The first article in this series provided an introduction to the topics of blur sensitivity, blur discrimination and blur adaptation (see Optician 09.09.22). The terms blur sensitivity, blur discrimination and blur adaptation were defined, and a short review of scientific research in this area was presented. This, the second article, will consider the mechanisms underlying blur adaptation and then discuss the relevance of blur sensitivity, blur adaptation and blur discrimination to clinical practice. Blur can introduce changes in the visual perception of defocused images, can produce short-term changes in the structure of the eye, and can alter electrical activity in the retina and visual cortex. Research in these areas will be discussed.
The mechanism of blur adaptation
The phenomenon of improved visual performance in the recognition of defocused targets following a period of adaptation to blur is well documented.1 The underlying mechanisms for blur processing have been the subject of much research, with studies of the visual cortex2 and retina3 suggesting they be considered as sites of interest.
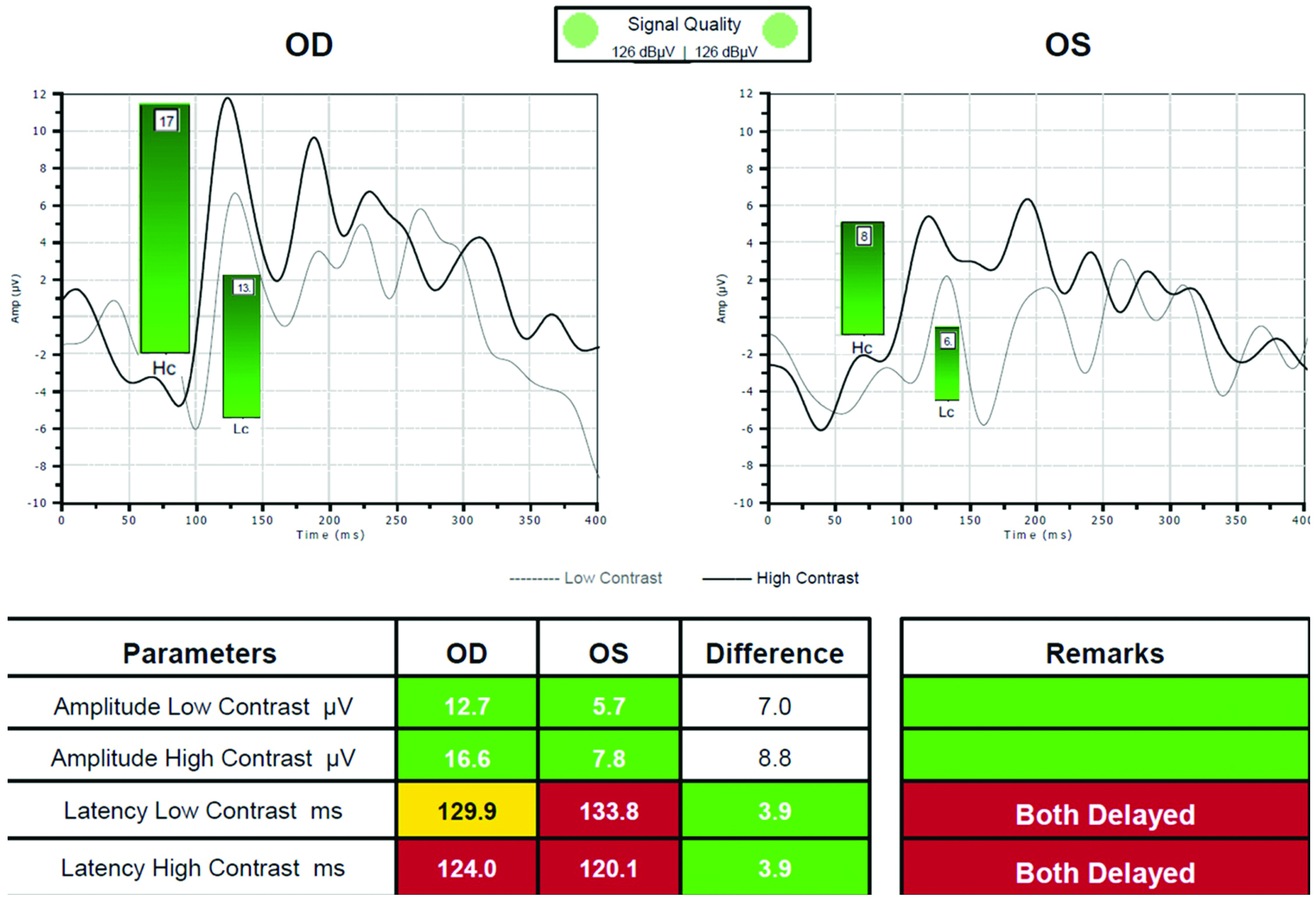
Electrical activity in the visual pathway can be used as a measurement of changes induced by periods of adaptation to blur. Changes in activity within the visual cortex due to blur can be assessed by measurement of the visual evoked potential (VEP), using electrodes placed on the scalp (figure 1).
