Background
Age-related macular degeneration (AMD) is the most common cause of registrable sight impairment in the UK, accounting for approximately half of all registrations. As the name suggests, the disease presents as a degeneration of the central retinal tissue leading to distortion and loss of central vision.
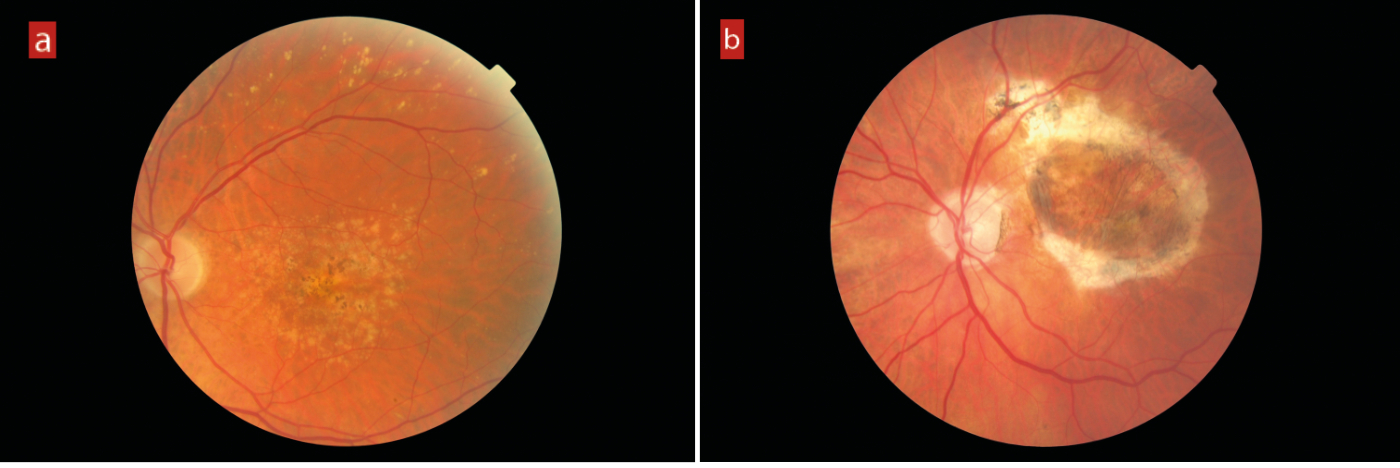
AMD is usually classified as either dry or wet. Dry AMD, which represents around 80% of cases, results from progressive atrophy of retinal tissue around the macular area, often initially indicated by the presence of drusen beneath the retina. Localised atrophy is seen as areas of hypopigmentation with which clumps of pigmentation may be associated. Extension of atrophic areas eventually leads to late-stage appearance described as geographic atrophy (figure 1).
Figure 1: (a) Early AMD showing extensive drusen, localised hypopigmented atrophy and areas of hyperpigmentation. (b) Geographic atrophy in a severely sight impaired patient
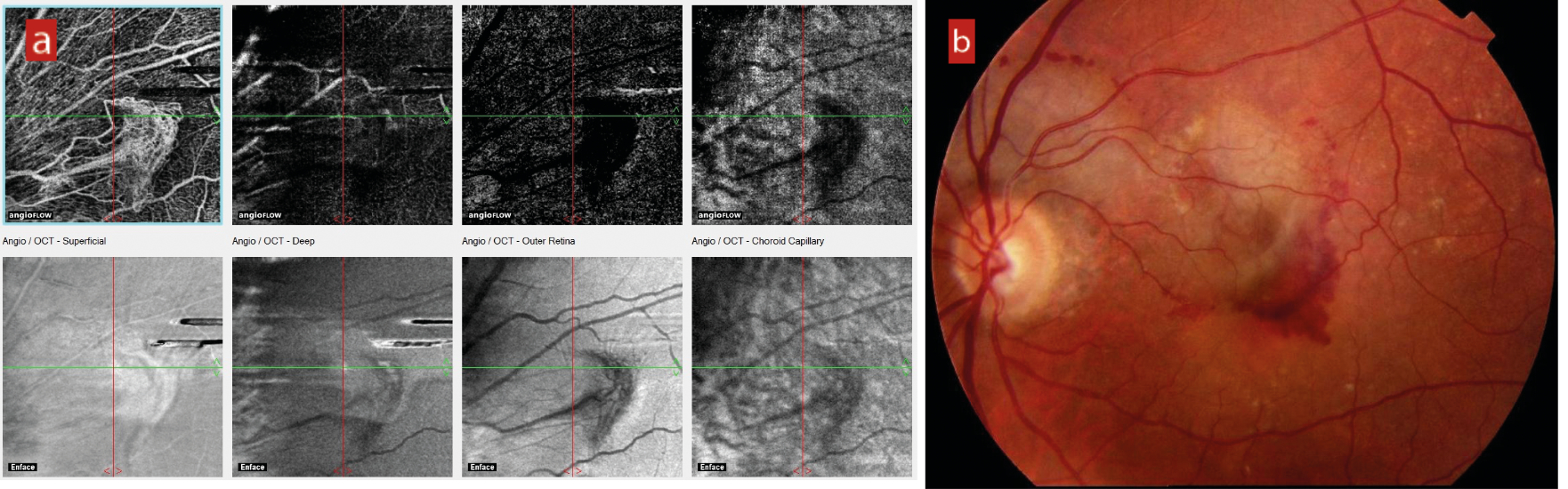
In around 20% of cases, serous fluid elevation of the retina from the underlying vascular supply causes ischaemia and a build-up of vascular endothelial growth factor (VEGF). Without prompt intervention with anti-VEGF injection, choroidal neovascularisation follows and inevitably leads to haemorrhaging and a rapid and profound loss of central acuity (figure 2).
