Dr Chris Amos reports on two trials where an antibacterial case was tested against a regular high density polyethylene contact lens case, and offers advice on case care and management
Back in the early 1990s, contact lens case contamination was a hot issue. It should be again. The emergence of no-rub multipurpose solutions with added viscosity agents for comfort has increased the likelihood for lens case residue build-up - potential food for micro-organisms. With the simplification of lens care, eye care practitioners may mistakenly shortcut patient education on lens care even though patient instruction has been proved to significantly reduce lens and lens case contamination, and eye infection.1 Because good lens care practices need to be constantly enforced, this is an important responsibility of the eye care practitioner.
Contrary to previous market trend predictions, and despite the adoption of daily disposable lenses and extended wear lenses, many patients re-use contact lenses and lens care systems. While the latest solutions have more anti-microbial potency, the contamination level in lens cases remains high.2 It makes no difference whether lenses are new or old, conventional, planned replacement or disposable, they can all become contaminated once stored in a contaminated lens case.
Contact lens cases are prone to contamination in the first instance because bacteria generally thrive as colonies on surfaces. To accomplish this, bacteria have the ability to change from a mobile 'planktonic' form into an attached 'sessile' phenotype3,4 during which they may secrete a polysaccharide slime or glycocalyx and produce cellular surface structures, such as type IV pili, that enable the bacteria to irreversibly bind to surfaces.5 They generally tend to do this under environmentally challenging conditions when deprived of nutrients.6
The combination of living and dead organisms and secreted glycocalyx thus forms what is known as the 'biofilm'. Bacteria traditionally account for less than one third of a biofilm's composition and a majority of the film consists of exopolymeric substances.7 Biofilms are found on other medical devices such as catheters and are significant because the glycocalyx and outer cell layers protect micro-organisms against disinfectants, making them much harder to kill than their planktonic phenotype. Worse still, clumps of resistant sessile cells and daughter cells shed from the biofilm can potentially start the colonisation process all over again on a new surface such as a contact lens.
It is generally accepted that bacteria growing on a surface have several competitive advantages. Binding to a surface can provide the cell with a constant carbon and energy source and surface-bound development often imparts greater resistance to environmental stress factors. Adhesion of bacteria to implanted biomaterials and tissue surfaces is a significant event in the pathogenesis of human infection.8 Biofilms on contact lens cases also provide a food source for Acanthamoeba and can be responsible for a build-up of endotoxins9 and exotoxins.
Endotoxins are lipopolysaccharides unique to the cell walls of Gram-negative bacteria (such as Pseudomonas aeruginosa) that, if transferred into the eye, can activate macrophages to produce inflammatory cytokines, thus triggering an immune response. Because endotoxins are simply cell wall material, they can still present a hazard even if the originating bacteria from whence they came have been killed. Exotoxins are proteins secreted by bacteria with specific lytic, cytotoxic, or pharmacological modes of action and may also accumulate in a contact lens or have an effect after the bacteria producing them have been killed.
Although contact lens care products are effective against planktonic bacteria, they are less so against resistant sessile organisms and have not been specifically formulated to prevent biofilm formation. The chief concern with the presence of biofilm on lens cases is that it has been associated with microbial keratitis in contact lens wearers.10,11 However, some progress has been made in making the lens case surfaces less attractive to bacteria.
The MicroBlock antimicrobial lens case was introduced into the EU market last year following extensive in-vitro efficacy and cytotoxicity testing that demonstrated significant bactericidal activity against clinical isolates of the most significant ocular pathogens P aeruginosa, and S marcescens, with superior performance to other marketed lens cases.12 The antimicrobial activity of MicroBlock is achieved by a glass powder additive that releases silver ions in the presence of moisture through ion exchange. The additive is incorporated during the injection moulding process and is present throughout the whole thickness of the plastic. It cannot be worn away and is effective on both the inside and outside of the case.
Over the last few years a diverse range of products using silver ion-based antimicrobial surface treatments has appeared in the marketplace, largely driven by consumer demand. These include dishwashers, refrigerators and other appliances, building products, food processing, packaging, and preparation surfaces, heating, ventilating, air conditioning and water filtration and delivery systems, touch screens, ballpoint pens, credit cards and medical appliances.