Figure 1: Visual evoked potential (VEP) results showing delayed transfer for both high and low contrast targets (courtesy of Kirit Patel)
Retinal responses to blur can be measured by assessment of the electroretinogram (ERG) using an electrode placed along the margin of the lower eye lid. The pattern ERG shows a characteristic waveform of electrical activity of the retina consisting of a positive peak at around 50 milliseconds, and a negative trough at around 100 milliseconds (figure 2). The position in time of peaks and troughs in the waveform and the amplitude of the signal can be investigated in relation to different visual stimuli. Exposure to blurred scenes has been shown to change the characteristic pattern of electrical activity within the visual system,4 and to functional magnetic resonance imaging (fMRI) of the visual cortex (figure 3).5 Generalised increases in the latency of electrophysiological signals and reductions in neural activity seen via functional imaging techniques are observed when the retina is exposed to blur.
Figure 2: Electroretinogram (ERG) example. Initial pattern ERG (left side) suggested the retinal ganglion cells at the macula (parvocellular) were under distress and not firing efficiently. The right eye was worst affected, and this was reflected in the VEP plot where there is latency to high contrast stimuli. The later pattern (right side) showed that the macular ganglion cells have bounced back to show a normal response to visual stimuli (courtesy of Kirit Patel)
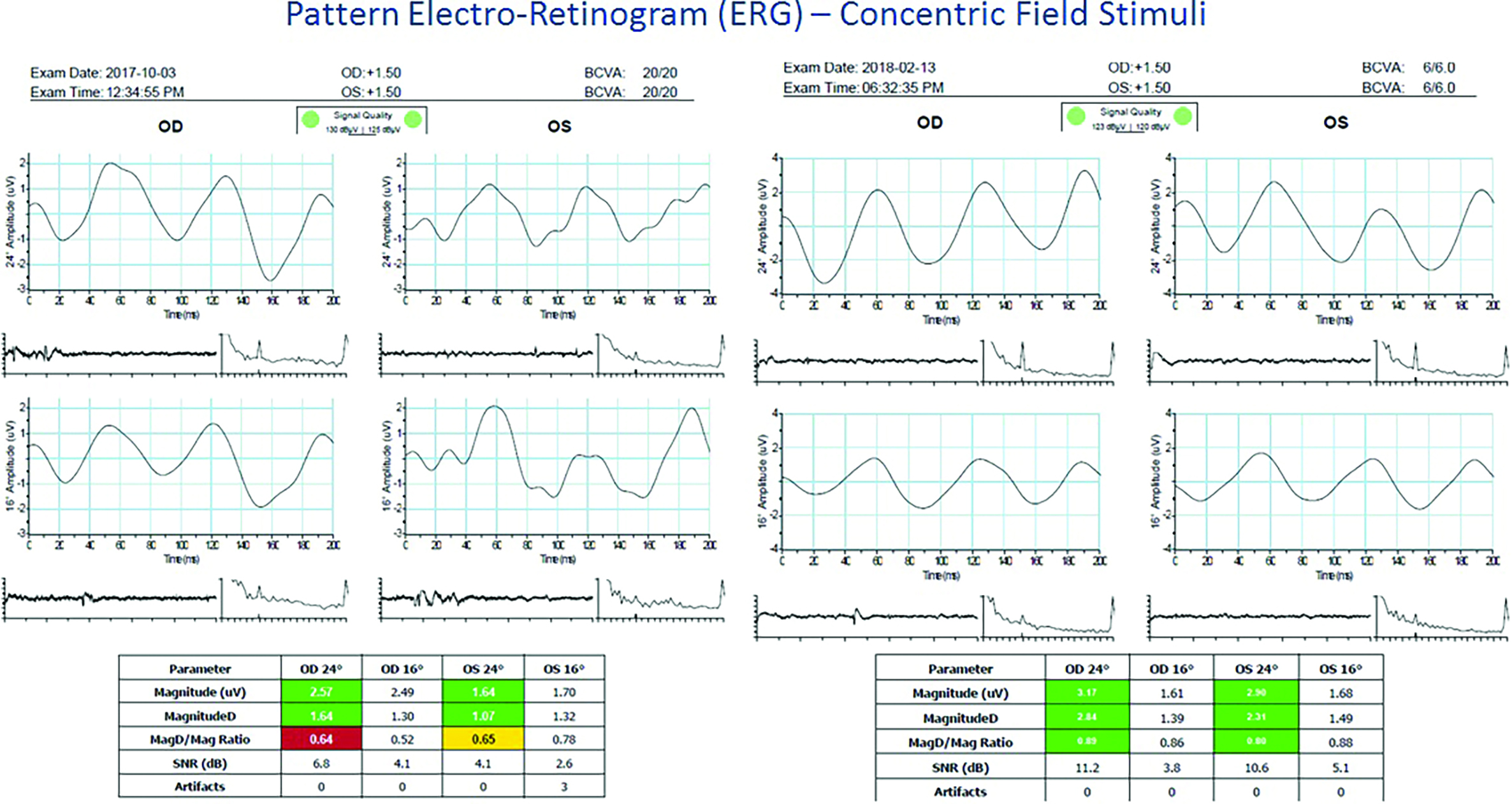
Figure 3: Functional magnetic resonance interferometry (fMRI). Orange area signifies activity

The neurological basis for blur adaptation is thought to lie in the ability of the visual system to change the signal gain to optimise the perception of a visual scene. The human visual system possesses spatial frequency specific channels,6 and it is thought that the signal gain of these channels can be modified by a period of blur adaptation.1, 7 For example, the placing of a low powered positive spherical lens in front of an emmetropic or optimally corrected ametropic eye will reduce the contrast of high spatial frequency information within a visual scene. The gain of channels tuned to high spatial frequencies will undergo adaptation to attempt to optimise the clarity of the scene. Alternatively, the gain of channels responsive to low spatial frequencies could be reduced in order to restore the balance with higher frequency channels. Current thinking is that a mix of these two approaches is utilised in the blur adaptation process.