While the increasing use of OCT in primary care practice and prompt referral for anti-angiogenic treatment has significantly reduced the level of sight loss due to wet AMD, it is still important to remember that, even with treatment, most wet AMD patients suffer some sight loss while the slow, progressive sight loss of dry AMD remains resistant to attempts at treatment. As such, with increasing life-expectancy, AMD represents a major cause of sight loss and economic cost to society, independent from the high cost of anti-angiogenic treatment itself. Loss of central vision is strongly associated with depression, falls and loss of independence, all of which represent a significant burden upon health care costs.
Figure 2: (a) Choroidal neovascularisation (CNV) seen with OCT-angiography. (b) Haemorrhaging subsequent to CNV
AMD is a multifactorial degenerative condition. The reasons for the photoreceptor damage and loss are still a matter for debate and on-going research, but most authorities suggest a combination of inflammatory responses, involve Bruch’s membrane changes, vascular insufficiency, genetics and oxidative stress.
As understanding of the nature of AMD improves, the need to identify reliable biomarkers for identification and assessment along with better awareness of risk factors for the disease and its progression is increasingly important in the absence of cheap and effective treatments. After a brief discussion of the epidemiology of AMD, this first article will focus on the risk factors of the disease, either modifiable or non-modifiable.
Prevalence
While it is estimated that about 80% of people with AMD have non-neovascular or atrophic AMD, wet AMD is responsible for 90% of the severe visual acuity loss.1 Approximately 67 million people in Europe are currently affected by any AMD and, due to ageing population, this number is expected to increase by 15% by 2050. For any late AMD, prevalence is expected to increase by 20% to 12 million in the same time period.2 Worldwide, the number of people with AMD is predicted to increase from 196 to 288 million by 2040.3
In the UK, around a quarter of a million older adults in the UK have severe visual impairment or blindness due to advanced AMD.4 Roughly half of advanced AMD is due to neovascular AMD and about half is due to geographic atrophy.5 The prevalence of neovascular AMD is 1.2 to 6.3%, while the prevalence of geographic atrophy is 1.3 to 6.7%.6 AMD is more prevalent than glaucoma and diabetic retinopathy combined.7 With the prevalence of AMD being greater than many systemic diseases, it is also worth remembering the significant impact AMD can have on quality of life.8
AMD patients report that the condition has a significant impact upon their daily activities, particularly in low light conditions.9 With the examination methods most commonly used at present, many believe that ophthalmologists and optometrists are underdiagnosing AMD, potentially missing almost 25% of patients with already showing clinically significant signs.10 This will be discussed further in part 2 of this series.
There are a great many influences upon the expression and progression of AMD. Some key ones are summarised in table 1. A good number of these are modifiable as indicated and will be the main focus of the remainder of this article.11
Table 1: Some important risk factors for AMD and their modifiability11
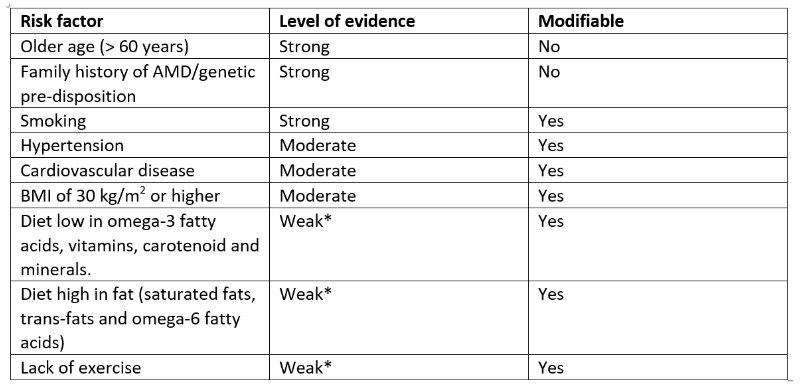
*Although a higher level of evidence is currently lacking, it may be nonetheless prudent for clinicians to
advise patients of the potential risk of AMD associated with lifestyle factors, including diet and a lack of exercise.
