Each of these adnexal structures are essential for healthy functioning and protection of the ocular surface.2
Despite being constantly exposed to potential microbes, shearing forces of blinking, air currents and low humidity, the ocular surface maintains its functional integrity with the help of a pre-corneal film, also known as the tear film.3,4
The tear film alters the anti-microbial activity, thereby providing the ocular surface with an elaborate defence system.5 Disorders of the ocular surface are commonly seen in ophthalmic clinics and include dry eye disease (DED), blepharitis, Meibomian gland dysfunction (MGD) and ocular allergies.6
A significant overlap in signs and symptoms has been reported in various ocular surface diseases.7 Due to the multifactorial nature of these ocular surface diseases, their pathophysiology remains poorly understood and can lead to a significant negative impact on daily activities.8
It has been reported that patients suffering from blepharitis and other ocular surface diseases are at a higher risk of developing anxiety and depression leading to a negative impact on the overall quality of life.9
Microbial flora and the ocular surface
The ocular surface is rich in nutrients and serves as an excellent medium for the growth of a diverse range of microorganisms.10 It has been reported that patients suffering from blepharitis are heavily infested with microbes, compared to normal individuals.11
Despite this, the ocular surface is not inflamed under normal conditions.12 It has been stipulated that the ocular surface flora has co-evolved with humans and therefore humans have developed a unique innate immune response towards commensals and potentially pathogenic microorganisms.13
This discrimination between normal microbial flora and pathogenic organisms is achieved via microbial-associated molecular patterns (MAMPs). These MAMPs are a group of pattern recognition receptors (PRR) that can distinguish between molecules produced by microbes and host cells.12
PRR can be intracellular – nucleotide-binding oligomerization domain (NOD)-like receptors (NLR) and cell surface sensors – Toll-like receptors (TLR).14 The TLR activates the secretion of pro-inflammatory cytokines and interferon (IFN)-α and IFN-β when the healthy integrity of the ocular surface is breached by microbes, resulting in inflammation.15
Blepharitis
In clinical terms, blepharitis is referred to as inflammation of the eyelids, with possible involvement of adnexal tissues.16 It is of utmost importance since blepharitis is one of the most common conditions encountered in eye care practice.17
The condition is generally reported as bilateral, remissive and chronic.18, 19 The preferred practice pattern developed by the AAO also classifies blepharitis based on the anatomical location as – (i) anterior blepharitis affecting the eyelashes, eyelid margin and eyelash follicles, and (ii) posterior blepharitis affecting the meibomian glands.20
Anterior blepharitis can be further sub-classified into bacterial (mostly staphylococcal), fungal (seborrheic) and parasitic (Demodex) forms.21 Whereas posterior blepharitis causes inflammation at the posterior lid margin, which may be associated with MGD, allergic or infectious forms of conjunctivitis and morphological changes in the eyelid.22, 23
Demodex blepharitis
There are over 100 species of Demodex mites, belonging to the class Arachnida and the subclass Acarina, that have been identified as obligatory commensals of the pilosebaceous unit in mammals.
The two main species of these microscopic mites found in humans are Demodex folliculorum, which inhabits eyelash follicles, and Demodex brevis, which resides in sebaceous glands.24, 25 Both species are translucent with four pairs of legs and short claws on each side.
The life cycle of Demodex lasts about two weeks (14.5 days), non-uniformly spread evolving through the different stages of ovum, larva, protonymph, deutonymph and then an adult. These mites are more active during the dark because they are semi-transparent in appearance and bright light can easily oxidise their inner structure; this is when most of the movement and mating are believed to occur.
Demodex mites feed on epithelial cells and sebum and can travel up to 16mm in one hour. It is estimated that an average healthy individual typically hosts between 1,000 and 2,000 Demodex mites on their body at any given time.26
Due to the eyeball being recessed compared to the surrounding facial structures, it is less accessible for daily cleaning, which makes it easy for mites to thrive near the eyelids. At the surface level, Demodex mites play an aetiological role in causing various ocular surface diseases, including chronic blepharitis, DED, MGD and chalazion (meibomian cyst) among others.