The location of blur adaptation processes within the visual cortex is supported by research that has demonstrated interocular transfer of adaptive effects, where blur adaptation procedures applied to one eye have been shown to produce improvements in defocused visual acuity in the fellow eye. Kompaniez and colleagues examined the effect of different levels of blur exposure between the two eyes on blur adaptation parameters.8 It was found that adapting stimuli presented to one eye could bring about adaptation in the contralateral eye. When differential blur levels were presented to the eyes, the sharper of the two stimuli dominated the adapting process. Based on their data, the authors suggest that the eyes cannot adapt individually to differential levels of blur.
This finding is consistent with a central location, the visual cortex, for blur adaptation effects within the human visual system.
Contrast sensitivity and blur adaptation
It follows that blur adaptation effects can be apparent in the measurement of contrast sensitivity.9, 10 Rajeev and Metha measured contrast sensitivity under 2.00 DS of myopic defocus before and after a 30-minute blur adaptation period.10 Following adaptation, contrast sensitivity was reduced at low spatial frequencies (0.5 and 1 cycle per degree) and increased at higher spatial frequencies (8 and 12 cycles per degree). It has also been shown that as well as general reductions in the contrast sensitivity function, it is possible to induce localised ‘notches’ in the response. These notches have been found in foveal vision,11 and more recently around 20 degrees into the peripheral visual field.12
The assessment of the effect of a period of blur adaptation on contrast sensitivity needs careful consideration from an experimental design point of view, due to the need for ‘top up’ adaptation sessions. The measurement of contrast sensitivity can be lengthy, and blur adaptation effects can be diluted if top up adaptation to blurred imagery is not included in the experimental design.