Non-modifiable risk factors
Age
Age is the strongest demographic risk factor associated with age-related macular degeneration.12 The older we are, the greater our risk of developing the condition. Around one in every 200 people has AMD at 60. However, by the age of 90 it affects one person in five. More specifically, the prevalence of late AMD in the UK among people aged 50 years or over is 2.4%. This increases to 4.8% in people aged 65 years or over, and 12.2% in people aged 80 years or over.6 The prevalence in people aged 75 to 85 years is over three times that of people aged 43 to 54 years.12
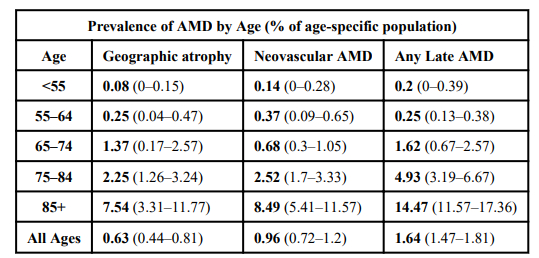
On average, we are living longer, so the number of people affected is increasing. Due to the time-dependent mechanisms of AMD, specifically the formation of drusen and choroidal neovascularization, age will likely remain the leading risk factor for the development of age-related macular degeneration. Table 2 combines data from four different studies estimating the prevalence of age-related macular degeneration by age group.13
Table 2: Pooled data on the relationship between age and prevalence of age-related macular degeneration. The single bold number represents the % of the population, in the given age range, with AMD; while the range of numbers within parentheses describes the ranges reported by the various studies
Ethnicity and gender
AMD is slightly more common in women than in men and may be less common in people with non-European ancestry compared with people with European ancestry.5 After accounting for age differences in different countries, the ethnic origin of the populations also has an impact and, within any multicultural population, variation in AMD prevalence remains between different ethnicities. AMD prevalence has been estimated at 12.3% in people with European ancestry, versus 10.4% (Hispanic), 7.5% (African) and 7.4% (Asian).3
Europeans have substantially higher prevalence of early AMD, but only slightly higher prevalence of late AMD. This suggests that Europeans are partially protected from progression from early to late disease, unlike those of Asian or African ancestry who are more likely to show disease characterised by a more direct progression to late AMD. Notably, beyond Europe, a shift in global patterns of AMD is under way. Asia is predicted to be the home for over half of the world’s late AMD cases in the future and, therefore, AMD can no longer be considered primarily a European disease.3
Family history and genetics
Research shows us that, when a parent has AMD, the increased odds that their offspring will develop the disease over their lifespan is 28 times, while the odds are 12 times greater when a sibling is affected.14 These odds ratios are even higher when factors such as age and smoking are included in the risk calculation.
AMD has numerous genetic links, most notably the CFH and ARMS2 genes. Research is continuing, showing future potential for using genetic testing to improve early diagnosis in those likely to develop and be at greater risk of progression to late AMD, as well as in helping to differentiate those patients that may or may not benefit from nutritional supplement intervention.
The use of genetic testing for AMD in research is well established. However, in a clinical setting, it is not currently advocated for aiding patient recommendations by a number of professional bodies and many researchers.15-18 Despite this, some recent research suggests that certain specific genetic combinations respond better to nutritional intervention than others.19
Once the exact role of genetic testing for eye care is established, incorporating it into clinical practice will offer an additional benefit in the UK as we enter an era of customised patient care. It should improve our understanding of the likelihood of a patient developing AMD and potentially help to pinpoint which supplements might offer the greatest benefit. Such testing could either be offered through NHS care pathways or as a private lab test offered by the practice.
In conclusion, as more than 80% of patients with late-stage AMD have a genetic predisposition, their first-degree relatives, particularly siblings, should have regular routine eye examinations.
Modifiable risk factors
The most commonly cited modifiable risk factors for AMD in eye care practice are smoking, poor diet and exposure to ultraviolet (UV) and short wavelength light. Each of these will be considered in turn.
Smoking
Several epidemiological studies across the world have demonstrated how smoking is a major modifiable risk factor. It is frequently associated with more severe forms of AMD,20-23 and increases the likelihood of both eyes being affected.24
Current smokers have a two to four-fold increase in risk of developing AMD when compared to patients that never smoked.25-27 Current and former smokers were found to have an increased prevalence of late AMD, although a more recent study also found an association of early AMD features with smoking.28 Levels of smoking are classified as pack-years. The number of pack-years smoked better reflects the amount of exposure over a lifetime of smoking. To calculate pack-years of smoking, the average number of cigarettes smoked per day is divided by 20 to give packs per day and multiplied by the total number of years of smoking. The increased AMD risk appears to be higher in those patients who have smoked 20 pack-years and more.23, 29 There is bad news for people living with smokers too: passive smoking; living with a smoker for five years or more, increases the risk for AMD among non-smokers.25, 30
Cigarette smoke promotes molecular and pathological changes that may establish the ideal macular microenvironment for the development of AMD23 and has been linked to cellular changes in all retinal layers,22 particularly the retinal pigment epithelium (RPE).23, 31 These cellular changes involve a variety of mechanisms, including vascular inflammation and endothelial dysregulation,23, 32 oxidative damage,33-35 toxic damage, and histopathological changes.23, 36
Cigarette smoke also induces ‘pro-inflammatory’ changes in the RPE, through an important part of the immune system: increased expression of complement activation products with reduced expression of complement regulators.37 Cigarette smoke contains a large number of pro-oxidant compounds that increase oxidative stress, resulting in damage to the RPE and the alterations in the metabolic support of the RPE, ultimately causing apoptosis of the photoreceptors.38 Oxidative stress is also thought to be pivotal in lipofuscin and drusen formation.23 Examples of pro-oxidant compounds in cigarette smoke include nicotine and cadmium, though the most abundant is hydroquinone, which is also found in processed foods, plastic containers and atmospheric pollutants.39
Nicotine, specifically, has been found to cause vasoconstriction and is thought to impair choroidal blood flow.32 Hydroquinone is thought to be a key factor in the pathogenesis of dry AMD as well in the development of choroidal neovascularisation.35 Cigarette smoke can also cause toxic damage of the mitochondrial DNA within the RPE cell, contributing to the formation of drusen in individuals who are cigarette smokers.23
Although electronic cigarettes deliver lower levels of carcinogens than do conventional cigarettes, and therefore may pose less cancer risk to users (albeit not zero cancer risk), they still expose users to ultrafine particles and other toxins that may substantially increase cardiovascular and non-cancer lung disease risk.