These mites can be lethal among pets (especially in dogs when causing Demodectic mange), whereas they have been termed as an opportunistic pathogen (microbes that become virulent in immune-compromised individuals) among humans.25 This has been supported in various studies since the clinical role of Demodex mites in causing blepharitis was reported, though the pathogenesis is uncertain.27
Demodex has also been associated with a key pathogenic role in other dermatological conditions such as rosacea, follicular pityriasis, perioral dermatitis, basal cell carcinoma and demodicosis gravis.28, 29
On the cellular level, a symbiotic relationship is commonly found among various multicellular organisms whereby they either benefit from each other (mutualism) or depend on the host for their survival (parasitism). A similar relationship has been reported between Demodex mites and Bacillus oleronius where Demodex mites act as a carrier for the bacterium. Even after their death, Demodex mites can activate inflammatory cascades through toxins released by Bacillus oleronius.
Previous investigations indicated that these bacterial toxins can activate the host immune system.30 Additionally, research has demonstrated that antigenic proteins extracted from this bacterium can stimulate inflammatory responses.31 The prevalence of Demodex in humans increases with age. While infants are free from Demodex infestation, their transmission is believed to occur via direct close contact with adults.
Demodex has been investigated and reported to be found in all ethnic groups across the globe. It has been reported that Demodex affects more than 80% of individuals over the age of 60 years and can reach as high as 100% in individuals over the age of 70 years.32
Additionally, poor ocular hygiene has also been attributed to higher Demodex prevalence.33 There has been a lot of debate about the role that sex plays in the prevalence of Demodex. While some studies indicate males are affected, others believe females are more affected. However, it has also been reported that Demodex infestations remain independent of sex as sex is the lowest risk factor for Demodex infestation.34
Furthermore, studies have also shown a high infestation rate of Demodex mites in immune-compromised patients suffering from diabetes, HIV, and renal diseases.35, 36 This further indicates the opportunistic nature of the pathogen and highlights the importance of taking preventive measures.
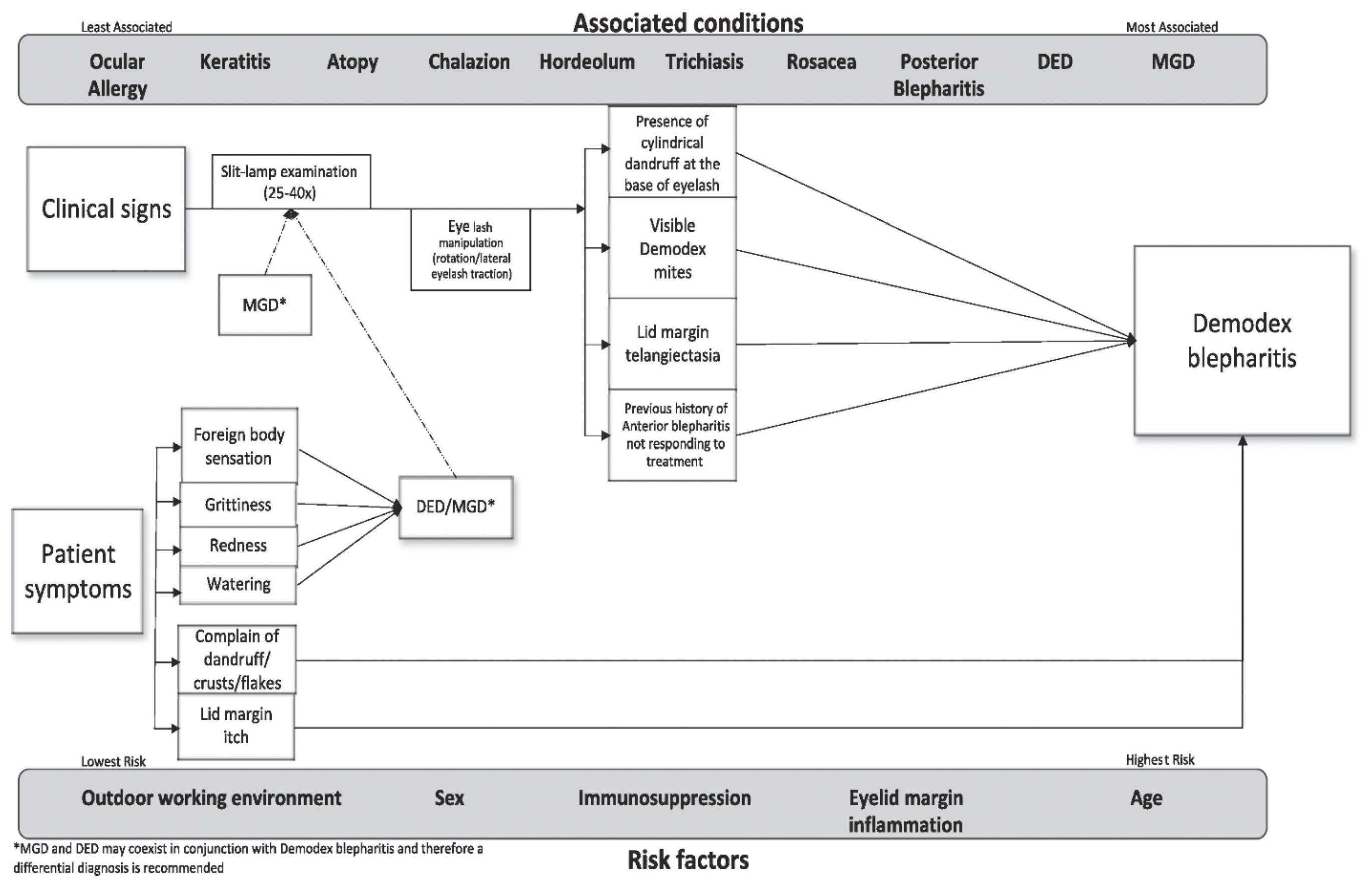
Clinical diagnosis (signs and symptoms)
Demodex blepharitis is a significant cause of ocular discomfort and is a potential cause for an unstable tear film. Demodex blepharitis remains an under-diagnosed condition in clinical practice37 and its diagnosis and management could vary among different professionals.38 The role of Demodex mites in causing a disease remains widely debated. This is because, though Demodex is present in all individuals, it does not necessarily cause a disease, since Demodex is considered to contribute to the normal ecology of the eyelid.39
Therefore, the mere presence of Demodex may not be a reliable indicator of the disease. However, over-colonisation of Demodex mites on the eyelids may lead to symptoms of ocular irritation, lid margin itch, and inflammation along the eyelid margin, subsequently leading to chronic blepharitis. Demodex diagnosis can be based on one or a combination of the presence of multiple presenting signs and symptoms.