Blur and structural changes in the eye
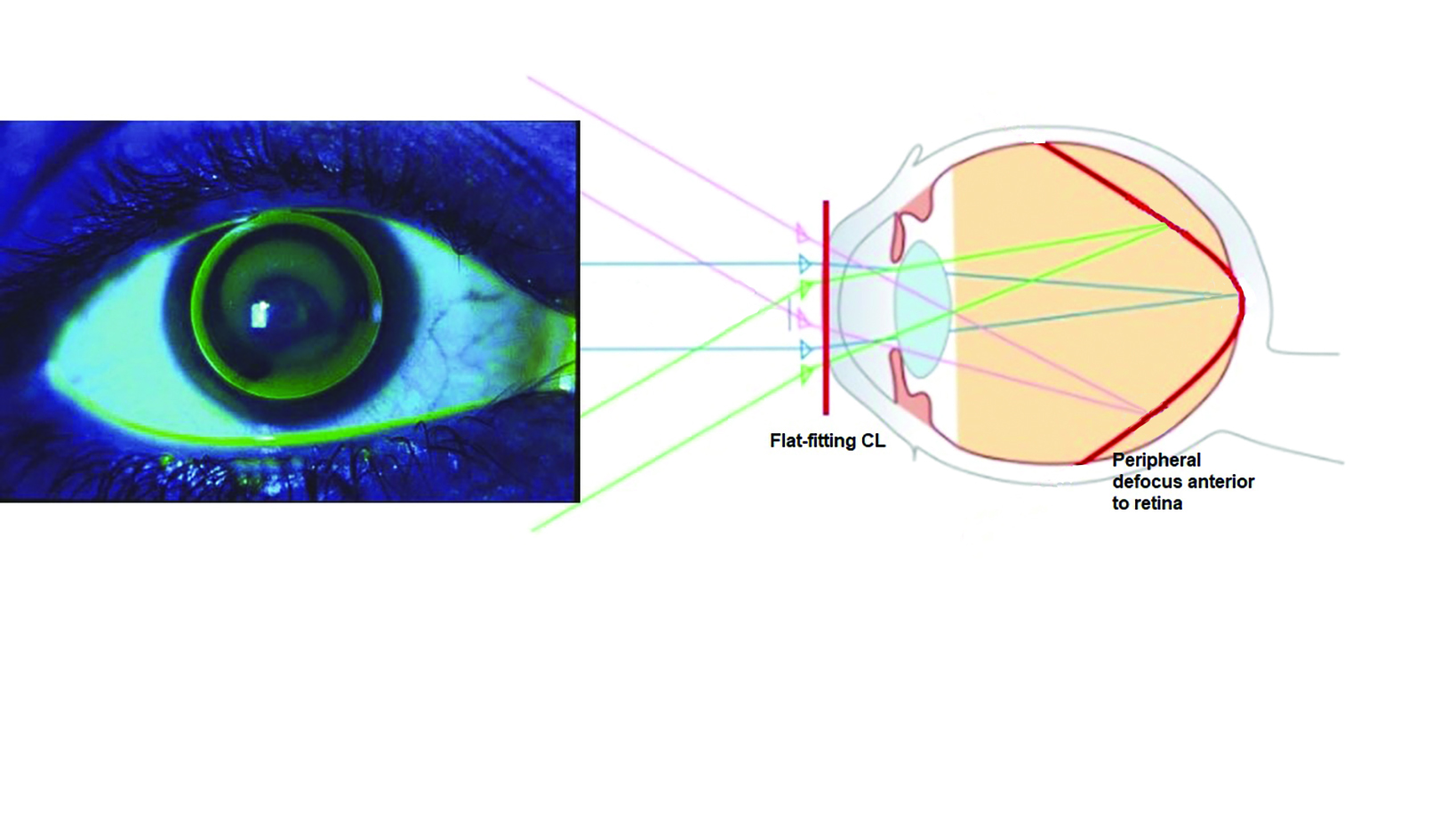
It has been shown that myopic blur can control the growth of the human eye, and thus offer an effective method of controlling myopia. Work in orthokeratology showed that a clear image at the fovea, accompanied by a peripheral image shell in front of (and so myopic with respect to) the retina, produced a slowing in axial eye growth (figure 4).13 Contact lenses14 and spectacle lenses15, 16 utilising a myopic peripheral image shell have also demonstrated the usefulness of retinal blur in myopia control.