There is conclusive evidence that, in addition to nicotine, most electronic cigarettes contain and emit numerous potentially toxic substances, although it emits fewer toxicants than combustible tobacco products. For a further review, see Optician 21.06.19 - 'E-cigarettes: a guide for eye care practitioners'.
Diet and AMD
Eye care professionals (ECPs) should consider advice relating to any modifiable risk factors plus the potential merit of modifying their diet and/or taking some form of antioxidant supplementation. A recent review of nutrition and lifestyle, relating to the risk of AMD progression show beneficial effects in dietary patterns rich in green leafy vegetables and other sources of zeaxanthin and lutein, as well as omega-3 from oily fish.40 If this is not achievable through diet alone, it reasonable to recommend dietary supplements that include these nutrients, with special consideration given to the form and dosage of omega-3 recommendation.
In the original Age-Related Eye Disease Study (AREDS)41 and in AREDS 2,42 participants who benefitted from antioxidant vitamin and mineral supplementation were those who had either intermediate AMD or advanced AMD in one eye. The rate of development of advanced AMD at five years was reduced by 25% in the participants using the combination treatment of high dose antioxidant vitamins with zinc and copper.
There is no evidence from the AREDS results to support the use of antioxidant vitamin and mineral supplements for patients who have less than intermediate AMD, an important finding often overlooked when professionals refer to this source of evidence in their daily practice.
The AREDS 2 study results demonstrated that, in patients at high risk for progression, there was no statistically significant difference associated with supplementation with the original AREDS formula versus each of the other modifications on AMD progression. Interestingly, secondary analysis pointed to lutein and zeaxanthin playing a role for reducing the risk of progression to advanced AMD when given without beta carotene and may represent an appropriate substitute for beta-carotene.43 In the same analysis, patients with very low dietary intake of lutein plus zeaxanthin experienced a further 20% reduction in AMD risk by taking the AREDS2 formulation. Finally, there was no significant effect of lowering the zinc levels (25mg) on the reported risk of progression to advanced disease.43
However, it needs to be kept in mind that the AREDS 2 formula is a deliberately high dose, where many of the components are greatly in excess of the Nutrient Reference Value (NRV). When considering long-term supplementation, some people may have reason to avoid one or more of the supplements evaluated in the original AREDS or AREDS2.
Of particular interest is the dosage of vitamin E. Vitamin E is a fat-soluble vitamin, possessing antioxidant properties that protects the cell membrane from free radical damage. The levels of vitamin E in particular are controversial to some as the dosage found in AREDS formulations (400 IU) are significantly higher than the NRV (17.9 IU). The SELECT study demonstrated a 17% relative increased risk of developing prostate cancer in otherwise healthy men using vitamin E at higher dosages.44 The SELECT study findings have been applied to AREDS patients and did not demonstrate a harmful effect of vitamin E on incidence of prostate cancer, due possibly to the interaction with other supplementation found in the AREDS formulations. Discussion between the ECP and the patient of the potential adverse effects of high doses of antioxidant vitamins and minerals recommended by AREDS2, including an increased risk of genitourinary conditions, is warranted and may benefit from additional review by the patient’s GP.41
In summary, there is strong evidence for the use of an AREDS2 formula antioxidant vitamin and mineral supplements for patients who have intermediate AMD and are at risk of progression. Also, discussion of lifestyle modification is important and a lower zinc dose than that in the AREDS2 formulation could be considered.45
For patients with a few drusen, so not yet showing a high risk of progress, specific studies on how the risk of progression in mild AMD is influenced by diet and lifestyle are few, but several population studies show some benefit for overall risk.
On a more general note, the link between AMD and dietary habits is well established. More recently, the impact of processed, nutrient-deficient food gradually displacing whole, unprocessed, nutrient dense food in recent decades, upon the incidence of AMD has become apparent.46 This is all the more concerning as studies based in the US suggest that 63% of the food consumed there is processed, with just 12% from plant sources and 25% from animal sources.47
Another important consideration. Since the elderly make up a significant proportion of our patient base, it is important to recognise that ageing impacts the gastrointestinal (GI) tract.
This has a knock-on effect on the pro-inflammatory mechanism influencing nutrients reaching the retina. As one ages, a variety of processes, including reduced stomach hydrochloric acid secretion and a loss of the beneficial bifidobacteria in the gut occur.45 This, in turn, diminishes the ability to breakdown and absorb nutrients.
