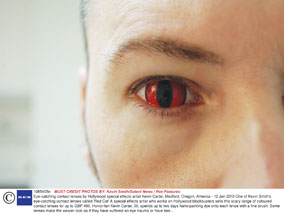
European body Euromcontact is conducting a research project on the potential eye health risks of plano cosmetic contact lenses by testing them against EU standards for product safety.
The European federation of national associations and international manufacturers of contact lenses and care products is encouraging practitioners to buy plano lenses (and the solution supplied with the lenses) from unfamiliar manufacturers and unregulated suppliers and send them for assessment. This is part of its initiative to have the lenses included as medical devices under the EC Medical Devices Directive. The results will be presented to the European Commission in advance of the review of the Medical Devices Directive.
Register now to continue reading
Thank you for visiting Optician Online. Register now to access up to 10 news and opinion articles a month.
Register
Already have an account? Sign in here