Figure 4: Myopic peripheral retinal image blur in orthokeratology
Research studies have shown that the human eye can respond structurally to imposed blur over a relatively short timescale. Delshad and colleagues measured axial eye length, using a low coherence optical biometer and choroidal thickness by OCT, to assess the effects of defocusing lenses on eye structure.17 It was found that the axial length and choroidal thickness could respond to the imposed defocus; this being achieved using +3.00 DS and -3.00 DS spherical lenses.
Imposition of hypermetropic defocus, using the -3.00 DS lens condition, caused a seven micron increase in the axial length of the eye and a four micron thinning of the choroid. In the myopic defocus condition (+3.00 DS lenses being used to achieve this), the axial length shortened by eight microns and the choroid increased in thickness by eight microns. This data demonstrates the ability of the eye to respond to blur and to move the retina in an appropriate direction to reduce the level of defocus, at least in the short term. The mechanism by which these short-term responses to retinal blur may translate into effective myopia management remains unclear at present.
There is considerable interest in myopia management in terms of safety and efficacy of interventions. This was clearly evident at the recent International Myopia Conference held in Rotterdam, with more than 700 delegates from over 40 counties present. The methods of myopia management that utilise retinal blur as an axial eye length growth rate controller are demonstrating effectiveness, as shown by Chamberlain and colleagues for contact lenses18 and Bao and colleagues for spectacle lenses.16
Many research questions remain, however, and the coming years should see a drive towards answering questions around the exact mechanisms by which interventions achieve their myopia controlling effect, identification of patients likely to benefit from myopia management interventions, and methods to optimise the myopia controlling effects for individual patients.
The ability of a patient to tolerate blur in the retinal image are of obvious importance when selecting patients for myopia management treatments. Research evidence to date, conducted largely on children, shows a good level of tolerance to myopia management spectacles and contact lenses. Further work is now required to test tolerance levels in adults, since myopia management interventions may also be beneficial to adults developing myopia or those undergoing further progression following a period of stable myopia. Research studies on the effect of blur on the structure of the eye and on the performance of the visual system are highly relevant to the clinical control of myopia. As clinicians, we need to be aware of the potential changes to visual performance parameters and structural metrics that may be produced alongside myopia management. It may be the case that these short-term changes in the eye could be useful in determining whether a patient will respond to myopia management strategies; much more research in this area is needed, and there are certainly interesting times ahead.
Blur adaptation and refraction
We have all, no doubt, encountered the patient who, during the subjective refraction process, gives answers to our questions that demonstrate poor repeatability. This can manifest itself, for example, as an inability to notice the blur induced by additional positive sphere power over and above the final refraction, or the giving of conflicting responses during the crossed-cylinder test. Such patients may have poor blur sensitivity and blur discrimination.
Recognising that a patient has poor blur detection ability is an important clinical finding, and may be useful to the clinician when making spectacle prescribing decisions.
Another important clinical ramification of blur sensitivity and blur adaptation is the patient collecting their first myopic correction. A clinical scenario might be as follows.
Case Example
A 22-year-old patient with unremarkable ocular history and no previous spectacle or contact lens correct attends for a routine eye examination and claims to have always had good vision until recently. They are now undertaking much more close work in their job and have noticed that their distance vision is not quite so good.