Obesity
Obesity accounted for over a million hospital admissions in the UK last year and represents a major risk factor for various systemic diseases,48 in addition to being a risk factor for AMD49, 50 by virtue of its association with pro-inflammatory processes.51
Abdominal obesity is a key risk factor for AMD risk with waist-hip ration (WHR) being a more reliable indicator than body mass index (BMI).52 A higher WHR is associated with a greater risk of AMD progression.53, 54 To further strengthen the argument, a reduction in WHR is associated with reduced odds of AMD
development.55
Adipose tissue is a storage site for carotenoids, in particular abdominal adipose tissue.56 The implication is, therefore, that the greater the abdominal obesity, the fewer carotenoids will be available to supply the macula.57 Of particular interest, it would seem that those with a high BMI (>25) had less lutein reaching the retina than those with lower BMI.58
In summary, obesity is a strong risk factor for AMD. Lutein is stored in adipose tissue and this can limit the efficacy of ingested lutein, whether sourced through diet or supplements.
UV and blue light
There seems to be a widespread misapprehension that elderly phakic patients should be advised about UV protection to maintain macular health. While it was certainly the case in the days of aphakia for macula exposure to UV to be implicated in maculopathic progression and so warranting UV filters in any correction, the poor penetration of shorter wavelengths, along with the increasing brunescence of the crystalline lens with age (figure 4), means that phakic older people do not run any increased risk of UV or shorter wavelength phototoxic damage at the macula. Obviously, UV protection is essential to minimise cataract progression and the risk of adnexal and ocular surface lesions, such as basal cell carcinoma, pterygia and pinguecula.
Figure 4: Brunescence of the ageing crystalline lens protects the macula from short wavelength light damage
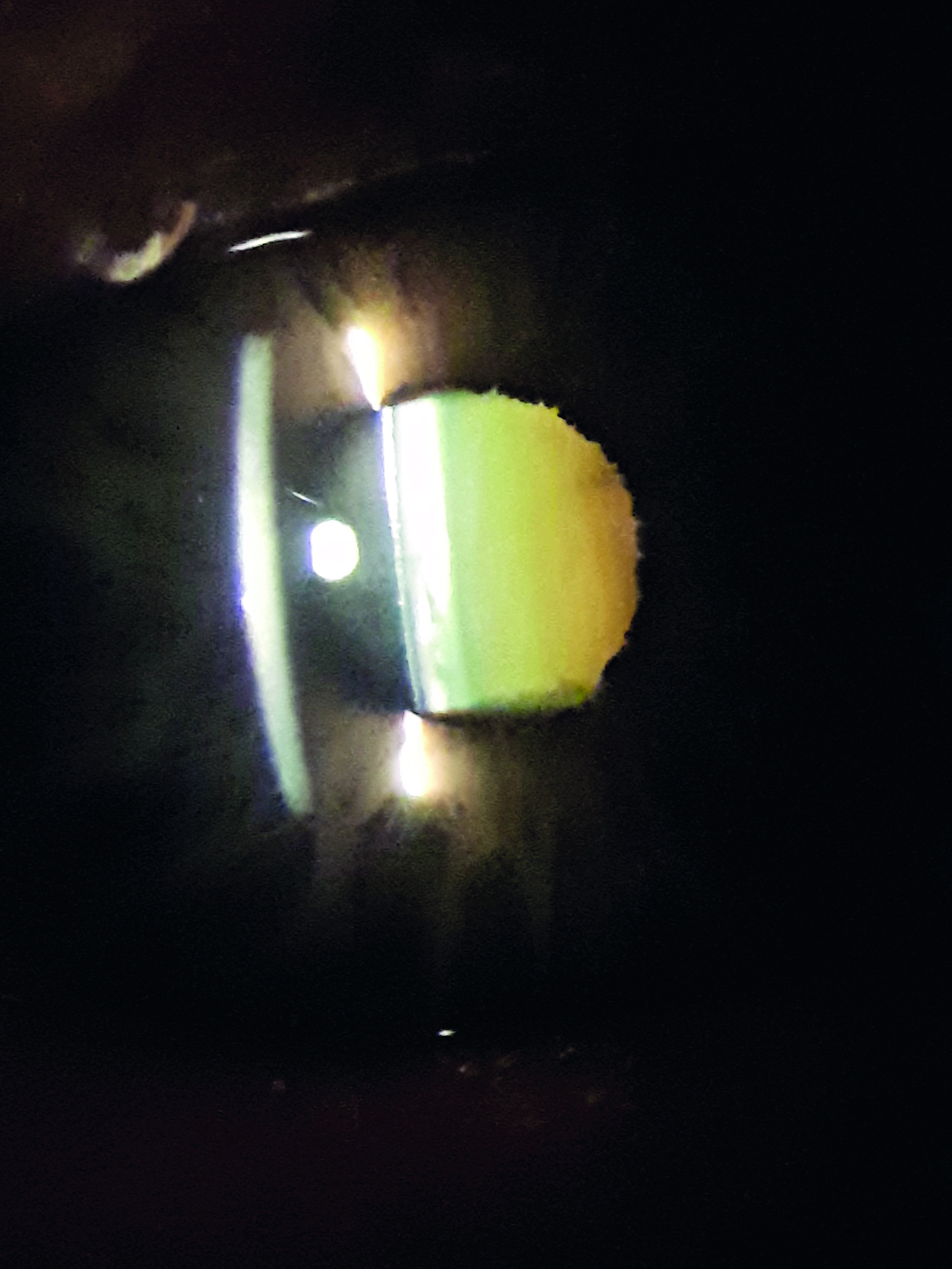
That said, studies have suggested that, while there appeared to be no association between current sun exposure and the development of AMD, a significant association was found between sun exposure (over eight hours daily) in younger life and both early AMD and late AMD.59 This lends some support to the suggestion that the people whose macula health might most benefit from UV and short visible light protection are those with clear crystalline lenses, larger pupils and long hours outdoors; in other words, the young.