Itching (lid margin) is the most common symptom observed in Demodex blepharitis as reported by the patients as well as agreed upon by the experts.34, 40, 41 This lid margin itch has been attributed to an increased activity of Demodex during the night. Additional symptoms, such as redness, tearing, grittiness and foreign body sensations, can also be observed.
Cylindrical dandruff (CD) at the base of the eyelash, sometimes also referred to as lash collarettes is pathognomonic of Demodex blepharitis.28 The CD is believed to form from micro-abrasions in eyelash follicles. These micro-abrasions happen when Demodex uses its claws to scrape the internal walls of the lash follicles.42 This is the most common clinical feature observed in Demodex infestation.
Conventionally, eyelash epilation followed by a microscopic examination was used to detect the presence of Demodex. However, recently, more improved eyelash manipulation techniques have been developed, such as eyelash rotation, cylindrical dandruff removal, lateral eyelash traction and in vivo confocal microscopy.43
These techniques involve simply choosing an eyelash with CD under high slit-lamp magnification (25x-40x) and then, either rotating it with the help of sterile forceps clockwise and anti-clockwise, or under gentle tension, alternating the grasped eyelash in nasal and temporal directions.43, 44 These methods stimulate mite tails to emerge from the follicle opening and can be viewed in vivo.
Certain conditions such as MGD and DED are often associated with Demodex. These conditions can also co-exist and therefore efforts should be made towards a differential diagnosis as well. Additionally, rosacea and age can also be associated and/or act as a risk factor for Demodex blepharitis.
Inflammation and telangiectasia of the lid margin disfigure the natural shape of the patient’s eyelids making them feel less confident and unattractive, which can affect their social life in turn.45 These signs and symptoms correlate strongly in symptomatic patients.
Therefore, the presence of signs and symptoms, along with risk factors and associated conditions, are likely to be better indicators of Demodex infestation and should be considered while making a diagnosis as shown in figure 1.
Figure 1: Algorithm for clinical diagnosis of Demodex blepharitis34
Treatment
Demodex infestation does not have gold standard management guidelines and remains a topic of debate in the published literature. Therefore, various products and their formulations, such as mercury oxide ointment, sulphur ointment, topical and systemic ivermectin, various concentrations of tea tree oil (TTO), terpinen-4-ol (active ingredient in TTO), intense pulse light, manuka honey, okra-based products and more recently, and FDA-approved 0.25% lotilaner ophthalmic solution, have been used to combat Demodex blepharitis.46-49
It has been suggested that Demodex management should be aimed at reducing CD, itching, and the mite population by both chemical and mechanical measures. TTO in various concentrations (5-50%) has been extensively used to treat Demodex.