On examination, the following is found:
Unaided vision (logMAR):
- R 0.18
- L 0.20
Subjective refraction:
- R -1.00 DS (-0.10)
- L -1.00 / -0.25 x 180 (-0.12)
They are dispensed with spectacles for distance vision and are advised to wear them for driving and ‘as required’ for other activities. The patient returns after two months claiming that their vision without spectacles is now much worse and they have difficulty coping without the spectacles.
On re-examination, the refraction is unchanged but unaided visions are R 0.32 and L 0.34; roughly one-and-a-half lines worse than the unaided vision found at the initial examination. The unaided visions found at re-examination are more in keeping with what might be expected, given the degree of myopia. The unaided vision recorded at the initial examination is better than what might have been expected and is probably due to some degree of blur adaptation.
Given the research evidence on blur adaptation, it may be useful to discuss this with the patient when prescribing their first myopic correction, as this may help to manage the expectations of the patient and to prepare them for the likely change in perception of their unaided vision.
Technological advances in refraction
We have seen that the blur perception processes in play within the human visual system are amenable to adaptation. As a consequence, the responses that patients may give to criterion-dependent questions asked in the refraction procedure can change under different visual conditions.
Recent advances in the armoury of clinical refraction equipment include refraction devices that gather data on patient responses throughout the refraction process (figure 5). This may allow the device to check the repeatability of patient responses, perhaps yielding useful clinical data along similar lines to false positive and false negative metrics from a visual field test.
Figure 5: The Essilor Vision-R 800, an example of a semi-automated refraction system capable of adapting the correcting lenses to patient response reliability
At the end of the refraction process, the response that the patient gives to that final presentation of +0.25 DS can be crucial. With a good observer, the clinician can have confidence on whether to prescribe the additional +0.25 DS or not. With a poor observer in the chair, this becomes much more problematic. If your refraction system can gather data on the quality of answers that the patient gives to the many questions that we ask during subjective refraction, this could help us in our clinical decision-making.
Refraction devices are becoming available that have the facility to monitor the repeatability of a patient’s responses to questions asked during subjective refraction. As this technology develops, and when combined with advances in objective refraction techniques, the clinical confidence that clinicians have in their refraction results for the ‘uncertain’ patient should increase. It may be the case that such systems can provide a measure of a patient’s sensitivity to blur and their ability to tolerate and adapt to blur. This aspect may help eye care professionals in the selection of spectacle and contact lenses, and could help to reduce cases of non-tolerance of a vision correction appliance.
Blur adaptation and ocular pathology
Can central vision degradation resulting from central vision loss alter the blur adaptation process? Vera-Diaz and colleagues examined blur adaptation in 12 patients with central vision loss.19 The patients’ vision loss was caused by a range of pathologies, including dry and wet AMD, myopic degeneration and juvenile macular degeneration. Visual acuity of the participants ranged from 0.48 to 1.20 logMAR. For the blur adaption task, the participants used their preferred retinal locus to view adapting and test images. The preferred retinal locus ranged from 1.9 to 19.9 degrees in eccentricity from the fovea.
The psychophysical testing paradigm was similar to the procedure used by Webster and colleagues;1 an adapting image of a human face that was artificially blurred or sharpened was viewed for 30 seconds and then a test image (the same human face with a different level of blur processing applied to it) was viewed for 500 milliseconds. The participant was asked to judge whether the test image was ‘too blurry’ or ‘too sharp’. There were around 100 test image exposures per adapting image. This process was also conducted parafoveally in a smaller group of visually normal participants for comparison purposes.
The study found that adaptation to blur was measured reliably in patients with central vision loss, with similar patterns of adaptation being found in the visually normal participants. The study concluded that it was important to consider the blur adaptation processes that are in play in the visual systems of low vision patients when prescribing visual aids. Adaptation effects may have implications for the optimisation of vision rehabilitation systems that utilise image enhancement processing, and may influence the subjective tolerance of such systems (figure 6).
Figure 6: The success of image enhancement and augmentation systems, such as the eSight3 shown here, may be influenced by blur adaptation

Final thoughts
In summary, in these articles we have seen that the response of the human visual system to blur can be assessed in a number of ways. The visual system can adapt to blur, and subsequent responses can be modified by such adaptation. From a structural viewpoint, the eye can change in length and the choroid can alter in thickness in response to imposed defocus. Research around blur sensitivity, blur discrimination, blur adaptation, and structural responses to blur are of relevance to the expanding area of myopia management.