Owing to the complexity in quantifying sun exposure differences among people living in the same location, some have criticised the many studies that reported an association between sunlight exposure and AMD.60
Finally, while modern intraocular lenses (IOLs) are designed to protect the macula of a pseudophake from UV exposure and potential phototoxicity, there is still a paucity of evidence for any such benefit from IOLs that block blue light.61
Clinical communication
The importance of effective clinical communication is key to patient engagement and, with regard to educating patients about possible ways of limiting the progression of a common and, as yet, untreatable eye disease, an essential part of eye care practice.
The majority of patients are comfortable, and have the expectation, of discussing diet and smoking status during their eye exam.62 Sadly, a significant percentage of AMD patients express dissatisfaction with their clinicians largely around a lack of education regarding nutrition and lifestyle choices.63
Most clinicians ask about smoking status. However, follow-up may be lacking, losing the opportunity to discuss the importance of smoking cessation or sign posting patients appropriately. This has the potential to leave more than 60% of patients feeling that their clinician had not been given adequate advice about AMD, often feeling a general lack of support.64 Most worrying for the profession is the risk that any clinical recommendation may be diluted by the public perception of optometry prioritising the retail business needs over their healthcare role, potentially degrading the profession’s credibility and the people’s trust in future optometrist recommendations.
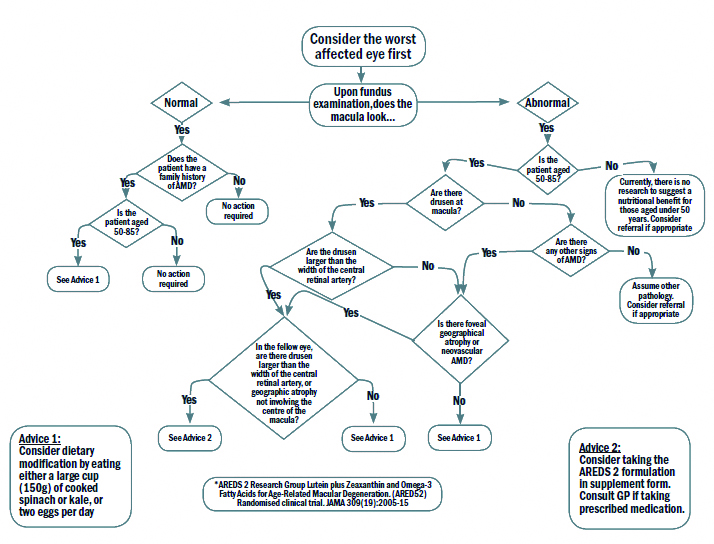
There are a variety of options for the clinician to gain confidence when managing a patient with AMD, one such is the ‘Nutrition advice for people with, or at risk of AMD clinical decision-making tool,’ developed at Aston University65 and previously reported on in Optician 17.02.17. This decision tree (figure 5) can form a valuable aid in conjunction with discussion around modifying risk factors where applicable.
Figure 5: The clinical decision making flowchart65
The use of educational material can help initiate and guide the discussion. The patient’s understanding of the advice given is important and knowing that changing their diet can slow the progression of their AMD allows patients to feel more supported and also better involved in their own health.
Conclusion
As there is currently no cure for AMD, the importance of lifestyle recommendations to halt, or at least slow, the disease progression cannot be understated. Preventing degenerative diseases through a blend of the frequent and regular monitoring of eye and vision health, coupled with educating patients that disease processes occur not just as a function of age, but through unhealthy lifestyle choices. These can be addressed through wellness and nutritional strategies and allow the clinician to offer a more personalised health care plan for patients.
- The next article in this series will focus on clinical assessment of the macula.
- Dr Rohit Narayan is a therapeutic optometrist with a specialist interest in holistic optometry.