In the silver ion exchange process, environmental ions such as sodium (present in moisture) are taken up by the silver carrier material in exchange for the release of silver ions, while maintaining a stable overall charge. The non-reactive nature of the exchange enables incorporation into virtually any manufacturing process. With reported contact lens case contamination rates of 20-80 per cent,13 there is as much a need for effective bactericidal treatment of the lens case as for the lens itself - perhaps even more so.
We tested MicroBlock in the field, where it was exposed to a larger variety of bacteria than in laboratory testing to see how it performed against a regular high density polyethylene contact lens case.
Methods and Materials
To demonstrate the degree of effectiveness under various regimens typically adopted by contact lens wearers under real world conditions, two clinical studies were conducted with a primary goal of comparing microbial contamination in the MicroBlock anti-bacterial lens case compared to a standard control lens case after one month of use.
In the first trial, half the subjects emptied and rinsed the MicroBlock lens case with fresh SOLO-care Aqua multipurpose solution and then re-closed the lens case (Regimen 1), while the other half did the same except the lens case was refilled with fresh SOLO-care Aqua and then closed (Regimen 2). The control case was left open to air dry. In the second trial, all the subjects emptied and rinsed out both the MicroBlock and control lens cases with fresh SOLO-care Aqua multipurpose solution and then re-closed both lens cases.
The clinical protocols were developed in accordance with generally accepted guidelines for the investigation of medical devices in human subjects in the United States and complied with the Declaration of Helsinki. Both trials were planned as one-month prospective, randomised, open label, contralateral trials consisting of initial baseline and a final one-month visit and were conducted under Good Clinical Practices.
All trial subjects were selected from the subject populations at two investigational sites - one in Des Plaines, Illinois and the other in Duluth, Georgia. Subjects were enrolled after independent ethics committee protocol approval had been received at the sites and all subjects underwent informed consent procedures. Inclusion criteria specified the subjects to be previous multipurpose solution users (not hydrogen peroxide) and subjects continued with their current lenses. Each visit included visual acuity measurement, symptoms assessment and slit-lamp examination. Additionally, in the first trial, each subject completed a questionnaire regarding current lens case management and antimicrobial lens case concepts.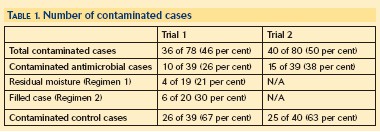
At the end of the study, lens cases were submitted for culturing within a few days of collection (thus minimising attenuating bacterial growth in transit over time). Lens case bowls were swabbed and the swabs plated onto various media including blood agar, Chocolate II agar with IsoVitaleX, and Tryptic Soy Agar (TSA). Recovered microbes were identified using an industry standard bioMrieux Vitek machine. This machine uses cards containing many miniature wells of various substrates (such as fructose and lactose sugars) that a given microbe selectively ferments. The microbe can then be identified according to which substrates were fermented by looking up a database. Gram staining and biochemical assays (such as catalase and oxidase tests) are first conducted to identify the overall class of organism - Gram negative, bacillus etc - and basic sub-category type within these classes, so that the appropriate card for the bioMrieux Vitek machine is selected. Cases were not screened for anaerobic micro-organisms and no quantitative analysis was performed. However, isolated species were identified.
Both trials had an overall sample size of 40 subjects which gave sufficient statistical power for a McNemar's test (with <03B1>=0.05 two-sided significance level) to determine whether the proportion of contaminated versus non-contaminated cases is different between test and control cases.
Results
In both trials the antibacterial lens cases had a statistically significantly lower degree of bacterial contamination than the control lens cases - 26 per cent versus 67 per cent in the first trial and 38 per cent versus 63 per cent in the second (Table 1 and Figure 1). A majority of micro-organisms isolated were members of the normal skin flora, primarily Staphylococcus species. This is consistent with historical literature reports.14 Gram-negative organisms associated with corneal infection were found in the control cases: one Pseudomonas aeruginosa isolate in both trials and two Serratia marcescens isolates in the second trial (Figure 2). Pseudomonas aeruginosa and Serratia marcescens were not found in the MicroBlock case.
Register now to continue reading
Thank you for visiting Optician Online. Register now to access up to 10 news and opinion articles a month.
Register
Already have an account? Sign in here