However, higher concentrations of TTO could be toxic to the ocular surface and have been advised to be used only under expert supervision. For chronic cases, it is possible that the ocular surface microflora may form a biofilm and Demodex can potentially use this biofilm as a defensive shield and enhance their survival against topical demodectic products.
Therefore, eyelid debridement could be extremely useful in removing this biofilm prior to treatment with topical anti-demodectic products. This could also enhance the penetration and efficacy of anti-demodectic products. Since the life cycle of Demodex is about two weeks, the primary treatment aims to cover two life cycles of the mite (four to six weeks).
Therefore, it is recommended that mechanical removal of CD followed by 5% TTO twice a day helps in greater improvement in symptoms and would form the primary treatment modality.34, 42 The use of anti-demodectic agents at night may effectively eliminate more mites and prevent their mating, thus reducing the likelihood of their offspring.
If there are improvements in signs and symptoms following this, the patient should be carefully monitored for any reoccurrences. Additionally, a cyclic treatment regime could also be incorporated as well, where after two weeks, the treatment is paused for a week to provide sufficient time for the eggs to hatch and then resume for another two weeks. This cyclic treatment has been shown to break the vicious cycle of reoccurrences.50
The efficacy of the treatment should be reviewed between two to six weeks after commencing the treatment. This would enable the practitioners to monitor the outcome of the primary treatment. Any improvement in signs and symptoms could be considered as treatment success. If the first line treatment is not effective, a more rigorous approach could be employed wherein the frequency of lid cleaning could be increased followed by in-practice treatment with a higher concentration of TTO, IPL and additional pharmacological treatment.
- Dr Nikhil Sharma is a lecturer in vision sciences at Glasgow Caledonian University, his PhD thesis focused on Demodex blepharitis.
References
- Korb DR, Association BCL. The tear film: structure, function and clinical examination [Internet]. Oxford: Butterworth-Heinemann; 2002.
- Lee WB, Mannis MJ. 1 - Historical Concepts of Ocular Surface Disease [Internet]. Holland EJ, Mannis MJ, Lee WB, editors. Ocular Surface Disease: Cornea, Conjunctiva and Tear Film. London: W.B. Saunders; 2013. p. 3–10. Available from: http://www.sciencedirect.com.gcu.idm.oclc.org/science/article/pii/B9781455728763000018
- Bron AJ, Tiffany JM, Gouveia SM, Yokoi N, Voon LW. Functional aspects of the tear film lipid layer. Exp Eye Res [Internet]. 2004;78(3):347–60. Available from: http://www.sciencedirect.com/science/article/pii/S0014483503003038
- Rolando M, Zierhut M. The Ocular Surface and Tear Film and Their Dysfunction in Dry Eye Disease. Surv Ophthalmol. 2001;45(2):S203–10.
- Flanagan JL, Willcox MDP. Role of lactoferrin in the tear film. Biochimie [Internet]. 2009;91(1):35–43.
- Djalilian AR. Ocular Surface Disease A Case-Based Guide. 1st ed. Cham: Springer International Publishing; 2018.
- Nichols K. Blepharitis and dry eye: a common, yet complicated combination: the diseases often co-present, which may confound treatment priorities.(Dry Eye)(Disease/Disorder overview). Review of Optometry. 2010;147(8):39.
- Smith Janine A. The Epidemiology of Dry Eye Disease: Report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007) [Internet]. Vol. 5, The Ocular Surface. 2007. p. 93–107. Available from: http://www.sciencedirect.com/science/article/pii/S1542012412700824
- Chiang CC, Laks J, Lin CL, Tsai YY, Peng CL, Liao YT, et al. Patients with Blepharitis Are at Elevated Risk of Anxiety and Depression. Vol. 8, PLoS ONE. 2013. p. e83335.
- Armstrong RA. The microbiology of the eye. Ophthalmic and Physiological Optics. 2000;20(6):429–41.
- Groden L, Murphy B, Rodnite J, Genvert GI. LID FLORA IN BLEPHARITIS. Cornea; Cornea. 1991;10(1):50–3.
- Miller D, Iovieno A. The role of microbial flora on the ocular surface. Curr Opin Allergy Clin Immunol. 2009;9(5):466–70.
- Ueta M. Innate Immunity of the Ocular Surface and Ocular Surface Inflammatory Disorders. Cornea. 2008;27 Suppl 1(1):S31–40.