Useful links
- Smoking cessation services; www.nhs.uk/better-health/quit-smoking
- Dietary advice; www.hsph.harvard.edu/nutritionsource/healthy-weight/diet-reviews/mediterranean-diet
- Nutrition and nutrient intake; www.nutrition.org.uk/attachments/article/234/Nutrition%20Requirements_Revised%20Oct%202016.pdf
- Lifestyle advice for optometrists; www.college-optometrists.org/membership/free-patient-resources/patient-leaflets.html
- Healthy life advice; www.aop.org.uk/ot/in-practice/business-management/2018/06/25/healthy-me-healthy-you
References
- American Academy of Ophthalmology. Age-related macular degeneration preferred practice pattern. file:///C:/Users/william/Desktop/Downloads/Age-related%20Macular%20Degeneration%20PPP_2022%20Update.pdf (Accessed 10/11/22)
- Li JQ et al. (2020) Prevalence and incidence of age-related macular degeneration in Europe: a systematic review and meta-analysis. British Journal of Ophthalmology 104(8), 1077-1084
- Wong, WL et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. The Lancet Global Health, 2(2), pp. e106-e116
- Royal College of Ophthalmologists. (2013) Age-related macular degeneration: guidelines for management. http://www.rcophth.ac.uk
- Owen C et al. (2012) The estimated prevalence and incidence of late-stage age related macular degeneration in the UK. British Journal of Ophthalmology 96(5), 752-756
- NICE. (2018) Age-related macular degeneration. National Institute for Health and Care Excellence. http://www.nice.org.uk
- Klein R et al. (2011) Prevalence of age-related macular degeneration in the US population. Archives of Ophthalmology; 129(1):75-80
- Taylor DJ et al. (2016) How does age-related macular degeneration affect real-world visual ability and quality of life? A systematic review. BMJ Open;6: e011504
- Owsley C et al. Delays in rod-mediated dark adaptation in early age-related maculopathy. Ophthalmology. 2001 Jul;108(7):1196-202
- Neely DC et al. Prevalence of undiagnosed age-related macular degeneration in primary eye care. JAMA Ophthalmology, 2017, 135(6), pp.570-575
- Hart KM et al. Optometry Australia’s chairside reference for the diagnosis and management of age-related macular degeneration. Clinical and Experimental Optometry, 2020, 103(3), pp.254-264
- BMJ Best Practice (2018) Age-related macular degeneration. British Medical Journal. https://bestpractice.bmj.com
- Lambert NG et al. Risk factors and biomarkers of age-related macular degeneration. Progress in Retinal and Eye Research, 2016 Sep;54:64-102
- Shahid, H et al. Genetic Factors in AMD Study Group. Age-related macular degeneration: the importance of family history as a risk factor. British Journal of Ophthalmology, 2012, 96(3), pp.427-431
- Warwick A et al. Genetics and genetic testing for age-related macular degeneration. Eye (London). 2018 May;32(5):849-857
- McCarty CA et al. How do patients respond to genetic testing for age-related macular degeneration? Optometry and Vision Science. 2018, Mar;95(3):166-170
- Carneiro  et al. Nutritional and lifestyle interventions for age-related macular degeneration: a review. Oxidative Medicine and Cellular Longevity, 2017:6469138
- Hobbs RP et al. Nutrient supplementation for age-related macular degeneration, cataract, and dry eye. Journal of Ophthalmic and Vision Research. 2014;9(4):487-493
- Kaufman SR et al. Genetics and age-related eye disease study formulation interaction in neovascular age-related macular degeneration. Journal of Vitreoretinal Diseases, 2020, p.2474126420941713
- Ardourel JE. Risk factors associated with age-related macular degeneration: a case control study in the Age-Related Eye Disease Study report number 3. Ophthalmology, 2000, vol. 107, no. 12, pp. 2224–2232
- Carneiro A et al. Nutritional and lifestyle interventions for age-related macular degeneration: a review. Oxidative Medicine and Cell Longevity, 2017; 2017: 6469138
- Cano M et al. Cigarette smoking, oxidative stress, the antioxidant response through Nrf2 signalling, and age-related macular degeneration. Vision Research, Volume 50, Issue 7, 31 March 2010, Pages 652–664
- Velilla S et al. Smoking and age-related macular degeneration: review and update. Journal of Ophthalmology, 2013, vol. 2013, Article ID 895147
- Joachim N et al. Five-year progression of unilateral age-related macular degeneration to bilateral involvement: the Three Continent AMD Consortium Report. British Journal of Ophthalmology, Published Online First: 20 January 2017
- Smith W et al. Risk factors for age-related macular degeneration: pooled findings from three continents. Ophthalmology, 2001, vol. 108, no. 4, pp. 697–704
- Chakravarthy U et al., ‘Cigarette smoking and age-related macular degeneration in the EUREYE Study. Ophthalmology, 2007, vol. 114, no. 6, pp. 1157–1163
- Jager RD et al. Age-related macular degeneration. The New England Journal of Medicine, 2008, vol. 358, no. 24, pp. 2606–2617
- Brandl C et al. (2016). Features of age-related macular degeneration in the general adults and their dependency on age, sex, and smoking: results from the German KORA study. PLoS ONE 11(11): e0167181
- Delcourt C et al. Smoking and age-related macular degeneration: the POLA study. Archives of Ophthalmology, 1998, vol. 116, no. 8, pp. 1031–1035
- Smith W et al. Smoking and age-related maculopathy. The Blue Mountains Eye Study. Archives of Ophthalmology, 1996,1141518–1523
- Roth F et al. Key pathophysiologic pathways in age-related macular disease. Graefe’s Archive for Clinical and Experimental Ophthalmology, 2004, vol. 242, no. 8, pp. 710-716
- Zhu BQ et al. Hemodynamic and vascular effects of active and passive smoking. American Heart Journal, 1995, vol. 130, no. 6, pp. 1270–1275