It is the view of the author that fundamental aspects of vision science should be considered alongside clinical studies of myopia management efficacy in order to identify patients that will respond to myopia management interventions, to maximise efficacy of treatment, and to help to ensure safe practice. Blur adaptation processes appear to remain intact in patients suffering from central vision loss of retinal origin, which shows that these processes may be relevant when prescribing for low vision patients.
- Edward Mallen is Professor of Physiological Optics at the University of Bradford and Past President of the College of Optometrists. His research interests are in structural and functional aspects of myopia, neural adaptation to blur, ocular biometry and adaptive optics.
References
- Webster MA et al. Neural adjustments to image blur. Nature Neuroscience, 2002, 5, 839-40
- Zeri F et al. Immediate cortical adaptation in visual and non-visual areas functions induced by monovision. Journal of Physiology, 2018, 596: 253-266
- Panorgias A et al. Retinal responses to simulated optical blur using a novel dead leaves ERG stimulus. Investigative Ophthalmology and Vision Science, 2021, Aug 2;62(10):1
- Sokol S. and Moskowitz A. Effect of retinal blur on the peak latency of the pattern evoked potential. Vision research, 1981, 21(8), pp.1279-1286
- Mirzajani A et al. Effect of lens-induced myopia on visual cortex activity: a functional MR imaging study. American Journal of Neuroradiology, 2011, 32(8), pp.1426-1429
- Blakemore C et al. On the existence of neurones in the human visual system selectively sensitive to the orientation and size of retinal images. The Journal of Physiology, 1969, Jul 1;203(1):237-60
- Georgeson MA, Sullivan GD. Contrast constancy: deblurring in human vision by spatial frequency channels. The Journal of Physiology, 1975 Nov 1;252(3):627-56
- Kompaniez E et al. Adaptation to interocular differences in blur. Journal of Vision, 2013 May 31;13(6):19
- Ohlendorf A, Schaeffel F. Contrast adaptation induced by defocus – a possible error signal for emmetropization? Vision Research, 2009 Jan;49(2):249-56
- Rajeev N, Metha A. Enhanced contrast sensitivity confirms active compensation in blur adaptation. Investigative Ophthalmology and Vision Science, 2010 Feb;51(2):1242-6
- Guo H, Atchison DA, Birt BJ. Changes in through-focus spatial visual performance with adaptive optics correction of monochromatic aberrations. Vision Research, 2008 Aug 1;48(17):1804-11
- Jaisankar D et al. Defocused contrast sensitivity function in peripheral vision. Ophthalmic and Physiological Optics, 2022 Mar;42(2):384-92
- Cho P, Cheung SW. Retardation of myopia in orthokeratology (ROMIO) study: a 2-year randomized clinical trial. Investigative Ophthalmology and Vision Science, 2012 Oct 11;53(11):7077-85
- Anstice NS, Phillips JR. Effect of dual-focus soft contact lens wear on axial myopia progression in children. Ophthalmology, 2011 Jun;118(6):1152-61
- Lam CSY et al. Defocus Incorporated Multiple Segments (DIMS) spectacle lenses slow myopia progression: a 2-year randomised clinical trial. British Journal of Ophthalmology, 2020 Mar;104(3):363-368
- Bao J et al. One-year myopia control efficacy of spectacle lenses with aspherical lenslets. British Journal of Ophthalmology, 2022 Aug;106(8):1171-1176
- Delshad S et al. The human axial length and choroidal thickness responses to continuous and alternating episodes of myopic and hyperopic blur. PLoS One, 2020 Dec 2;15(12):e0243076
- Chamberlain P et al. Long-term effect of dual-focus contact lenses on myopia progression in children: A 6-year multicenter clinical trial. Optometry and Visiown Science, 2022 Mar 1;99(3):204-212
- Vera-Diaz FA et al. Blur adaptation to central retinal disease. Investigative Ophthalmology and Vision Science, 2017;58(9):3646-3655