- Kishore U. Target Pattern Recognition in Innate Immunity. 1st ed. New York, NY: Springer New York; 2009.
- Pearlman E, Johnson A, Adhikary G, Sun Y, Chinnery HR, Fox T, et al. Toll-like Receptors at the Ocular Surface. Ocul Surf. 2008;6(3):108–16.
- Navel V, Mulliez A, Benoist d’Azy C, Baker JS, Malecaze J, Chiambaretta F, et al. Efficacy of treatments for Demodex blepharitis: A systematic review and meta-analysis [Internet]. Vol. 17, The Ocular Surface. 2019. p. 655–69. Available from: http://www.sciencedirect.com/science/article/pii/S1542012419300655
- Smith RE, Flowers CW. Chronic blepharitis: a review. CLAO J. 1995;21(3):200.
- Di Zazzo A, Giannaccare G, Villani E, Barabino S. Uncommon Blepharitis. J Clin Med. 2024;13(3):710.
- Bruce Jackson W. Blepharitis: current strategies for diagnosis and management. Canadian Journal of Ophthalmology/Journal canadien d’ophtalmologie. 2008;43(2):170–9.
- Amescua G, Akpek EK, Farid M, Garcia-Ferrer F, Lin A, Rhee MK, et al. Blepharitis Preferred Practice Pattern®. Ophthalmology. 2019;126(1):P56–93.
- Murphy O, O’ Dwyer V, Lloyd-McKernan A. The effect of lid hygiene on the tear film and ocular surface, and the prevalence of Demodex blepharitis in university students. Contact Lens and Anterior Eye. 2019;<xocs:first xmlns:xocs=””/>.
- Nelson JD, Shimazaki J, Benitez-Del-Castillo M. J, Craig JP, Mcculley JP, Den S, et al. The international workshop on meibomian gland dysfunction: report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci. 2011;52(4):1930.
- Nijm LM. 8 - Blepharitis: Classification [Internet]. Holland EJ, Mannis MJ, Lee WB, editors. Ocular Surface Disease: Cornea, Conjunctiva and Tear Film. London: W.B. Saunders; 2013. p. 55–60. Available from: http://www.sciencedirect.com.gcu.idm.oclc.org/science/article/pii/B9781455728763000080
- Ionela GA, Alexandru MO, Geanina MN. Demodex folliculorum and D. brevis – A cause of facial dermatitis and blepharitis. J Biotechnol. 2014;185:S100.
- Lacey N, Ní Raghallaigh S, Powell F. Demodex Mites - Commensals, Parasites or Mutualistic Organisms? Dermatology. 2011;222(2):128–30.
- Litwin D, WenChieh C, Dzika E, Korycińska J. Human permanent ectoparasites; recent advances on biology and clinical significance of Demodex mites: narrative review article. Iran J Parasitol. 2017;12(1):12.
- Geerling G, Tauber J, Baudouin C, Goto E, Matsumoto Y, O’Brien T, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52(4):2050–64.
- Liu J, Sheha H, Tseng SCG. Pathogenic role of Demodex mites in blepharitis. Curr Opin Allergy Clin Immunol. 2010;10(5):505.
- Morrás PG, Santos SP, Imedio IL, Echeverría ML, Hermosa JMH, Morrás PG. Rosacea-like demodicidosis in an immunocompromised child. Pediatr Dermatol. 2003;20(1):28–30.
- Lacey N, Delaney S, Kavanagh K, Powell FC. Mite‐related bacterial antigens stimulate inflammatory cells in rosacea. British Journal of Dermatology. 2007;157(3):474–81.
- Li J, O’Reilly N, Sheha H, Katz R, Raju VK, Kavanagh K, et al. Correlation between ocular Demodex infestation and serum immunoreactivity to Bacillus proteins in patients with facial rosacea. Ophthalmology. 2010;117(5):870–7.
- Hom MM, Mastrota KM, Schachter SE Demodex. Optometry and Vision Science. 2013;90(7):e198–205.
- Lee SH, Chun YS, Kim JH, Kim ES, Kim JC. The relationship between demodex and ocular discomfort. Invest Ophthalmol Vis Sci. 2010;51(6):2906.
- Sharma N, Martin E, Pearce EI, Hagan S. A Delphi approach to establishing consensus on best practice for the diagnosis and treatment of Demodex blepharitis. Contact Lens and Anterior Eye [Internet]. 2023;102080. Available from: https://www.sciencedirect.com/science/article/pii/S1367048423003144
- Yagdiran Düzgün O, Aytekin S. Comparison of Demodex folliculorum density in haemodialysis patients with a control group. Vol. 21, Journal of the European Academy of Dermatology and Venereology. Oxford, UK: Blackwell Publishing Ltd; 2007. p. 480–3.