- Heeschen C et al. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nature Medicine, 2001, vol. 7, no. 7, pp. 833–839
- Pons M et al. Cigarette smoke-related hydroquinone dysregulates MCP-1, VEGF and PEDF expression in retinal pigment epithelium in vitro and in vivo. PLoS ONE, 2011, vol. 6, no. 2, Article ID e16722
- Pons M et al. Nicotine Increases the VEGF/PEDF Ratio in Retinal Pigment Epithelium: A Possible Mechanism for CNV in Passive Smokers with AMD. Investigative and Ophthalmological Visual Science, 2011 May; 52(6): 3842–3853
- Patton W et al. Retinal pigment epithelial cell DNA is damaged by exposure to benzo[a]pyrene, a constituent of cigarette smoke. Experimental Eye Research, 2002, vol. 74, no. 4, 513–522
- Kijlstra A et al. Age-related macular degeneration: a complementopathy? Ophthalmic Research, 2015;54(2):64-73
- Beatty S et al. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Survey of Ophthalmology, 2000, vol. 45, no. 2, 115-134
- DeCaprio AP. The toxicology of hydroquinone; relevance to occupational and environmental exposure. Critical Reviews in Toxicology, 1999, vol. 29, no. 3, 283-330
- Chapman NA et al. Role of diet and food intake in age-related macular degeneration: a systematic review. Clinical & Experimental Ophthalmology, 2019, Jan;47(1):106-127
- AREDS Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age- related macular degeneration and vision loss: AREDS report no. 8. Archives of Ophthalmology, 2001, vol. 119, no. 10, 1417–1436
- Chew EY et al. Long-term effects of vitamins C and E, beta-carotene and zinc on age-related macular degeneration. AREDS Report no. 35. Ophthalmology 2013; 120: 1604-1611
- The Age-Related Eye Disease Study 2 (AREDS2) Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age- related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA, 2013, vol. 309, no.19, 2005-2015
- Klein EA et al. Vitamin E and the risk of prostate cancer: results of the selenium and vitamin e cancer prevention trial (SELECT). JAMA, 2011; 306(14) 1549-1556
- Augood C et al. Oily fish consumption, dietary docosahexaenoic acid and eicosapentaenoic acid intakes, and associations with neovascular age-related macular degeneration. American Journal of Clinical Nutrition, 2008;88:398-406
- Mares-Perlman JA et al. Lutein and zeaxanthin in the diet and serum and their relation to age-related maculopathy in the third national health and nutrition examination survey. American Journal of Epidemiology, 2001;153:424–432
- Moeller SM et al. CAREDS Research Study Group: Associations betwewen intermediate age-related macular degeneration and lutein and zeaxanthin in the Carotenoids in Age-Related Eye Disease Study (CAREDS): Ancillary study of the Women’s Health Initiative. Archives of Ophthalmology, 2006;124: 1151–1162
- Gherghel D et al. Obesity and ocular health. OPTICIAN, 19.04.2019, pp 26-31
- Adams MK et al. Abdominal obesity and age-related macular degeneration. American Journal of Epidemiology, 2011, 173(11), pp.1246-1255
- Singh N et al. Prevention of age-related macular degeneration. The Asia-Pacific Journal of Ophthalmology, 2017, 6(6), pp.520-526
- Zhang QY et al. Overweight, obesity, and risk of age-related macular degeneration. Investigative Ophthalmology & Visual Science, 2016, 57(3), pp.1276-1283
- Noble RE. Waist-to-hip ratio versus BMI as predictors of cardiac risk in obese adult women. Western Journal of Medicine, 2001;174(4):240-241
- Seddon JM et al. Progression of age-related macular degeneration: association with dietary fat, trans-unsaturated fat, nuts, and fish intake. Archives of Ophthalmology, 2003, 121(12), pp.1728-1737
- Peeters A et al. Changes in Abdominal Obesity and Age-Related Macular Degeneration: The Atherosclerosis Risk in Communities Study. Archives of Ophthalmology. 2008;126(11):1554–1560
- Peeters A et al. Changes in the rates of weight and waist circumference gain in Australian adults over time: A longitudinal cohort study. BMJ Open, 2014, 4. e003667
- Chung HY et al. Site-specific concentrations of carotenoids in adipose tissue: relations with dietary and serum carotenoid concentrations in healthy adults. The American Journal of Clinical Nutrition, 2009, 90(3), pp.533-539
- Obana A et al. Effect of an antioxidant supplement containing high dose lutein and zeaxanthin on macular pigment and skin carotenoid levels. Scientific Reports, 2020, 10(1), pp.1-12
- Cannon B et al. Brown adipose tissue: function and physiological significance. Physiology Reviews, 2004 Jan;84(1):277-359
- Schick T et al. History of sunlight exposure is a risk factor for age-related macular degeneration. Retina, 2016; 36:787–90
- Smith W et al. Risk factors for age-related macular degeneration: Pooled findings from three continents. Ophthalmology. 2001b; 108:697–704
- Downie LE et al. Blue-light filtering intraocular lenses (IOLs) for protecting macular health. Cochrane Database of Systematic Reviews 2018, Issue 5. Art. No.: CD011977
- Downie LE et al. What do patients think about the role of optometrists in providing advice about smoking and nutrition? Ophthalmic and Physiological Optics, 2017; 37: 202–211
- Stevens R et al. Dietary analysis and nutritional behaviour in people with and without age-related macular disease. Clinical Nutrition ESPEN, 2015,10(3), e112-e117
- Taylor DJ et al. Measuring dynamic levels of self-perceived anxiety and concern during simulated mobility tasks in people with non-neovascular age-related macular degeneration. British Journal of Ophthalmology 2020;104:529-534
- Stevens R et al. Testing the impact of an educational intervention designed to promote ocular health among people with age-related macular degeneration. British Journal of Visual Impairment, 2018, 36(2), pp.110-127