- Yamashita LSF de F, Cariello AJ, Geha NMA, Yu MCZ, Hofling-Lima A. Demodex folliculorum on the eyelash follicule of diabetic patients. Vol. 74, Arquivos Brasileiros de Oftalmologia. Conselho Brasileiro de Oftalmologia; 2011. p. 422–4.
- Sharma N, Martin E, Pearce EI, Hagan S, Purslow C. Demodex Blepharitis: A Survey-Based Approach to Investigate Knowledge, Attitudes, and Practices Among Optometrists in India. Clin Optom (Auckl). 2023;55–64.
- Sharma N, Martin E, Pearce EI, Hagan S, Purslow C, Craig JP. Comparison of the Diagnosis and Management of Demodex Blepharitis Between Eye Care Practitioners in India and Australasia – A Survey-Based Comparison. Clin Optom (Auckl) [Internet]. 2024 [cited 2024 Oct 18];16:255–65. Available from: http://www.ncbi.nlm.nih.gov/pubmed/39359323
- Fromstein S, Harthan J, Patel J, Opitz D. Demodexblepharitis: clinical perspectives. Clin Optom (Auckl). 2018;10:57–63.
- Ayres BD, Donnenfeld E, Farid M, Gaddie IB, Gupta PK, Holland E, et al. Clinical diagnosis and management of Demodex blepharitis: the Demodex Expert Panel on Treatment and Eyelid Health (DEPTH). Eye. 2023;1–7.
- Sedzikowska A, Oseka M, Grytner-Ziecina B. Ocular symptoms reported by patients infested with Demodex mites. Acta parasitologica; Acta Parasitol. 2016;61(4):808–14.
- Murphy O, O’Dwyer V, Lloyd-McKernan A. The efficacy of tea tree face wash, 1, 2-Octanediol and microblepharoexfoliation in treating Demodex folliculorum blepharitis. Contact lens & anterior eye; Cont Lens Anterior Eye. 2018;41(1):77–82.
- Muntz A, Purslow C, Wolffsohn JS, Craig JP, Muntz A. Improved Demodex diagnosis in the clinical setting using a novel in situ technique. Cont Lens Anterior Eye. 2019;
- Murphy O, O’ Dwyer V, Lloyd-McKernan A. The Clinical Use of Eyelash Manipulation in the Diagnosis of Demodex folliculorum Blepharitis. Eye Contact Lens [Internet]. 2020;46. Available from: https://journals.lww.com/claojournal/fulltext/2020/01001/the_clinical_use_of_eyelash_manipulation_in_the.6.aspx
- Rhee MK, Yeu E, Barnett M, Rapuano CJ, Dhaliwal DK, Nichols KK, et al. Demodex blepharitis: A comprehensive review of the disease, current management, and emerging therapies. Eye Contact Lens. 2023;49(8):311–8.
- Bitton E, Aumond S. Demodex and eye disease: a review. Clinical and experimental optometry; Clin Exp Optom. 2021;104(3):285–94.
- Craig JP, Cruzat A, Cheung IMY, Watters GA, Wang MTM. Randomized masked trial of the clinical efficacy of MGO Manuka Honey microemulsion eye cream for the treatment of blepharitis [Internet]. The Ocular Surface. 2019. Available from: http://www.sciencedirect.com/science/article/pii/S1542012419301521
- Gao YY, Xu D lian, Huang li J, Wang R, Tseng SCG. Treatment of Ocular Itching Associated With Ocular Demodicosis by 5% Tea Tree Oil Ointment. Cornea; Cornea. 2012;31(1):14–7.
- Liu W, Gong L. Anti-demodectic effects of okra eyelid patch in Demodex blepharitis compared with tea tree oil. Experimental and therapeutic medicine; Exp Ther Med. 2021;21(4):338.
- Evren Kemer Ö, Karaca EE, Özek D. Efficacy of cyclic therapy with terpinen-4-ol in Demodex blepharitis: Is treatment possible by considering Demodex’s life cycle? Eur J Ophthalmol [Internet]. 2021 May 1 [cited 2024 Oct 21];31(3):1361–6. Available from: https://journals.sagepub.com/doi/full/10.1177/1120672120919085
